Investigation of Synthesis Mechanism, Optimal Hot-Pressing Conditions, and Curing Behavior of Sucrose and Ammonium Dihydrogen Phosphate Adhesive
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Procedures of SADP Adhesive
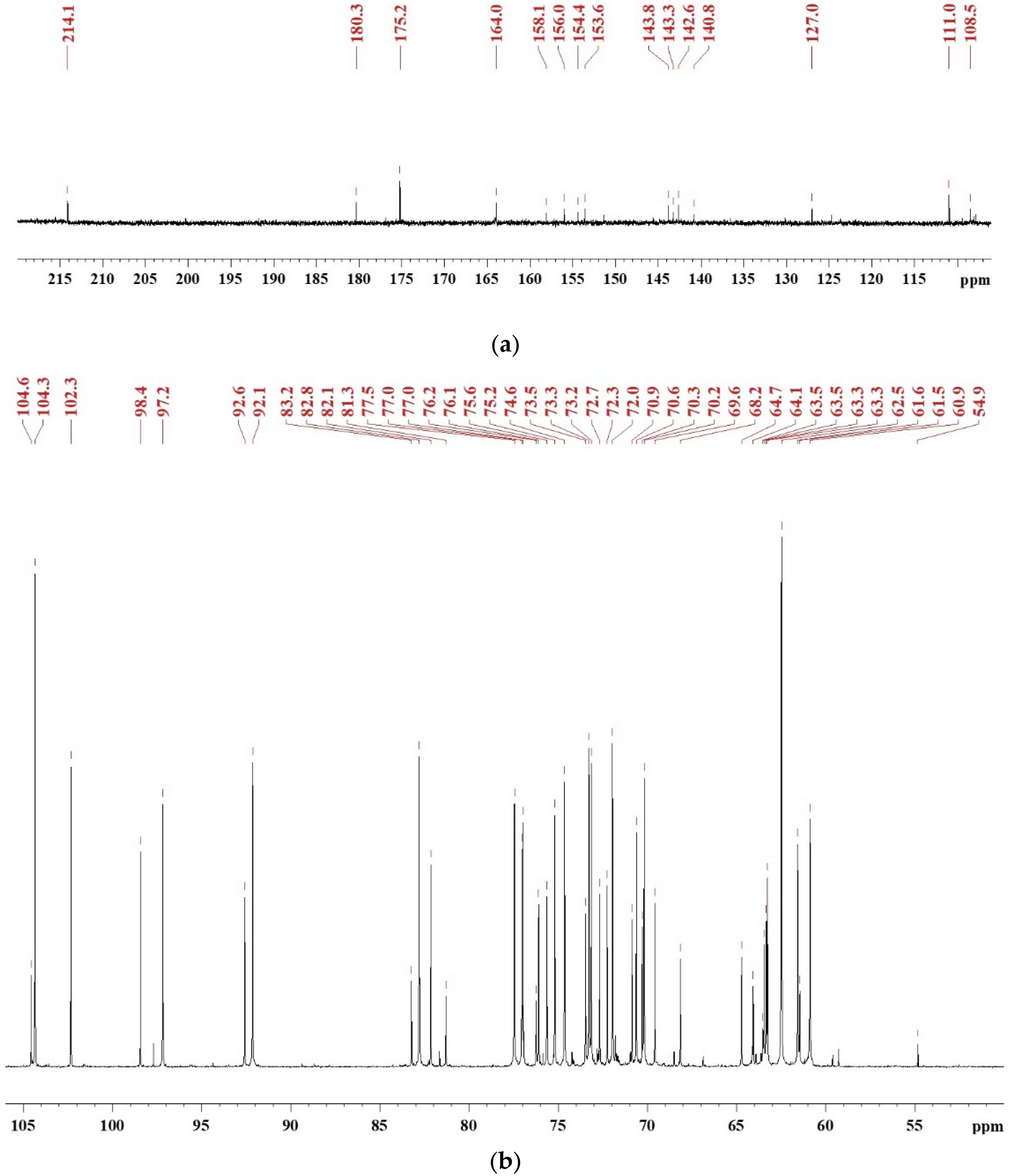
2.3. 13C Nuclear Magnetic Resonance (NMR) Analysis
2.4. Effects of Hot Pressing Conditions on the Bond Performance of SADP Adhesive
2.4.1. Manufacture of Plywood
2.4.2. Shear Strength Measurement
2.5. Curing Behaviour of SADP Adhesive
2.5.1. Thermal Analysis
2.5.2. Insoluble Mass Proportion
2.5.3. Fourier Transform Infrared Spectra (FT-IR)
2.5.4. Pyrolysis Gas Chromatography and Mass Spectrometry (Py-GC/MS)
3. Results and Discussion
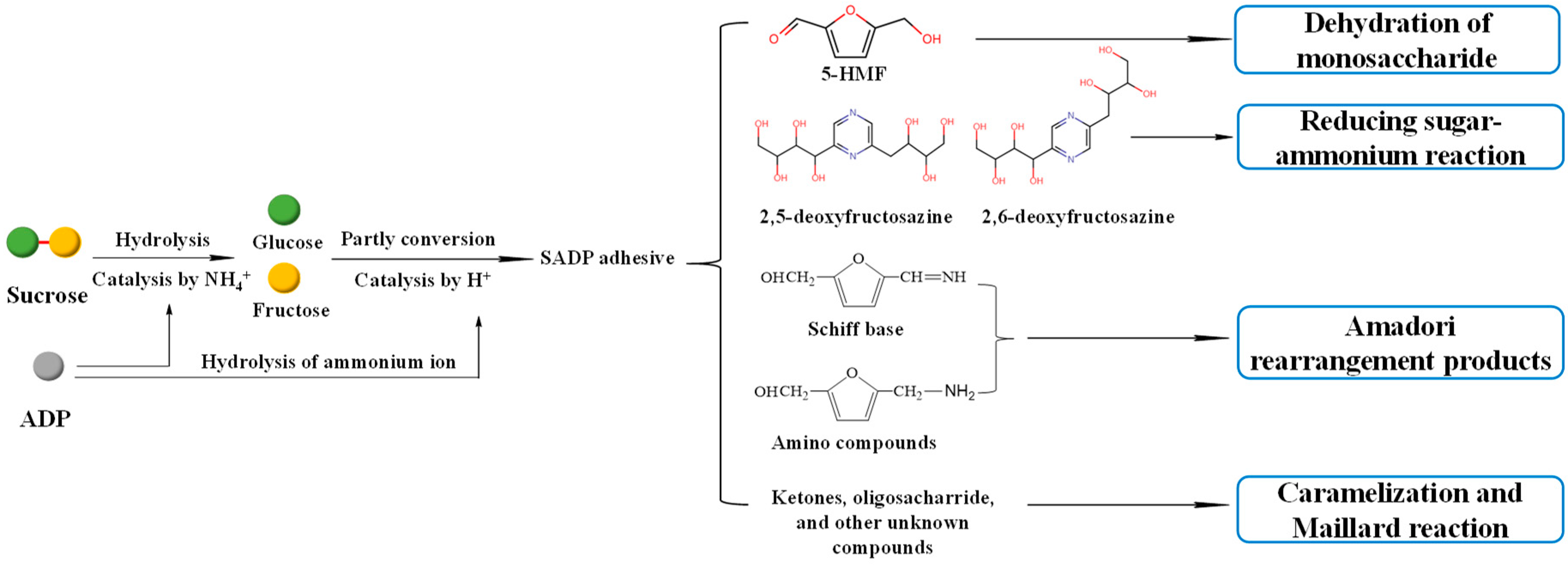
3.1. Synthesis Mechanism of SADP Adhesive
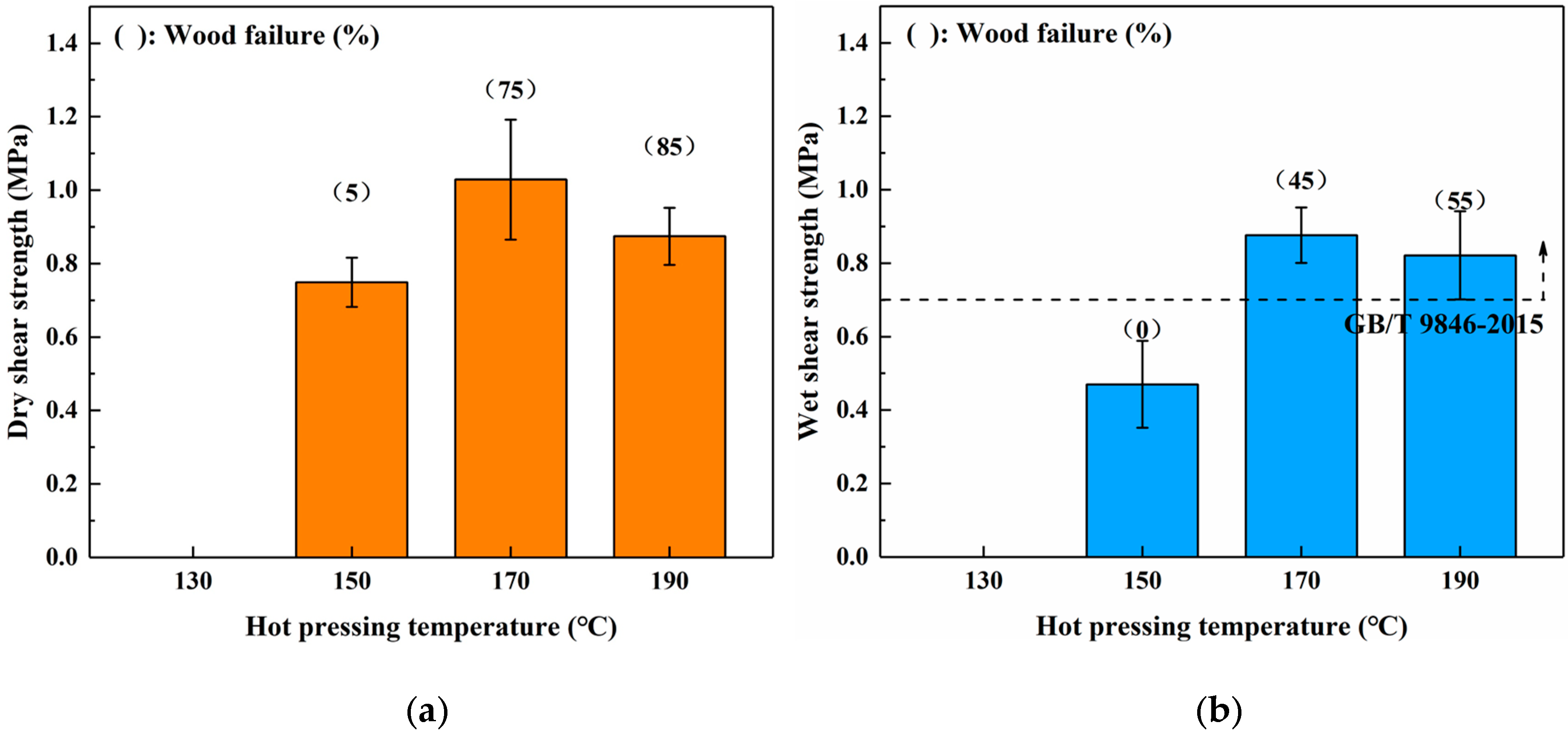
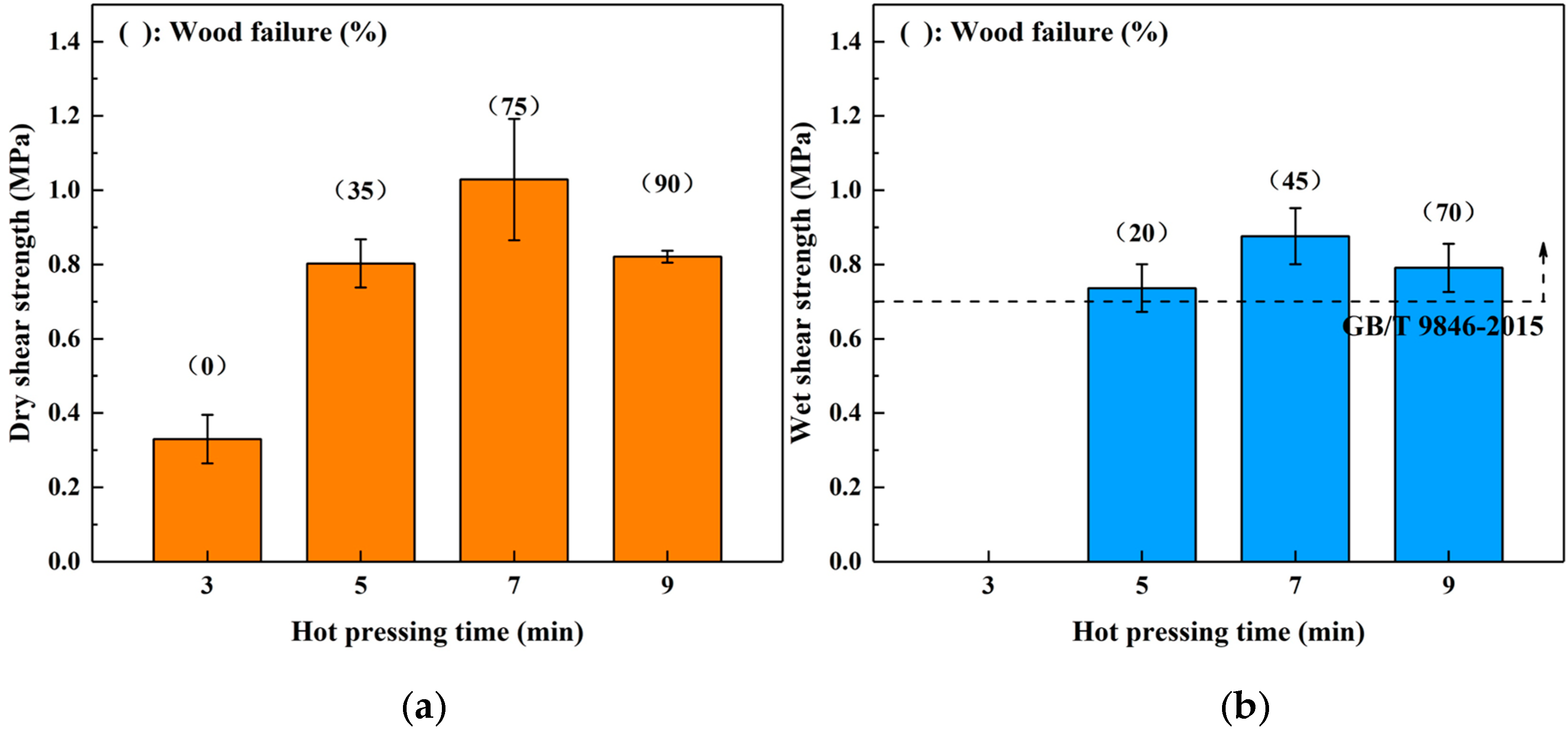
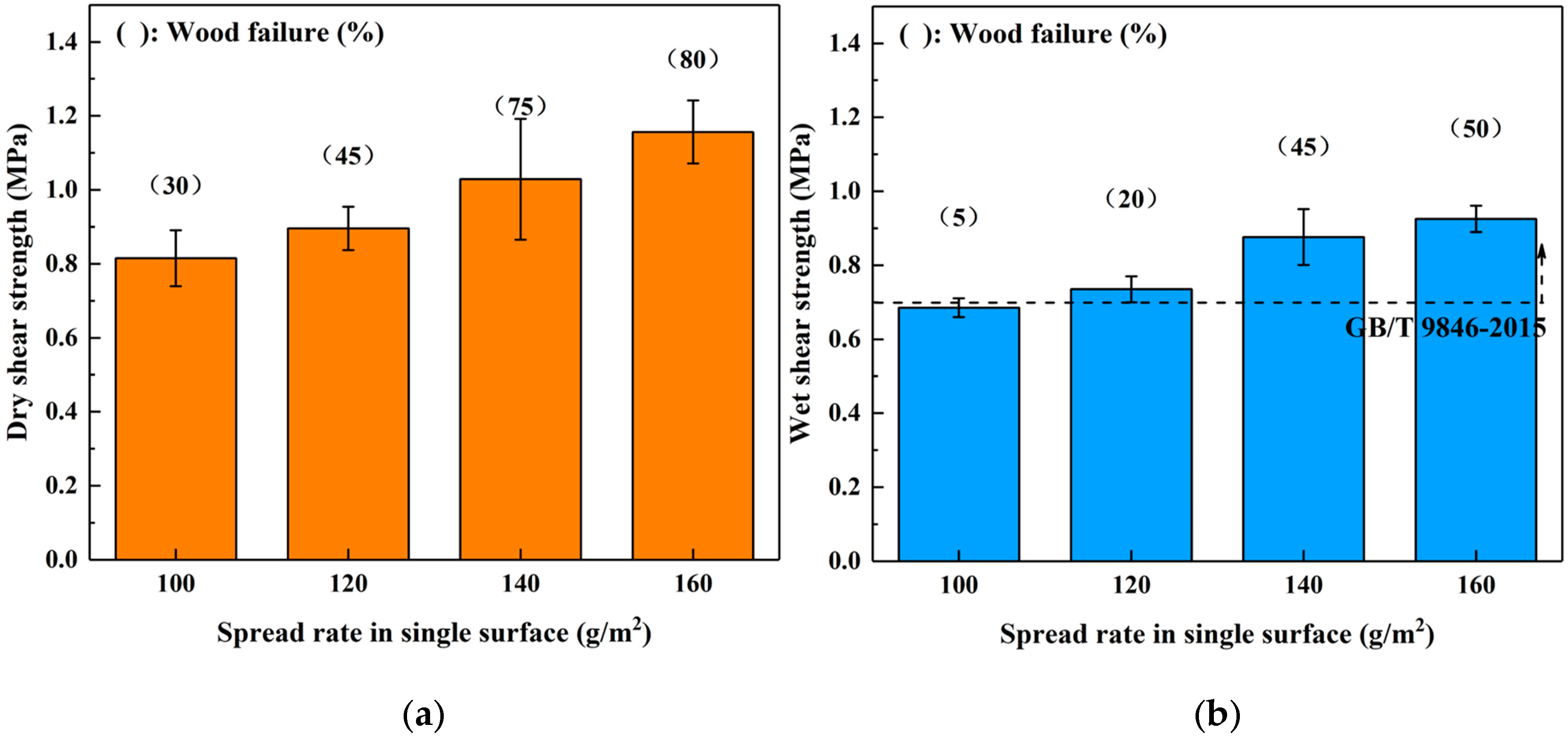
3.2. Effects of Hot Pressing Conditions on the Bond Performance
3.3. Curing Behavior of SADP Adhesive
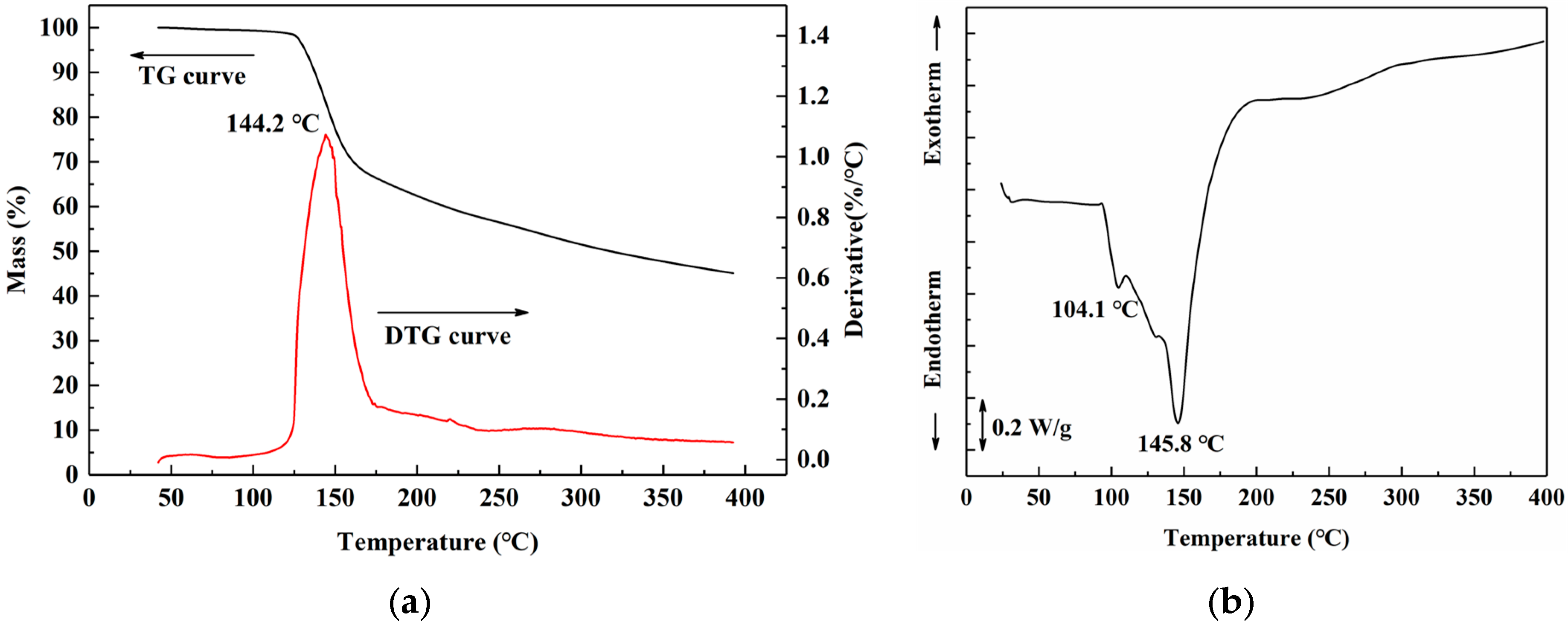
3.3.1. Thermal Analysis
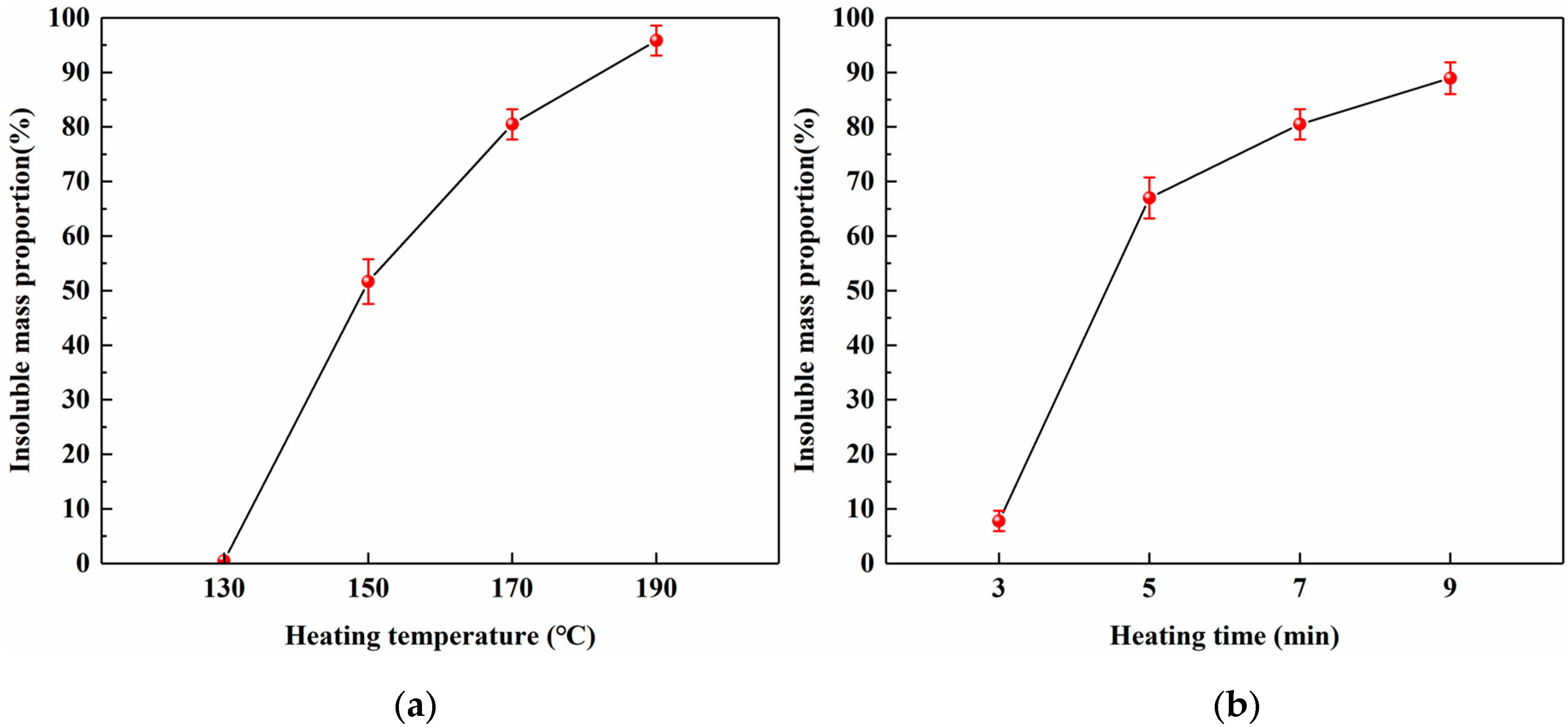
3.3.2. Insoluble Mass Proportion
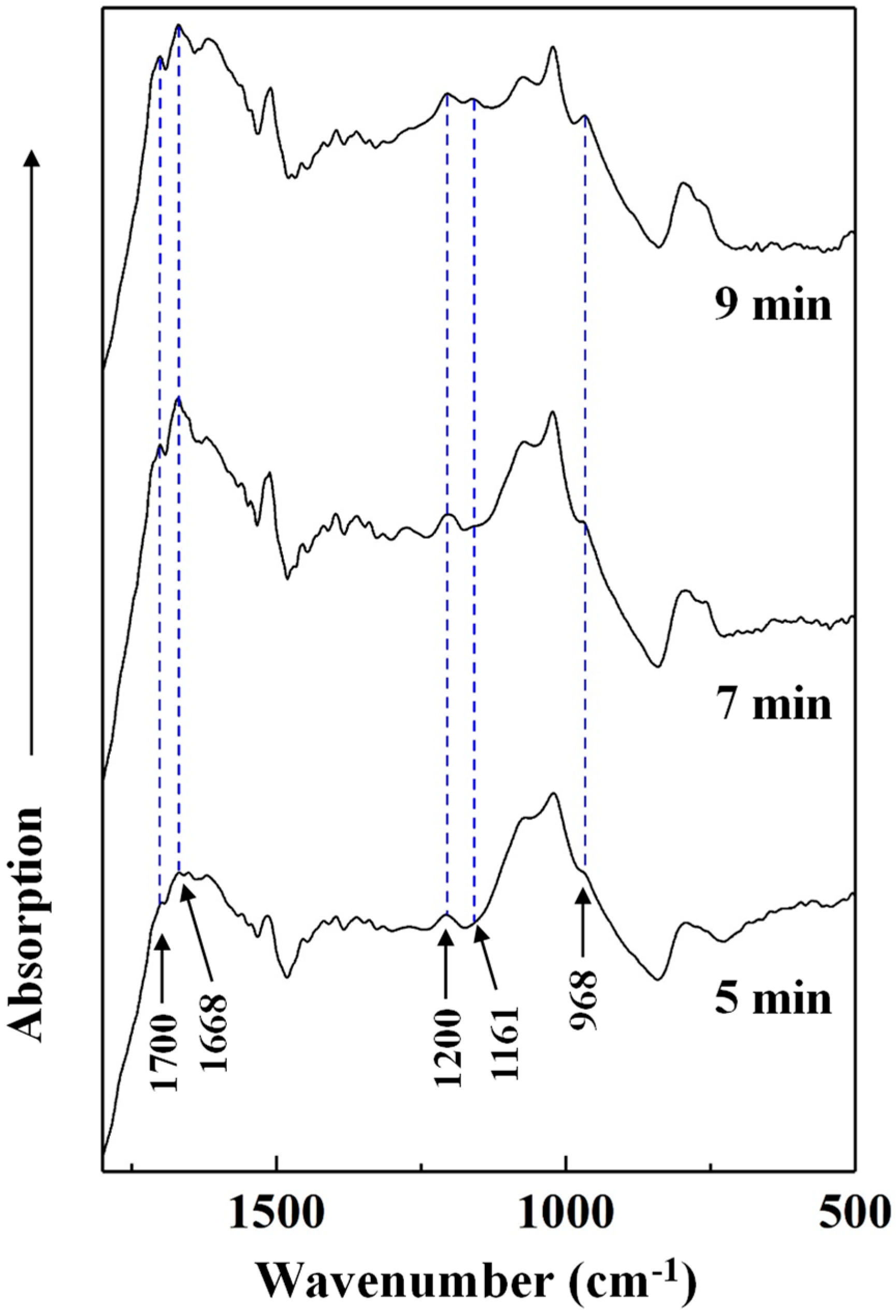
3.3.3. Chemical Change
3.4. Chemical Composition of Cured SADP Adhesive
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Xu, Y. Integrative process for sugarcane bagasse biorefinery to co-produce xylooligosaccharides and gluconic acid. Bioresour. Technol. 2019, 282, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.X.; Lin, W.Q.; Lai, C.H.; Li, X.; Jin, Y.C.; Yong, Q. Coupling the post-extraction process to remove residual lignin and alter the recalcitrant structures for improving the enzymatic digestibility of acid-pretreated bamboo residues. Bioresour. Technol. 2019, 285. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Q.; Lu, K.Y.; Yue, Y.Y.; Mei, C.T.; Huang, C.B.; Wu, Q.L.; Xu, X.W. Nanocellulose-templated assembly of polyaniline in natural rubber-based hybrid elastomers toward flexible electronic conductors. Ind. Crops Prod. 2019, 128, 94–107. [Google Scholar] [CrossRef]
- Yan, X.X.; Qian, X.Y.; Chang, Y.J.; Lu, R.; Miyakoshi, T. The effect of glass fiber powder on the properties of waterborne coatings with thermochromic ink on a Chinese fir surface. Polymers 2019, 11, 1733. [Google Scholar] [CrossRef]
- Tang, T.; Fu, Y. Formation of Chitosan/Sodium Phytate/Nano-Fe3O4 Magnetic Coatings on Wood Surfaces via Layer-by-Layer Self-Assembly. Coatings 2020, 10, 51. [Google Scholar] [CrossRef]
- Wu, Y.; Bian, Y.Q.; Yang, F.; Ding, Y.; Chen, K.X. Preparation and properties of chitosan/graphene modified bamboo fiber fabrics. Polymers 2019, 11, 1540. [Google Scholar] [CrossRef]
- Han, J.; Wang, S.; Zhu, S.; Huang, C.; Yue, Y.; Mei, C.; Xu, X.; Xia, C. Electrospun core-shell nanofibrous membranes with nanocellulose-stabilized carbon nanotubes for use as high-performance flexible supercapacitor electrodes with enhanced water resistance, thermal stability and mechanical toughness. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Ding, Q.Q.; Xu, X.W.; Yue, Y.Y.; Mei, C.T.; Huang, C.B.; Jiang, S.H.; Wu, Q.L.; Han, J.Q. Nanocellulose-mediated electroconductive self-healing hydrogels with high strength, plasticity, viscoelasticity, stretchability, and biocompatibility toward multifunctional applications. ACS Appl. Mater. Interfaces 2018, 10, 27987–28002. [Google Scholar] [CrossRef]
- Dong, H.; zheng, l.; Yu, P.; Jiang, Q.; Wu, Y.; Huang, C.; Yin, B. Characterization and application of lignin-carbohydrate complexes from lignocellulosic materials as antioxidant for scavenging in vitro and in vivo reactive oxygen species. ACS Sustain. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Han, J.Q.; Wang, H.X.; Yue, Y.Y.; Mei, C.T.; Chen, J.Z.; Huang, C.B.; Wu, Q.L.; Xu, X.W. A self-healable and highly flexible supercapacitor integrated by dynamically cross-linked electro-conductive hydrogels based on nanocellulose-templated carbon nanotubes embedded in a viscoelastic polymer network. Carbon 2019, 149, 1–18. [Google Scholar] [CrossRef]
- Xi, X.D.; Wu, Z.G.; Pizzi, A.; Gerardin, C.; Lei, H.; Zhang, B.G.; Du, G.B. Non-isocyanate polyurethane adhesive from sucrose used for particleboard. Wood Sci. Technol. 2019, 53, 393–405. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, S.J.; Kang, H.J.; Zhang, W.; Li, J.Z.; Zhang, S.F.; Huang, A.M. Reduction of energy consumption of green plywood production by implementing high-efficiency thermal conductive bio-adhesive: Assessment from pilot-scaled application. J. Clean. Prod. 2019, 210, 1366–1375. [Google Scholar] [CrossRef]
- Li, W.Z.; Mei, C.T.; Van den Bulcke, J.; Van Acker, J. The effect of water sorption/desorption on fatigue deflection of OSB. Constr. Build. Mater. 2019, 223, 1196–1203. [Google Scholar] [CrossRef]
- Li, W.Z.; Van den Bulcke, J.; Dhaene, J.; Zhan, X.X.; Mei, C.T.; Van Acker, J. Investigating the interaction between internal structural changes and water sorption of MDF and OSB using X-ray computed tomography. Wood Sci. Technol. 2018, 52, 701–716. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Xie, X.; Yuan, Z.; Cai, S.; Li, Y. Effect of phenol formaldehyde resin penetration on the quasi-static and dynamic mechanics of wood cell walls using nanoindentation. Nanomaterials 2019, 9, 1409. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Xie, X.; Cai, S.; Yuan, Z.; Li, Y. Multi-Scale evaluation of the effect of phenol formaldehyde resin impregnation on the dimensional stability and mechanical properties of pinus massoniana lamb. Forests 2019, 10, 646. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Wu, Z. Fabrication of Coatings with Structural Color on a Wood Surface. Coatings 2020, 10, 32. [Google Scholar] [CrossRef]
- Norback, D. An update on sick building syndrome. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 55–59. [Google Scholar] [CrossRef]
- Brightman, H.S.; Milton, D.K.; Wypij, D.; Burge, H.A.; Spengler, J.D. Evaluating building-related symptoms using the US EPA BASE study results. Indoor Air 2008, 18, 335–345. [Google Scholar] [CrossRef]
- Queneau, Y.; Jarosz, S.; Lewandowski, B.; Fitremann, J. Sucrose chemistry and applications of sucrochemicals. Adv. Carbohyd. Chem. Biochem. 2008, 61, 217–292. [Google Scholar] [CrossRef]
- Khan, R.; Jones, H. Sucrose chemistry: Its position as a raw-material for the chemical-industry. Sugar Ser. 1988, 9, 367–388. [Google Scholar]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard. J. Wood Sci. 2013, 59, 203–208. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Umemura, K. Investigation of a new natural particleboard adhesive composed of tannin and sucrose. J. Wood Sci. 2014, 60, 269–277. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Miao, Y.F.; Yang, Z.Q.; Wang, H.; Sang, R.J.; Fu, Y.C.; Huang, C.X.; Wu, Z.H.; Zhang, M.; Sun, S.J.; et al. Effects of sulfuric acid on the curing behavior and bonding performance of tannin-sucrose adhesive. Polymers 2018, 10, 651. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Hayashi, S.; Xu, W.; Wu, Z.H.; Tanaka, S.; Sun, S.J.; Zhang, M.; Kanayama, K.; Umemura, K. A novel eco-friendly wood adhesive composed by sucrose and ammonium dihydrogen phosphate. Polymers 2018, 10, 1251. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, S.; Wu, D.; Zhang, M.; Huang, C.; Umemura, K.; Yong, Q. Synthesis and characterization of sucrose and ammonium dihydrogen phosphate (SADP) adhesive for plywood. Polymers 2019, 11, 1909. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, M.; Umemura, K.; Zhao, Z. Investigation and characterization of synthesis conditions on sucrose-ammonium dihydrogen phosphate (SADP) adhesive: Bond performance and chemical transformation. Materials 2019, 12, 4078. [Google Scholar] [CrossRef]
- Szwergold, B.; Kappler, F.; Brown, T. Identification of fructose 3-phosphate in the lens of diabetic rats. Science 1990, 247, 451–454. [Google Scholar] [CrossRef]
- Thomas, N.L.; Birchall, J.D. The retarding action of sugars on cement hydration. Cem. Concr. Res. 1983, 13, 830–842. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Williams, L.D.; Ebede, C.C. Mechanism of the dehydration of D-fructose to 5-hydroxymethylfurfural in dimethyl sulfoxide at 150 °C: An NMR study. Carbohyd. Res. 2008, 343, 3021–3024. [Google Scholar] [CrossRef]
- Zhang, J.; Weitz, E. An in situ NMR study of the mechanism for the catalytic conversion of fructose to 5-hydroxymethylfurfural and then to levulinic acid using C-13 labeled D-fructose. Acs. Catal. 2012, 2, 1211–1218. [Google Scholar] [CrossRef]
- Horvat, J.; Klaic, B.; Metelko, B.; Sunjic, V. Mechanism of levulinic acid formation. Tetrahedron Lett. 1985, 26, 2111–2114. [Google Scholar] [CrossRef]
- Long, S.; Cao, Z.; Li, Y.L.; Yang, H.W.; Li, J.S.; Xiang, J.N. Synthesis of 2, 6-deoxyfructosazine and its decomposition of incense flavor in tobacco smoke. Fundam. Chem. Eng. 2011, 233–235, 78–81. [Google Scholar] [CrossRef]
- Wu, S.X.; Fan, H.L.; Zhang, Q.; Cheng, Y.; Wang, Q.; Yang, G.Y.; Han, B.X. Conversions of cellobiose and inulin to deoxyfructosazine in aqueous solutions. Clean-Soil Air Water 2011, 39, 572–576. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, X.H. Synthesis and properties of a novel crosslinked chitosan resin modified by L-lysine. React. Funct. Polym. 2008, 68, 1281–1289. [Google Scholar] [CrossRef]
- Kimura, H.; Nakahara, M.; Matubayasi, N. In Situ kinetic study on hydrothermal transformation of d-glucose into 5-hydroxymethylfurfural through d-Fructose with C-13 NMR. J. Phys. Chem. A 2011, 115, 14013–14021. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Razzaq, A. Mechanism of 1-(1-propylsulfonic)-3-methylimidazolium chloride catalyzed transformation of d-glucose to 5-hydroxymethylfurfural in DMSO: An NMR study. Carbohyd. Res. 2014, 386, 86–91. [Google Scholar] [CrossRef]
- Chauvin, M.F.; Megninchanet, F.; Martin, G.; Lhoste, J.M.; Baverel, G. The rabbit kidney tubule utilizes glucose for glutamine synthesis—A C-13 nmr-study. J. Biol. Chem. 1994, 269, 26025–26033. [Google Scholar]
- Yamamori, A.; Okada, H.; Kawazoe, N.; Onodera, S.; Shiomi, N. Synthesis of beta-d-fructopyranosyl-(2 → 6)-d-glucopyranose from d-glucose and d-fructose by a thermal treatment. Biosci. Biotechnol. Biochem. 2010, 74, 2130–2132. [Google Scholar] [CrossRef]
- Whitfield, M. The hydrolysis of ammonium ions in sea water—A theoretical study. J. Mar. Biol. Assoc. 2009, 54, 565–580. [Google Scholar] [CrossRef]
- Agyei-Aye, K.; Chian, M.X.; Lauterbach, J.H.; Moldoveanu, S.C. The role of the anion in the reaction of reducing sugars with ammonium salts. Carbohyd. Res. 2002, 337, 2273–2277. [Google Scholar] [CrossRef]
- Namiki, M. Chemistry of maillard reactions: Recent studies on the browning reaction mechanism and the development of antioxidants and mutagens. In Advances in Food Research; Chichester, C.O., Schweigert, B.S., Eds.; Academic Press: New York, NY, USA, 1988; Volume 32, pp. 115–184. [Google Scholar]
- Suarez-Pereira, E.; Rubio, E.M.; Pilard, S.; Mellet, C.O.; Fernandez, J.M.G. Di-d-fructose dianhydride-enriched products by acid ion-exchange resin-promoted caramelization of d-fructose: Chemical analyses. J. Agric. Food Chem. 2010, 58, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Saeman, J.F. Kinetics of wood saccharification—Hydrolysis of cellulose and decomposition of sugars in dilute acid at high temperature. Ind. Eng. Chem. 1945, 37, 43–52. [Google Scholar] [CrossRef]
- Gintner, Z.; Végh, A.; Ritlop, B. Determination of sucrose content of cariogenic diets by thermal analysis. J. Therm. Anal. 1989, 35, 1399–1404. [Google Scholar] [CrossRef]
- Eggleston, G.; TraskMorrell, B.J.; Vercellotti, J.R. Use of differential scanning calorimetry and thermogravimetric analysis to characterize the thermal degradation of crystalline sucrose and dried sucrose-salt residues. J. Agric. Food Chem. 1996, 44, 3319–3325. [Google Scholar] [CrossRef]
- Vaz, P.D.; Ribeiro-Claro, P.J.A. C-H center dot center dot center dot O hydrogen bonds in liquid cyclohexanone revealed by the vC=O splitting and the vC-H blue shift. J. Raman Spectrosc. 2003, 34, 863–867. [Google Scholar] [CrossRef]
- Perez, E.M.S.; Avalos, M.; Babiano, R.; Cintas, P.; Light, M.E.; Jimenez, J.L.; Palacios, J.C.; Sancho, A. Schiff bases from d-glucosamine and aliphatic ketones. Carbohyd. Res. 2010, 345, 23–32. [Google Scholar] [CrossRef]
- Zhao, X.F.; Peng, L.Q.; Wang, H.L.; Wang, Y.B.; Zhang, H. Environment-friendly urea-oxidized starch adhesive with zero formaldehyde-emission. Carbohyd. Polym. 2018, 181, 1112–1118. [Google Scholar] [CrossRef]
- Chis, A.; Fetea, F.; Matei, H.; Socaciu, C. Evaluation of hydrolytic activity of different pectinases on sugar beet (Beta vulgaris) substrate using FT-MIR spectroscopy. Not. Bot. Horti Agrobot. 2011, 39, 99–104. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.J. Curing behavior and viscoelastic properties of pine and wattle tannin-based adhesives studied by dynamic mechanical thermal analysis and FT-IR-ATR spectroscopy. J. Adhes. Sci. Technol. 2003, 17, 1369–1383. [Google Scholar] [CrossRef]
| Groups | Hot Pressing Temperature (°C) | Hot Pressing Time (min) | Spread Rate (g/m2) |
|---|---|---|---|
| Group 1 | 130 | 7 | 140 |
| 150 | |||
| 170 | |||
| 190 | |||
| Group 2 | 170 | 3 | 140 |
| 5 | |||
| 7 | |||
| 9 | |||
| Group 3 | 170 | 7 | 100 |
| 120 | |||
| 140 | |||
| 160 |
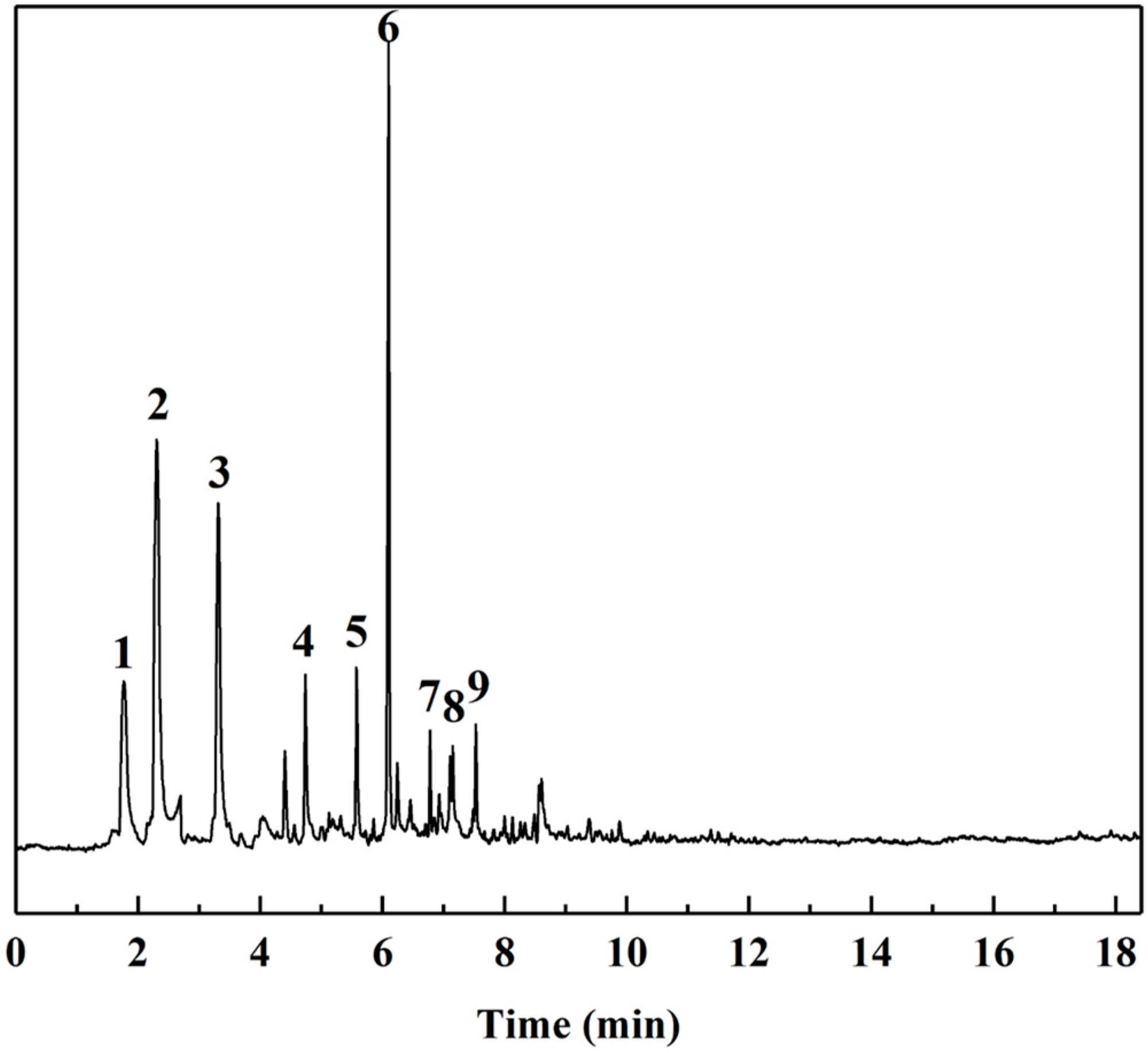
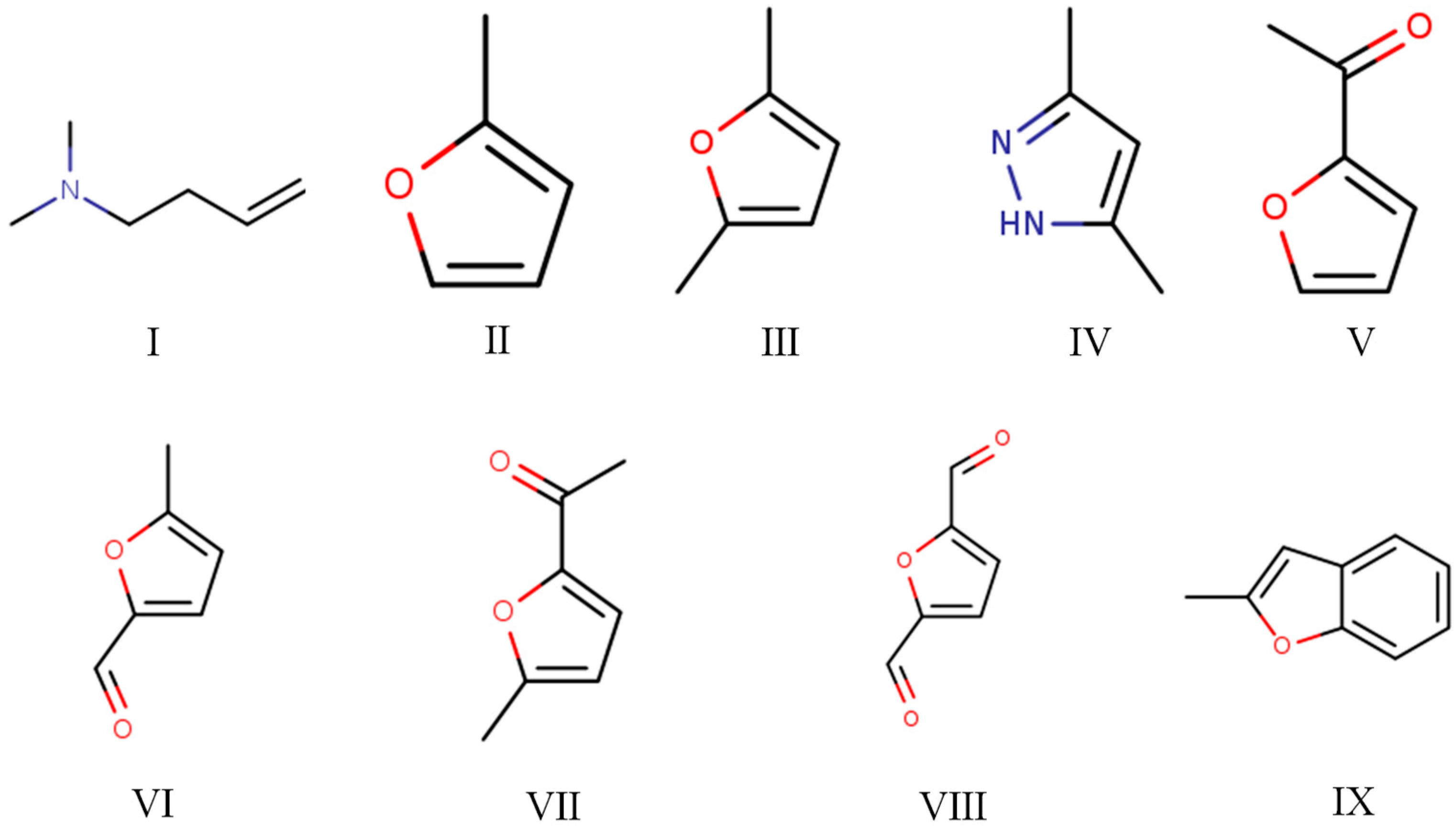
| Peak Number | RT (min) | SI | Compound | CAS | MW | Formula | Chemical Structure Number |
|---|---|---|---|---|---|---|---|
| 1 | 1.76 | 89 | N,N-dimethyl(3-butenyl)amine | 55831-89-5 | 99 | C6H13N | I |
| 2 | 2.30 | 96 | 2-Methylfuran | 534-22-5 | 82 | C5H6O | II |
| 3 | 3.31 | 97 | 2,5-Dimethylfuran | 625-86-5 | 96 | C6H8O | III |
| 4 | 4.74 | 83 | 3,5-Dimethylpyrazole (DMP) | 67-51-6 | 96 | C5H8N2 | IV |
| 5 | 5.58 | 96 | 2-Acetylfuran | 1192-62-7 | 110 | C6H6O2 | V |
| 6 | 6.10 | 97 | 5-Methylfurfural | 620-02-0 | 110 | C6H6O2 | VI |
| 7 | 6.78 | 96 | 2-Acetyl-5-methylfuran | 1193-79-9 | 124 | C7H8O2 | VII |
| 8 | 7.15 | 90 | 2,5-Furandicarboxaldehyde | 823-82-5 | 124 | C6H4O3 | VIII |
| 9 | 7.53 | 87 | 2-Methylbenzofuran | 4265-25-2 | 132 | C9H8O | IX |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Sakai, S.; Wu, D.; Chen, Z.; Zhu, N.; Gui, C.; Zhang, M.; Umemura, K.; Yong, Q. Investigation of Synthesis Mechanism, Optimal Hot-Pressing Conditions, and Curing Behavior of Sucrose and Ammonium Dihydrogen Phosphate Adhesive. Polymers 2020, 12, 216. https://doi.org/10.3390/polym12010216
Zhao Z, Sakai S, Wu D, Chen Z, Zhu N, Gui C, Zhang M, Umemura K, Yong Q. Investigation of Synthesis Mechanism, Optimal Hot-Pressing Conditions, and Curing Behavior of Sucrose and Ammonium Dihydrogen Phosphate Adhesive. Polymers. 2020; 12(1):216. https://doi.org/10.3390/polym12010216
Chicago/Turabian StyleZhao, Zhongyuan, Shunsuke Sakai, Di Wu, Zhen Chen, Nan Zhu, Chengsheng Gui, Min Zhang, Kenji Umemura, and Qiang Yong. 2020. "Investigation of Synthesis Mechanism, Optimal Hot-Pressing Conditions, and Curing Behavior of Sucrose and Ammonium Dihydrogen Phosphate Adhesive" Polymers 12, no. 1: 216. https://doi.org/10.3390/polym12010216
APA StyleZhao, Z., Sakai, S., Wu, D., Chen, Z., Zhu, N., Gui, C., Zhang, M., Umemura, K., & Yong, Q. (2020). Investigation of Synthesis Mechanism, Optimal Hot-Pressing Conditions, and Curing Behavior of Sucrose and Ammonium Dihydrogen Phosphate Adhesive. Polymers, 12(1), 216. https://doi.org/10.3390/polym12010216






