Preparation and Characterization of Semi-Alicyclic Polyimide Resins and the Derived Alignment Layers for Liquid Crystal Display Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
2.3. PI Resin Synthesis and Varnish Preparation
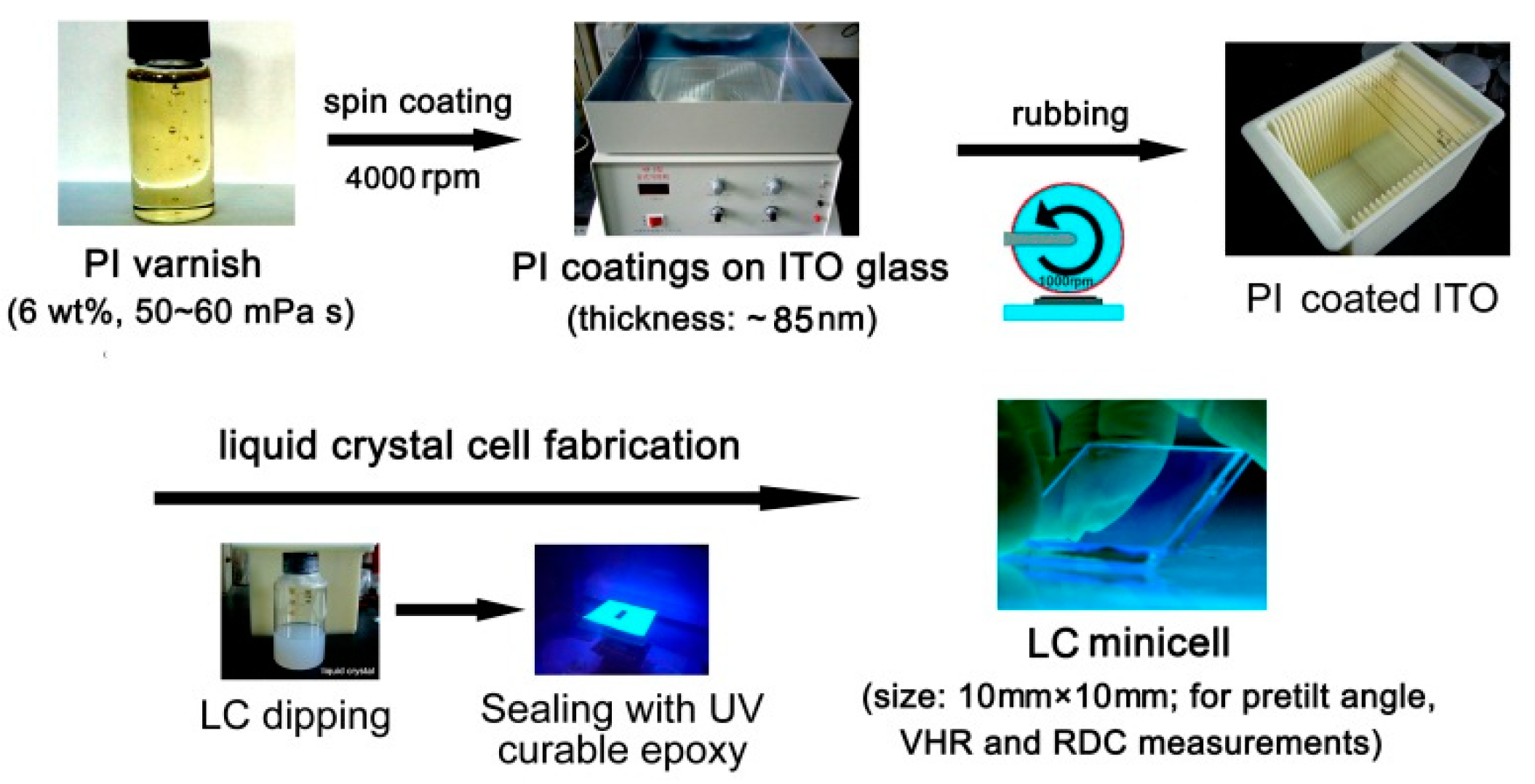
2.4. Fabrication of Liquid Crystal (LC) Minicells
3. Results and Discussion
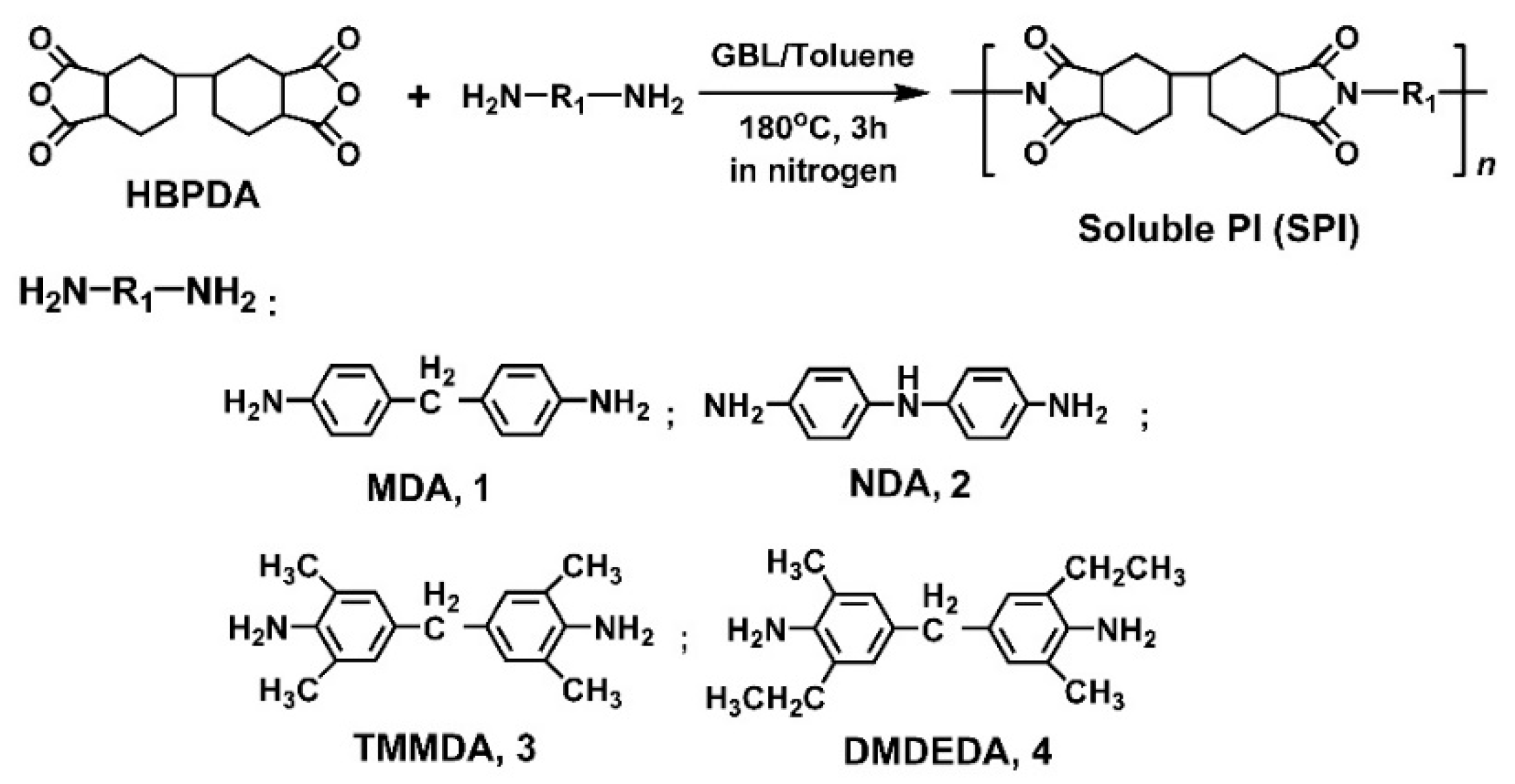
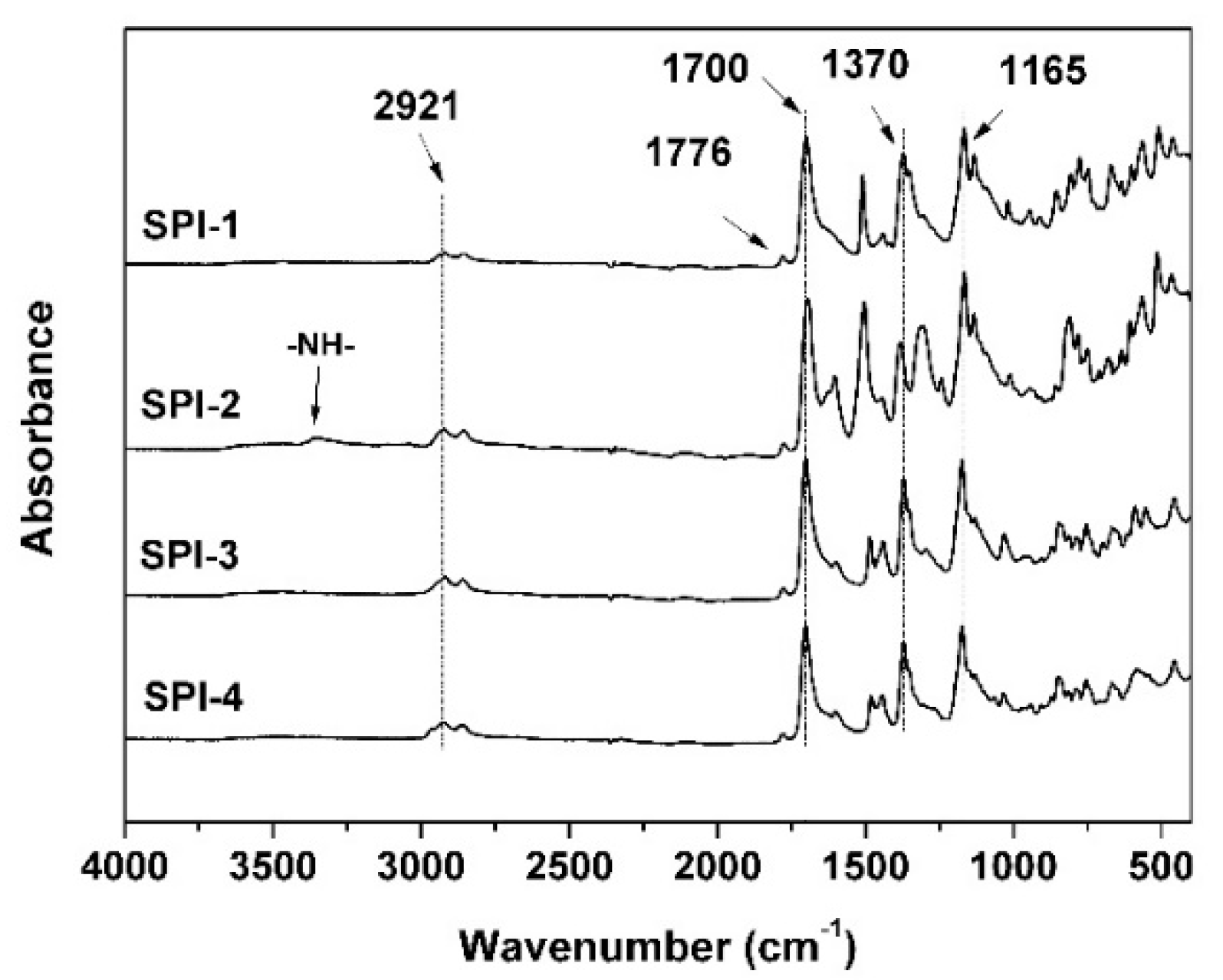
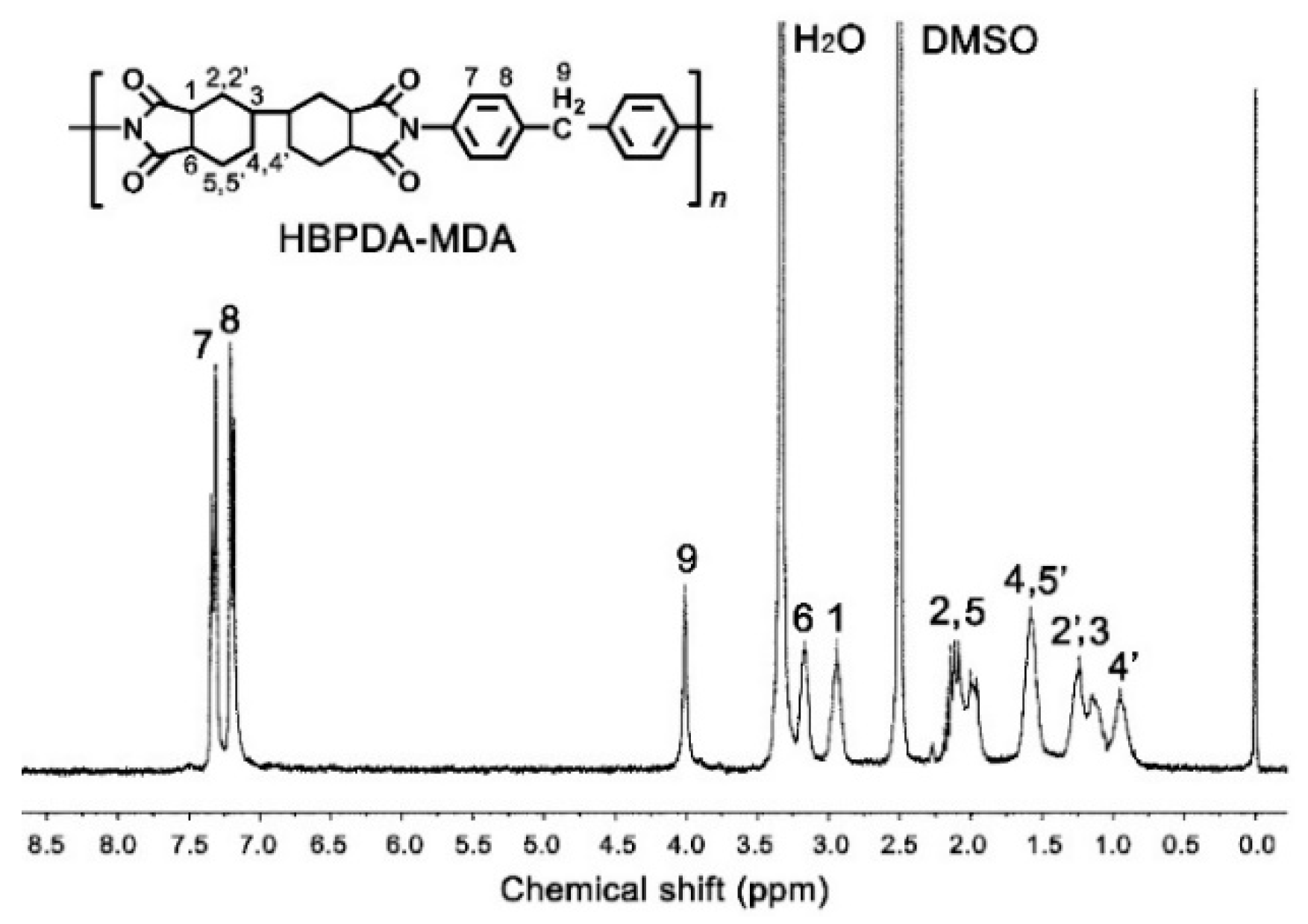
3.1. PI Synthesis and Alignment Agent Preparation
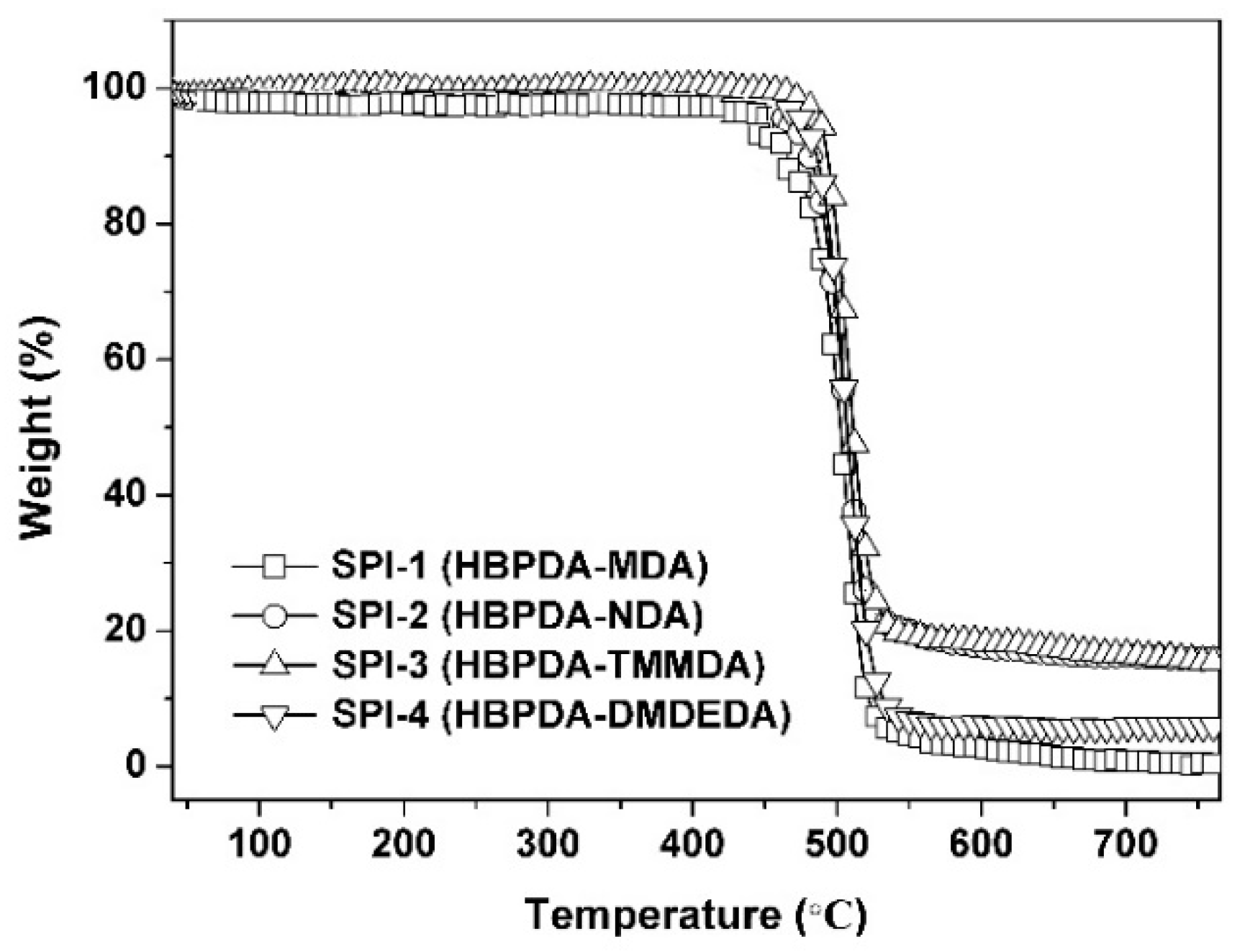
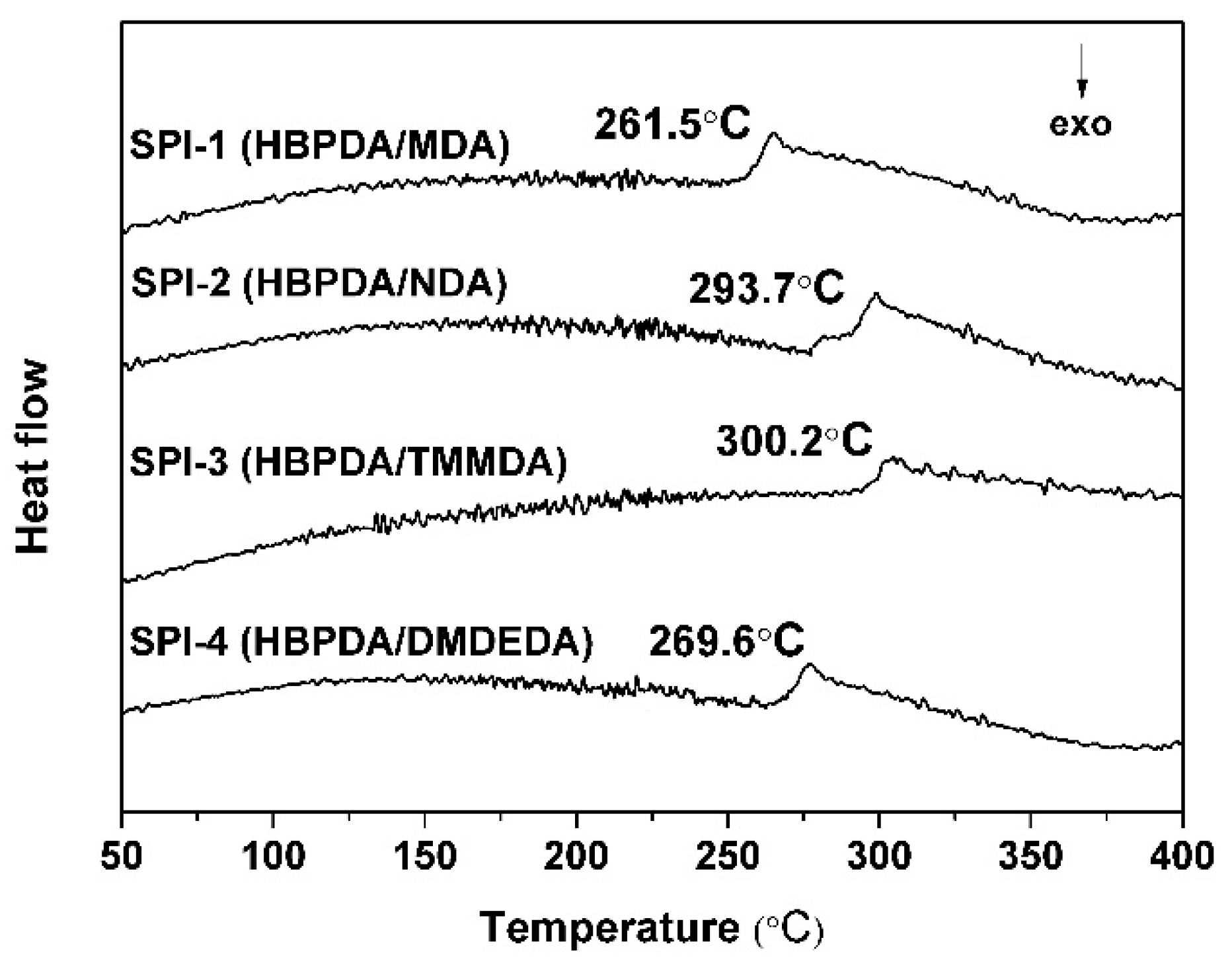
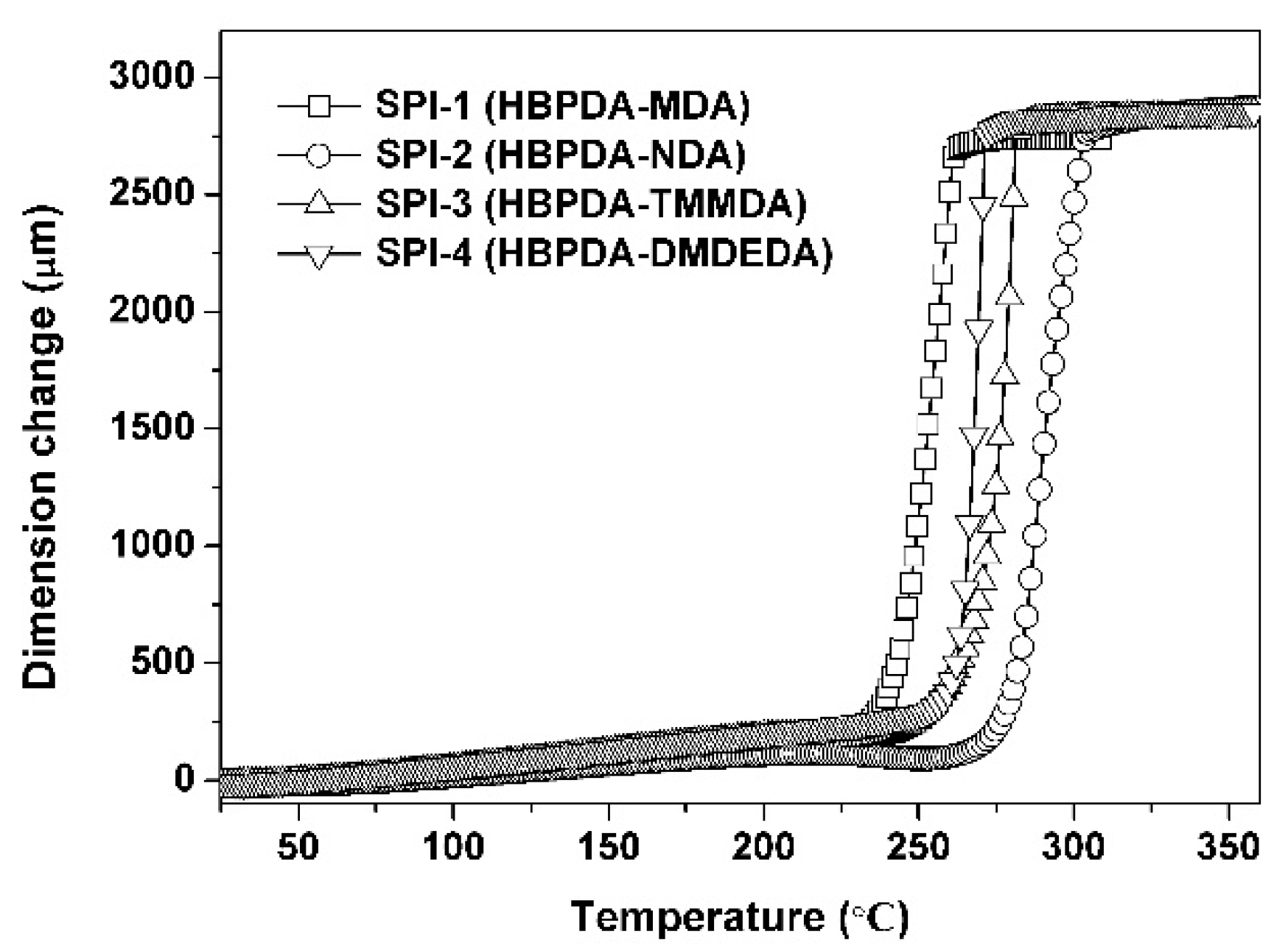
3.2. Thermal Properties
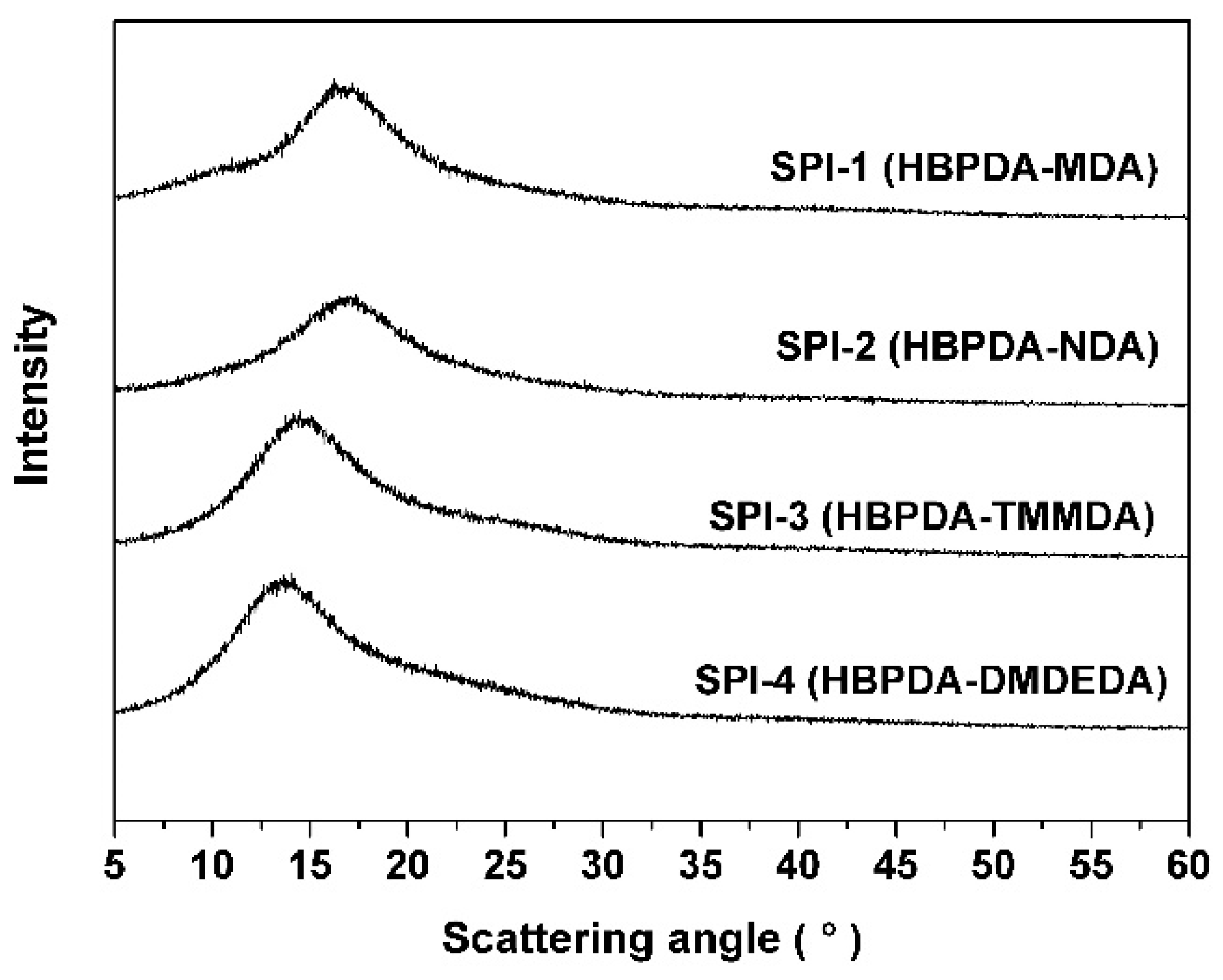
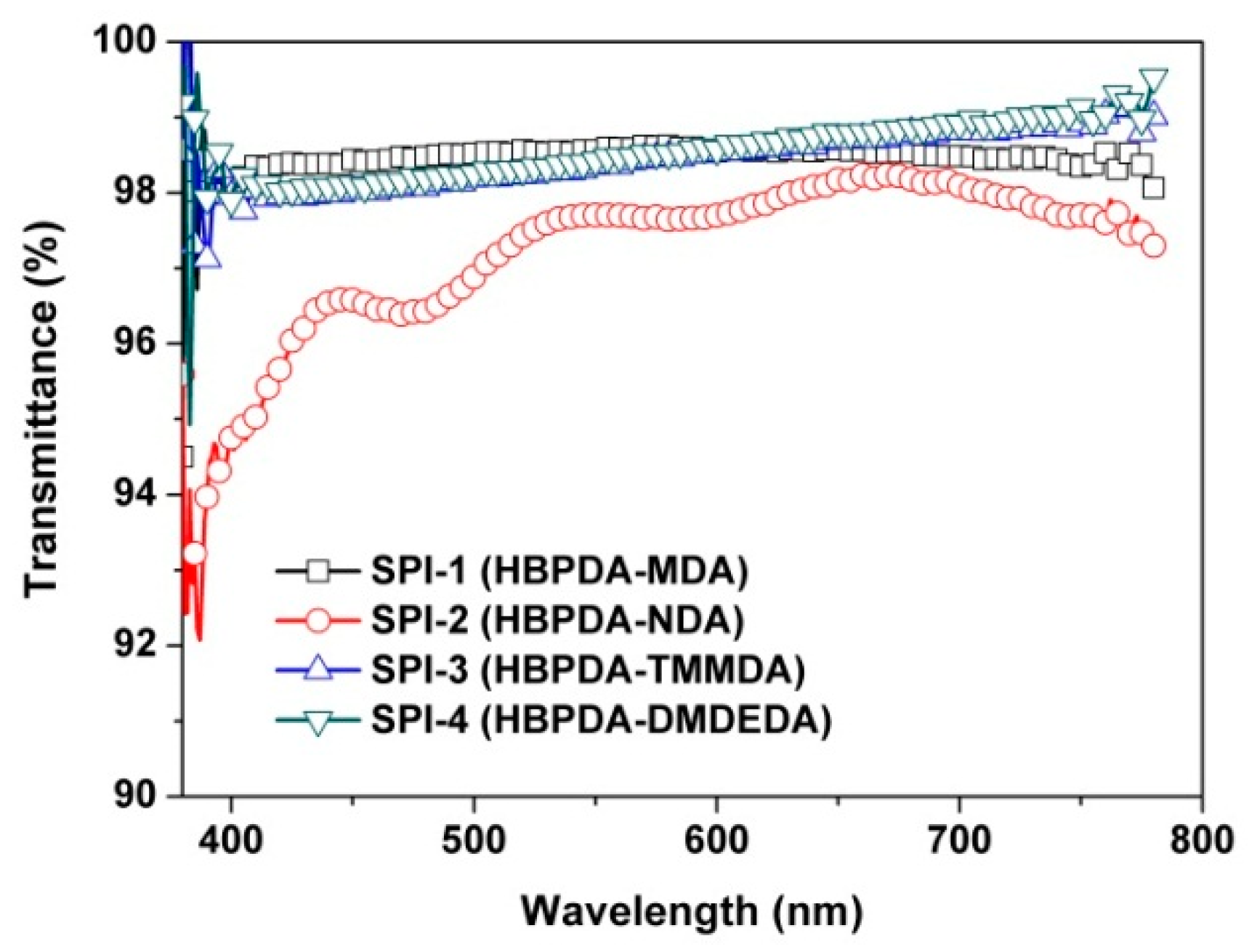
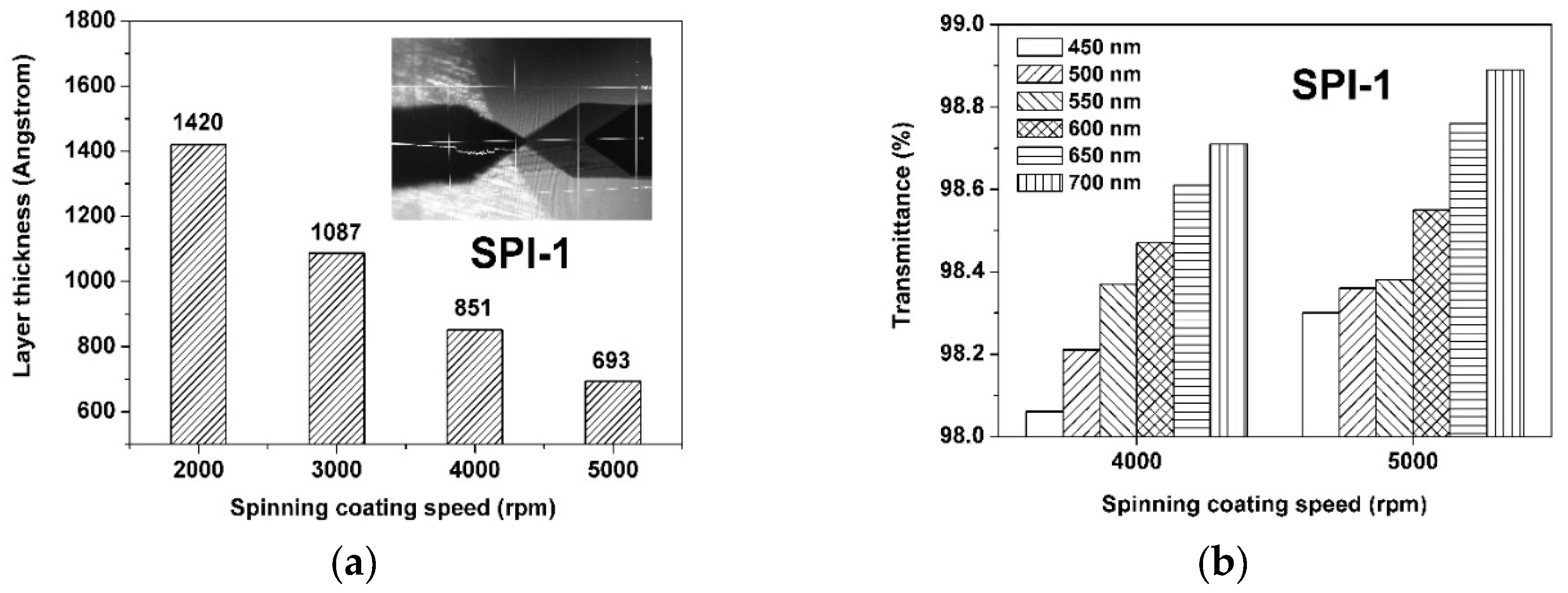
3.3. Optical Properties
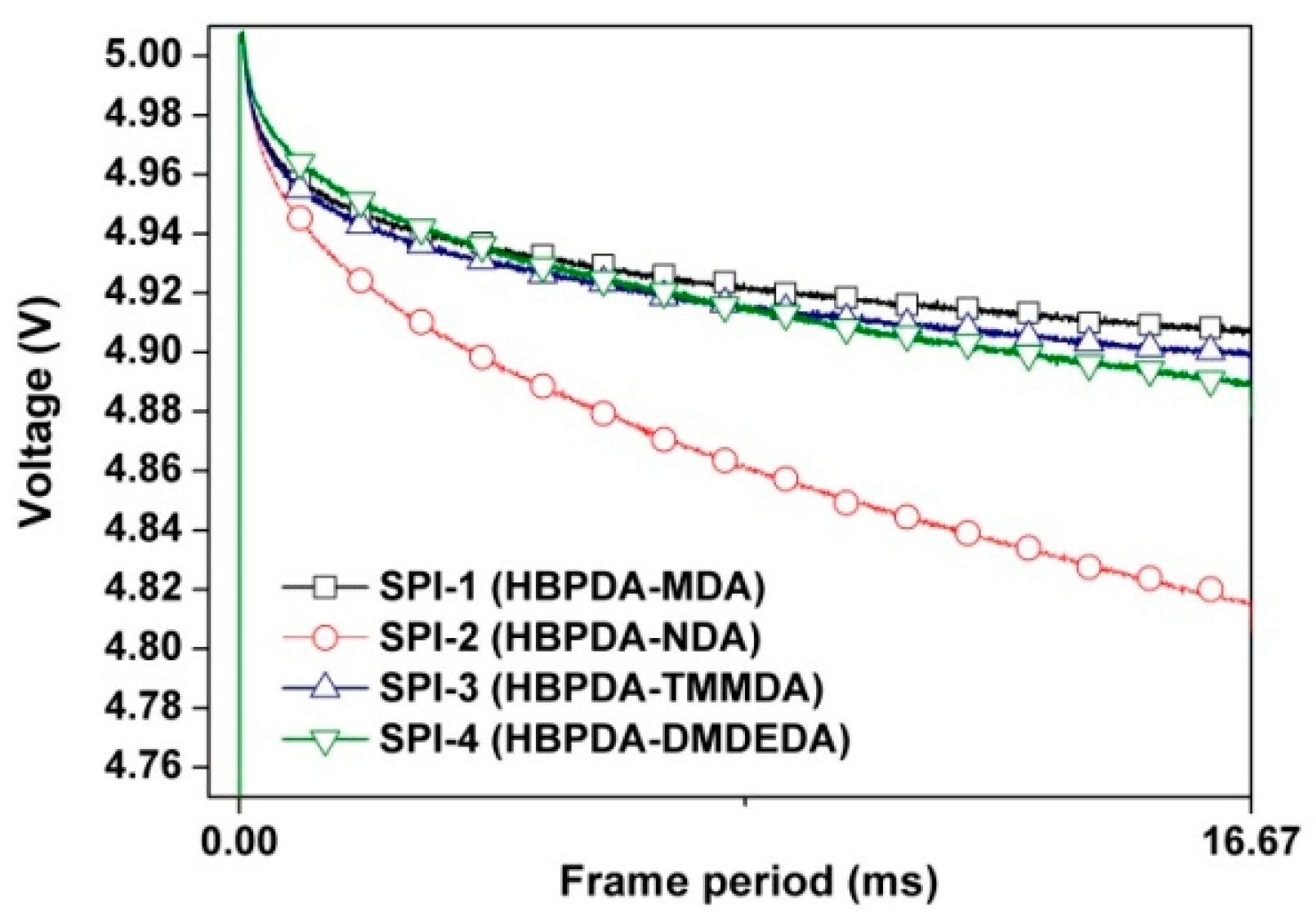
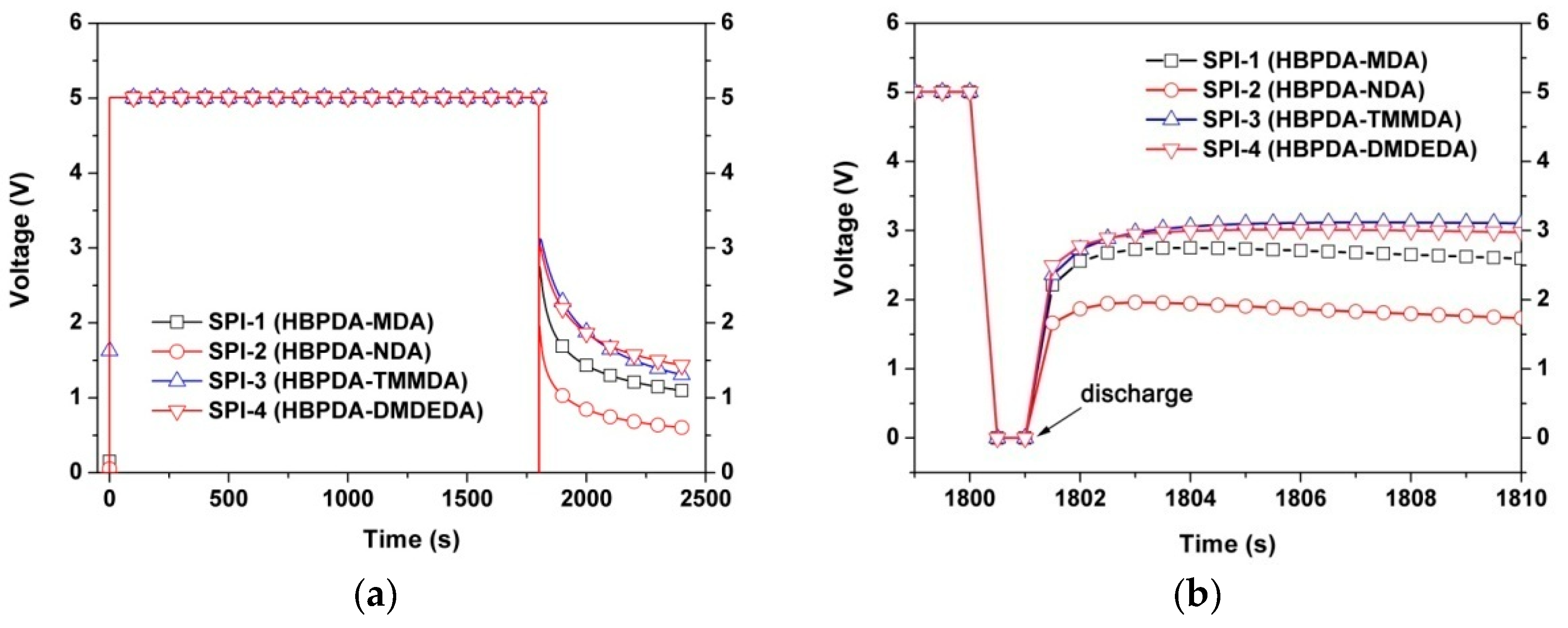
3.4. Optoelectronic Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bu, L.; Carbajal, L.; Chang, M.; Chen, J.; Johoson, J.C.; Kourtakis, K.; Lamontia, M.; Li, A.; Liao, K.; Liu, S.; et al. Advanced materials for flexible displays. SID Symp. Digest Tech. Pap. 2019, 50, 727–730. [Google Scholar] [CrossRef]
- Koo, J.H.; Kim, D.C.; Shim, H.J.; Kim, T.H.; Kim, D.H. Flexible and stretchable smart display: Materials, fabrication, device design, and system integration. Adv. Funct. Mater. 2018, 28, 1801834. [Google Scholar] [CrossRef]
- Chen, H.; Lee, J.; Lin, B.; Chen, S.; Wu, S.T. Liquid crystal display and organic light-emitting diode display: Present status and future perspectives. Light Sci. Appl. 2018, 7, 17168. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Hyodo, Y.; Jin, L.; Yamaguchi, Y.; Asozu, G.; Higano, E.; Higano, T.; Komura, S. High resolution IPS-LCDs fabricated with transparent polyimide substrates. SID Symp. Digest Tech. Pap. 2018, 49, 764–767. [Google Scholar] [CrossRef]
- Lee, T.R.; Kim, J.H.; Lee, S.H.; Jun, M.C.; Baik, H.K. Investigation on newly designed low resistivity polyimide-type alignment layer for reducing DC image sticking of in-plane switching liquid crystal display. Liq. Cryst. 2017, 44, 738–747. [Google Scholar] [CrossRef]
- Song, Y.Z.; Yuan, L.L.; Wang, Z.Y.; Yang, S.Y. Photo-aligning of polyimide layers for liquid crystals. Polym. Adv. Technol. 2019, 30, 1243–1250. [Google Scholar] [CrossRef]
- Rim, L.T.; Kim, J.H.; Jun, M.C.; Lee, S.H.; Baik, H.K. Optimisation of alignment materials for minimising residual retardation in in-plane switching liquid crystal display. Liq. Cryst. 2017, 44, 500–509. [Google Scholar] [CrossRef]
- Lin, G.J.; Chen, T.J.; Lin, B.R.; Liao, H.I.; Chang, Y.; Wu, J.J.; Yang, Y.J. Effects of pretilt angle on electro-optical properties of optically compensated bend liquid crystal devices with a mixed polyimide alignment layer. J. Disp. Technol. 2016, 12, 302–308. [Google Scholar] [CrossRef]
- Chen, P.A.; Yang, K.H. Ionic effects on electro-optics and residual direct current voltages of twisted nematic liquid crystal cells. Liq. Cryst. 2018, 45, 1032–1039. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.H.; Kwon, Y.R.; Ahn, S.H.; Srivastava, A.K.; Lee, S.H. Investigation on ion movement in the fringe-field switching mode depending on resistivity of alignment layer and dielectric anisotropic sign of liquid crystal. Liq. Cryst. 2015, 42, 486–491. [Google Scholar] [CrossRef]
- Ge, J.J.; Li, C.Y.; Xue, G.; Mann, I.K.; Zhang, D.; Wang, S.Y.; Harris, F.W.; Cheng, S.Z.D.; Hong, S.C.; Zhang, X.W.; et al. Rubbing-induced molecular reorientation on an alignment surface of an aromatic polyimide containing cyanobiphenyl side chains. J. Am. Chem. Soc. 2001, 123, 5768–5776. [Google Scholar] [CrossRef] [PubMed]
- Hahm, S.G.; Ko, Y.G.; Rho, Y.; Ahn, B.; Ree, M. Liquid crystal alignment in advanced flat-panel liquid crystal displays. Curr. Opin. Chem. Eng. 2013, 2, 71–78. [Google Scholar] [CrossRef]
- Dong, W.; Paek, S.H. Chemical structural effects of polyimides on the alignment and electro-optical properties of liquid crystal cells. Macromol. Res. 2004, 12, 251–257. [Google Scholar] [CrossRef]
- Momoi, Y.; Sato, O.; Koda, T.; Nishioka, A.; Haba, O.; Yonetake, K. Surface rheology of rubbed polyimide film in liquid crystal display. Opt. Mater. Exp. 2014, 4, 1057. [Google Scholar] [CrossRef]
- Nishikawa, M. Design of polyimides for liquid crystal alignment films. Polym. Adv. Technol. 2000, 11, 404–412. [Google Scholar] [CrossRef]
- Fukuro, H.; Sawahata, K.; Sato, T.; Endo, H. Optimal alignment materials and technologies for various LCDs. SID Symp. Digest Tech. Pap. 2000, 31, 434–437. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kwak, C.H.; Park, H.; Lee, J.H.; Jun, M.C.; Kang, I.B. Phase separation of Photo-Aligned polyimide blends for robust reliability. SID Symp. Digest. Tech. Pap. 2017, 48, 582–584. [Google Scholar] [CrossRef]
- Nishikawa, M. Development of novel polyimide alignment films for liquid crystal display televisions. J. Photopolym. Sci. Technol. 2011, 24, 317–320. [Google Scholar] [CrossRef][Green Version]
- Chang, C.J.; Chou, R.L.; Lin, Y.C.; Liang, B.J.; Chen, J.J. Effects of backbone conformation and surface texture of polyimide alignment film on the pretilt angle of liquid crystals. Thin Solid Films 2011, 519, 5013–5016. [Google Scholar] [CrossRef]
- Zhuang, Y.; Seong, J.G.; Lee, Y.M. Polyimides containing aliphatic/alicyclic segments in the main chains. Prog. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Lu, L.; Huang, Y.; Zhang, K.; Bos, P.; Liu, N.; Twieg, R.J.; Auman, B.; Bhowmik, A.; Bos, P. Reduced residual DC voltages in LCDs by using fluorinated polyimides. J. Soc. Inform. Dig. 2011, 19, 447–452. [Google Scholar] [CrossRef]
- Guo, Y.; Shen, D.; Ni, H.; Liu, J.; Yang, S. Organosoluble semi-alicyclic polyimides derived from 3,4-dicarboxy-1,2,3,4-tetrahydro-6-tert-butyl-1-naphthalene succinic dianhydride and aromatic diamines: Synthesis, characterization and thermal degradation investigation. Prog. Org. Coat. 2013, 76, 768–777. [Google Scholar] [CrossRef]
- Tsuda, Y.; Kojima, M.; Matsuda, T.; Oh, J.M. Soluble polyimides based on long-chain alkyl groups via amide linkages. Polym. J. 2008, 40, 354–366. [Google Scholar] [CrossRef]
- Zhi, X.X.; Bi, H.S.; Liu, J.G.; Gao, Y.S.; Zhang, Y.L.; Zhou, Y.C.; Zhang, Y.; Wu, X.; Zhang, X.M. Synthesis and characterization of soluble ester-containing polyimide alignment layers with high voltage holding ratio features and potential applications in TFT-LCDs. eXPRESS Polym. Lett. 2019, 13, 923–936. [Google Scholar] [CrossRef]
- Zhi, X.X.; Bi, H.S.; Gao, Y.S.; Liu, J.G.; Chen, J.; Gao, K.Y.; Zhang, X.M. Preparation and characterization of novel preimidized semi-alicyclic polyimide alignment layers with low curing temperature and high voltage holding ratio for TFT-LCDs. Chem. Lett. 2019, 48, 654–657. [Google Scholar] [CrossRef]
- Lee, W.C.; Chen, J.T.; Hsu, C.S.; Wu, S.T. Synthesis of alkyl-branched main chain copolyimides and their effect on the pretilt angles of liquid crystal alignment. Liq. Cryst. 2002, 29, 907–913. [Google Scholar] [CrossRef]
- Salley, J.M.; Frank, C.W. Charge Transfer in Aromatic Polyimides. In Polyimides: Fundamentals and Applications; Ghosh, M.K., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 279–307. [Google Scholar]
- Qi, L.; Guo, C.Y.; Huang Fu, M.G.; Zhang, Y.; Yin, L.; Wu, L.; Liu, J.G.; Zhang, X.M. Enhancement of solvent resistance of polyimide electrospun mat via the UV-Assisted electrospinning and photosensitive Varnish. Polymers 2019, 11, 2055. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Chen, W.T. Syntheses and properties of aromatic polyimides based on 1,1-bis [4-(4-aminophenoxy) phenyl]-1-phenyl-2,2,2-trifluoroethane and 1,1-bis[4-(4-aminophenoxy) phenyl]-1- phenyl-ethane. J. Polym. Res. 2003, 10, 95–103. [Google Scholar] [CrossRef]
- Jung, Y.; Yang, Y.; Kim, S.; Kim, H.S.; Park, T.; Yoo, B.W. Structural and compositional effects on thermal expansion behavior in polyimide substrates of varying thicknesses. Eur. Polym. J. 2013, 49, 3642–3650. [Google Scholar] [CrossRef]
- Medvedovski, E.; Alvarez, N.; Yankov, O.; Olsson, M.K. Advanced indium-tin oxide ceramics for sputtering targets. Ceram. Int. 2008, 34, 1173–1182. [Google Scholar] [CrossRef]
- Lee, S.H.; Bhattacharyya, S.S.; Jin, H.S.; Jeong, K.U. Devices and materials for high-performance mobile liquid crystal displays. J. Mater. Chem. 2012, 22, 11893–11903. [Google Scholar] [CrossRef]
- Ni, H.J.; Liu, J.G.; Wang, Z.H.; Yang, S.Y. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Arafune, R.; Sakamoto, K.; Ushioda, S.; Tanioka, S.; Murata, S. Importance of rubbing-induced inclination of polyimide backbone structures for determination of the pretilt angle of liquid crystals. Phys. Rev. E 1998, 58, 5914–5918. [Google Scholar] [CrossRef]
- Seo, J.; Lee, S.; Yoon, T.; Kim, J. Basic properties of cell fabricated by ion-beam treatment for in-plane switching LCD. J. Inform. Disp. 2006, 7, 12–18. [Google Scholar] [CrossRef]
- Ban, B.S.; Rim, Y.N.; Kim, Y.B. Pretilt angle for BTDA, PMDA and CPDA series polyimides with various side chain lengths. Liq. Cryst. 2000, 27, 125–130. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, W.J.; Lee, T.H.; Kim, J.E.; Suh, K.-S. Electrical conduction of polyimide films prepared from polyamic acid (PAA) and pre-imidized polyimide (PI) solution. eXpress Polym. Lett. 2007, 7, 427–432. [Google Scholar] [CrossRef]

| Items | PAA Type | SPI Type |
|---|---|---|
| Storage stability | Poor | Good |
| Printability | Good | Moderate |
| Curing temperature | Moderate to high | Low |
| Adhesion to substrate | High | Moderate |
| Rubbing endurance | High | Moderate to poor |
| Volume resistivity | Low | High |
| VHR feature of devices | Poor | High |
| RDC feature of devices | High | Poor |
| PI | ηinha (dL/g) | Molecular Weight b | Solubility c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn (g/mol) | Mw (g/mol) | PDI | NMP | DMAc | GBL | BC | DPM | ||
| SPI-1 | 1.06 | 55,407 | 104,198 | 1.88 | ++ | ++ | ++ | − | − |
| SPI-2 | 0.88 | 63,285 | 127,154 | 2.01 | ++ | ++ | ++ | − | − |
| SPI-3 | 0.86 | 38,683 | 72,209 | 1.87 | ++ | ++ | ++ | − | − |
| SPI-4 | 0.73 | 43,857 | 80,054 | 1.83 | ++ | ++ | ++ | − | − |
| PI | Tg (°C) a | T5% (°C) b | T10% (°C) b | Rw700 (%) c | CTE (×10−6/K) d |
|---|---|---|---|---|---|
| SPI-1 | 261.5 | 461.8 | 476.8 | 0.8 | 62.2 |
| SPI-2 | 281.7 | 468.3 | 481.5 | 16.4 | 53.2 |
| SPI-3 | 300.2 | 446.7 | 454.2 | 16.5 | 79.0 |
| SPI-4 | 269.6 | 476.1 | 486.0 | 5.9 | 81.6 |
| PI | da (Å) | T550b (%) | θpc (o) | VHR d (%) | RDC e (mV) |
|---|---|---|---|---|---|
| SPI-1 | 851 | 98.4 | 1.58 | 97.81 | 1098 |
| SPI-2 | 850 | 97.7 | 1.62 | 96.11 | 605 |
| SPI-3 | 855 | 98.3 | 1.92 | 97.58 | 1312 |
| SPI-4 | 853 | 98.2 | 1.97 | 97.57 | 1438 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, H.-s.; Zhi, X.-x.; Wu, P.-h.; Zhang, Y.; Wu, L.; Tan, Y.-y.; Jia, Y.-J.; Liu, J.-g.; Zhang, X.-m. Preparation and Characterization of Semi-Alicyclic Polyimide Resins and the Derived Alignment Layers for Liquid Crystal Display Technology. Polymers 2020, 12, 217. https://doi.org/10.3390/polym12010217
Bi H-s, Zhi X-x, Wu P-h, Zhang Y, Wu L, Tan Y-y, Jia Y-J, Liu J-g, Zhang X-m. Preparation and Characterization of Semi-Alicyclic Polyimide Resins and the Derived Alignment Layers for Liquid Crystal Display Technology. Polymers. 2020; 12(1):217. https://doi.org/10.3390/polym12010217
Chicago/Turabian StyleBi, Hong-sheng, Xin-xin Zhi, Peng-hui Wu, Yan Zhang, Lin Wu, Yao-yao Tan, Yan-Jiang Jia, Jin-gang Liu, and Xiu-min Zhang. 2020. "Preparation and Characterization of Semi-Alicyclic Polyimide Resins and the Derived Alignment Layers for Liquid Crystal Display Technology" Polymers 12, no. 1: 217. https://doi.org/10.3390/polym12010217
APA StyleBi, H.-s., Zhi, X.-x., Wu, P.-h., Zhang, Y., Wu, L., Tan, Y.-y., Jia, Y.-J., Liu, J.-g., & Zhang, X.-m. (2020). Preparation and Characterization of Semi-Alicyclic Polyimide Resins and the Derived Alignment Layers for Liquid Crystal Display Technology. Polymers, 12(1), 217. https://doi.org/10.3390/polym12010217






