The Effect of a Secondary Process on the Analysis of Isothermal Crystallisation Kinetics by Differential Scanning Calorimetry
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
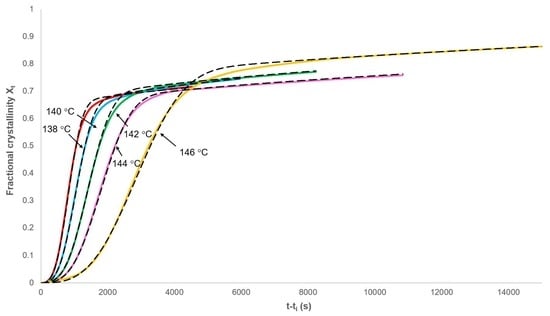
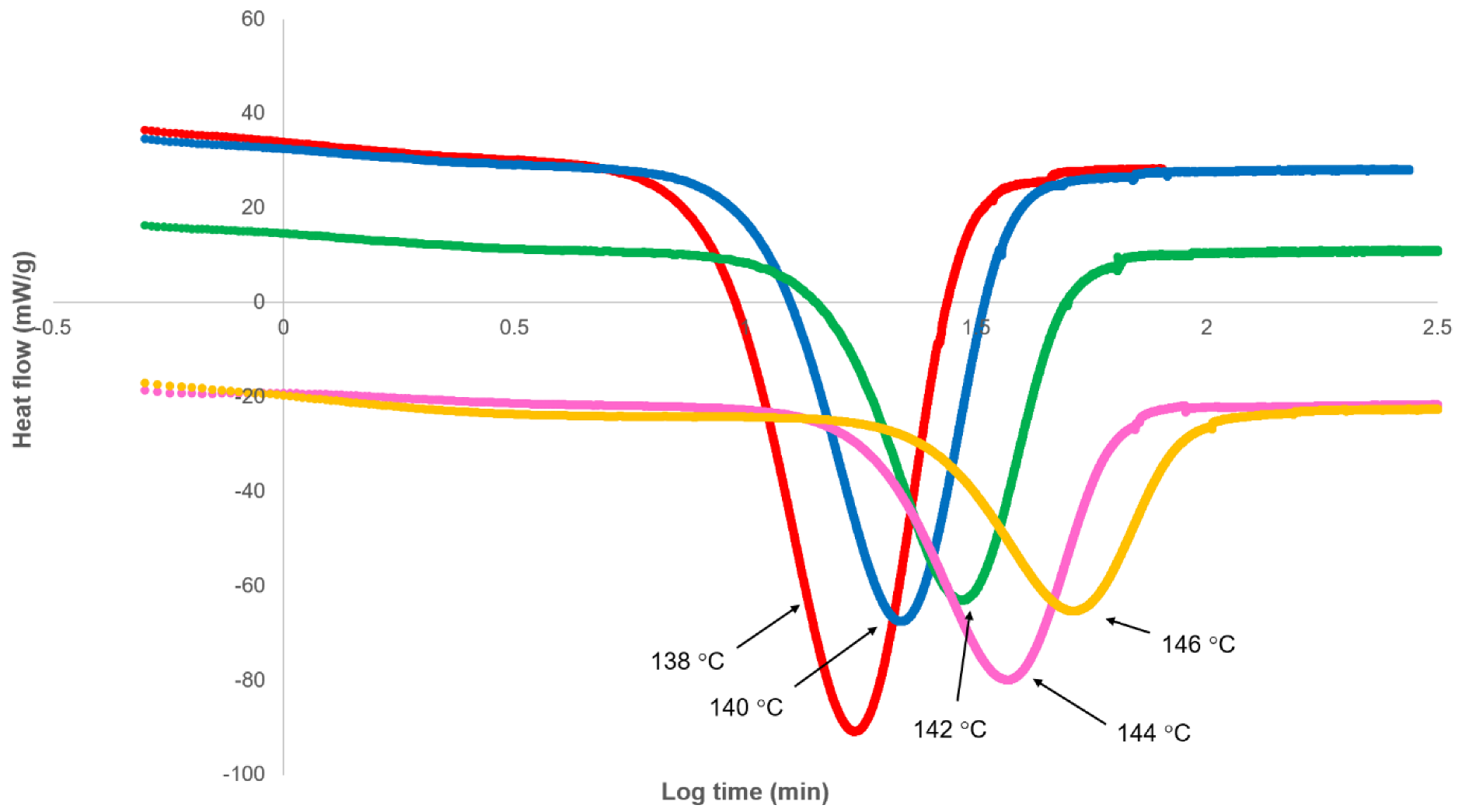
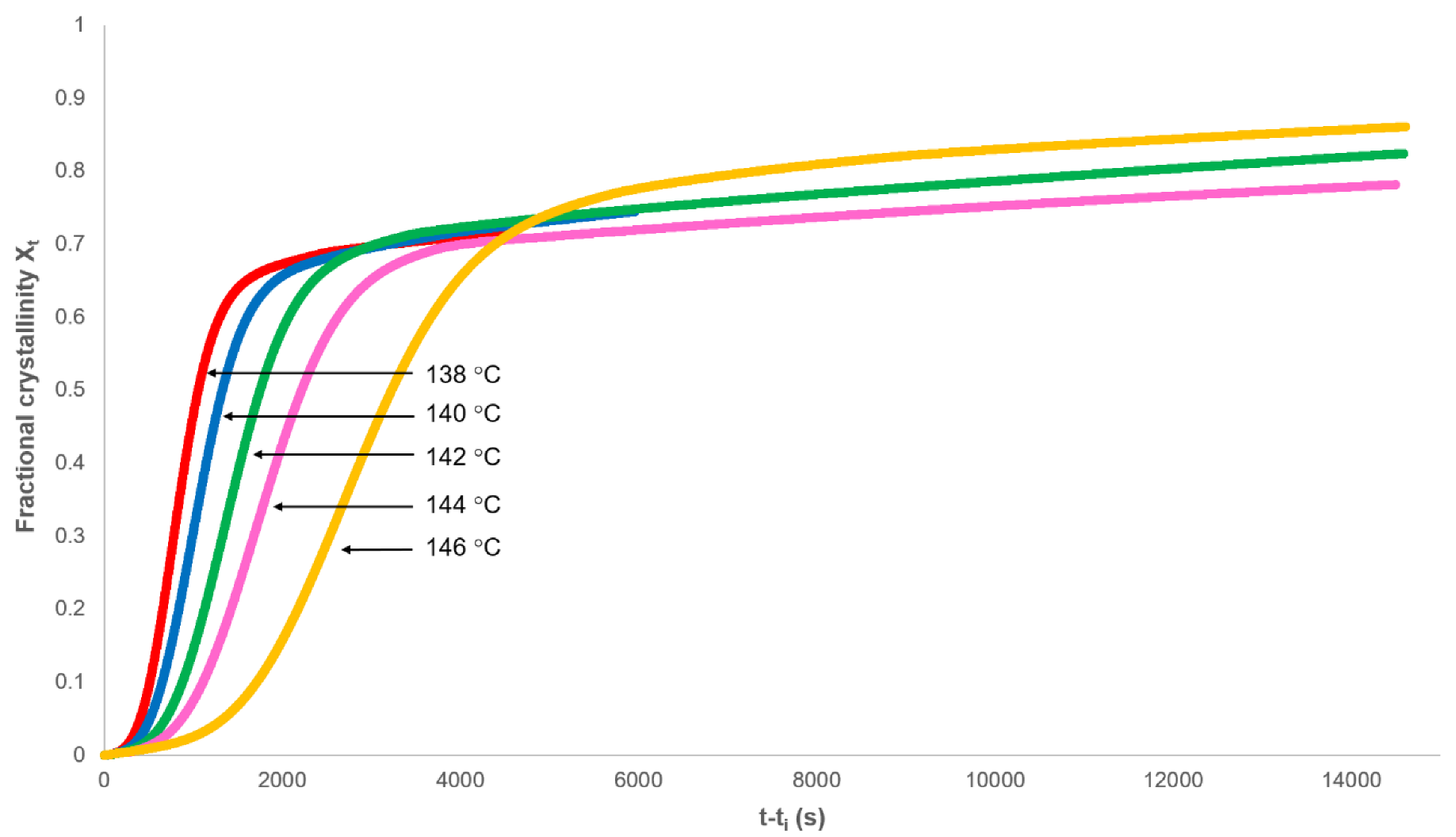
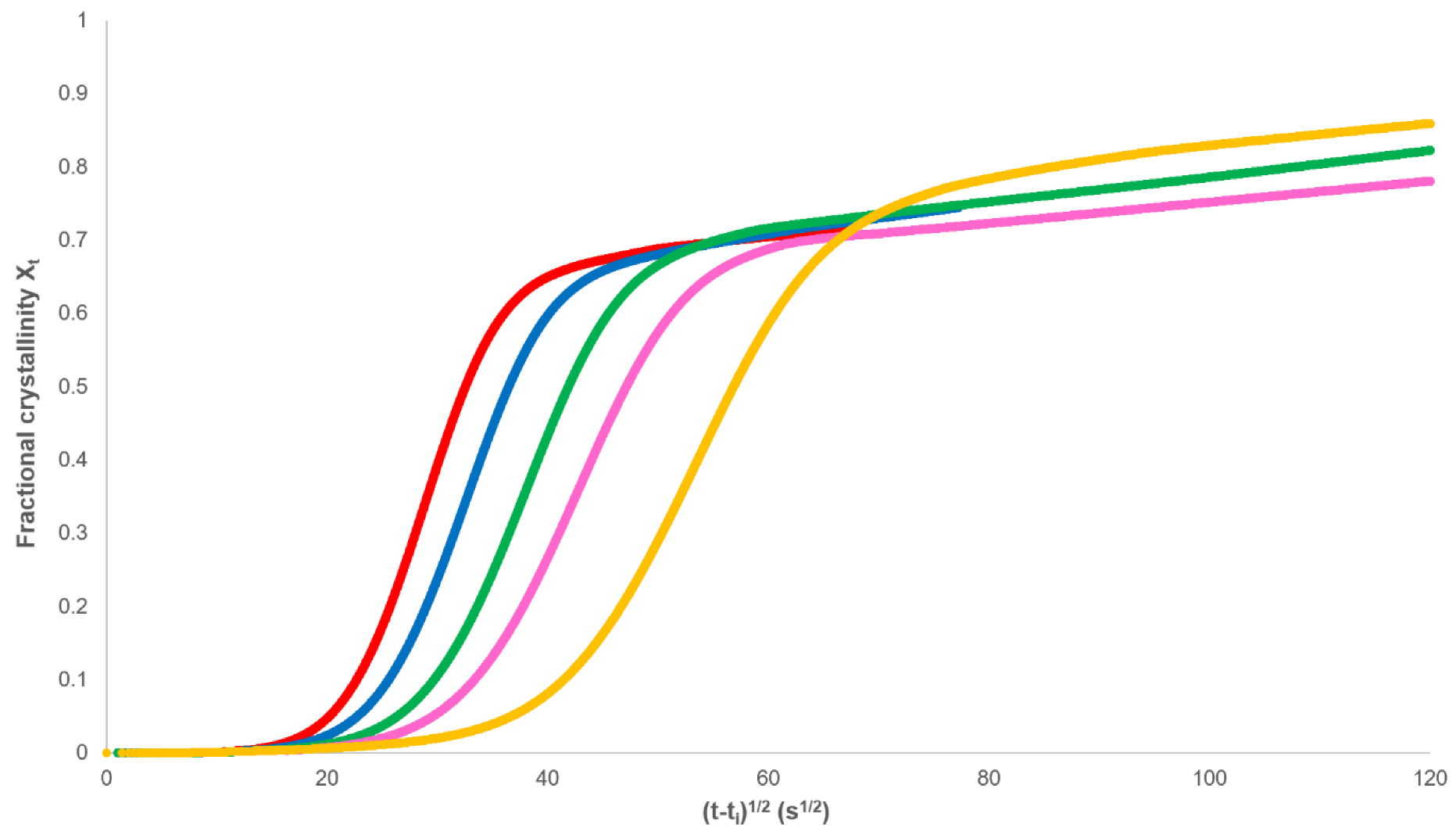
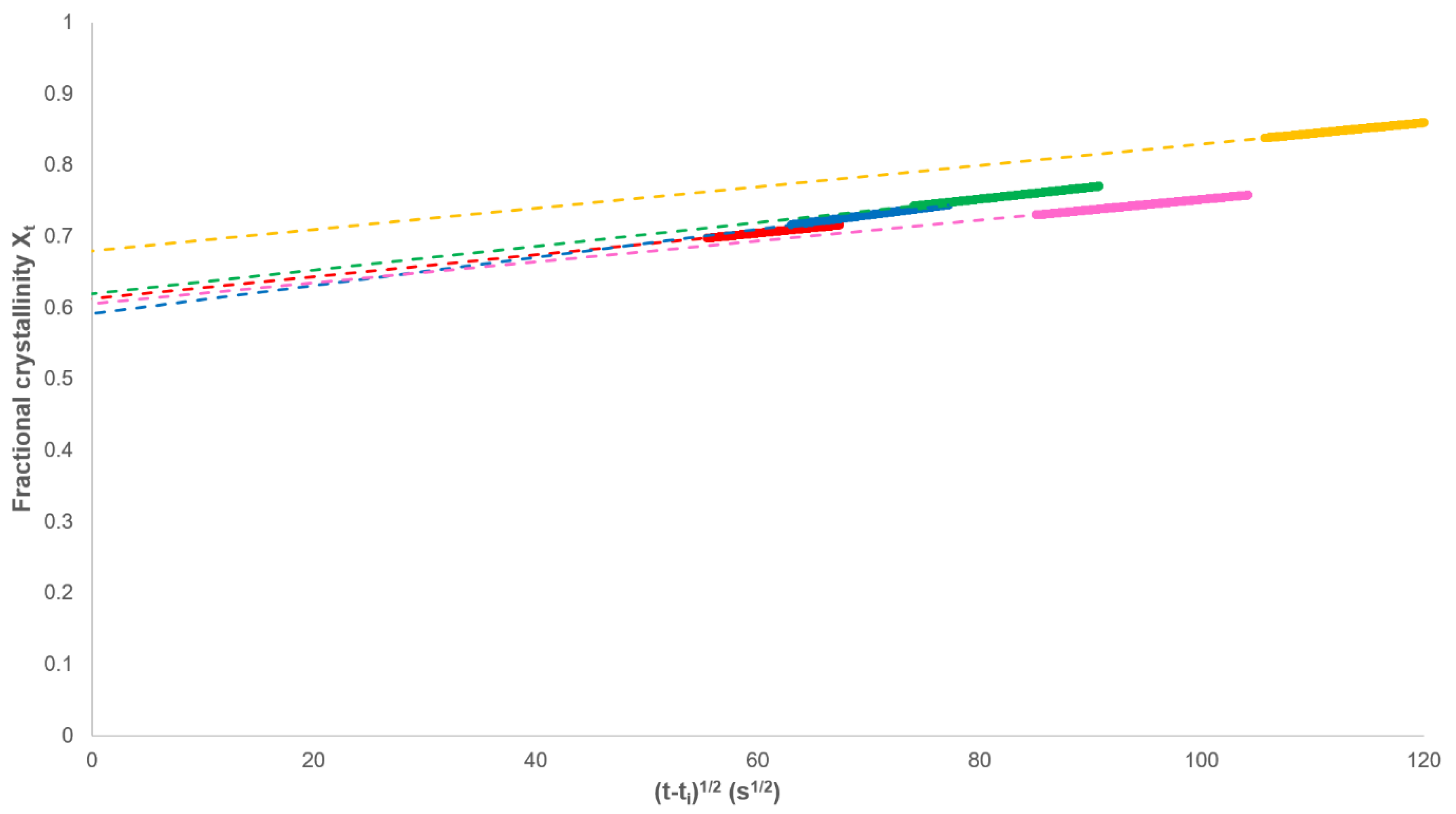
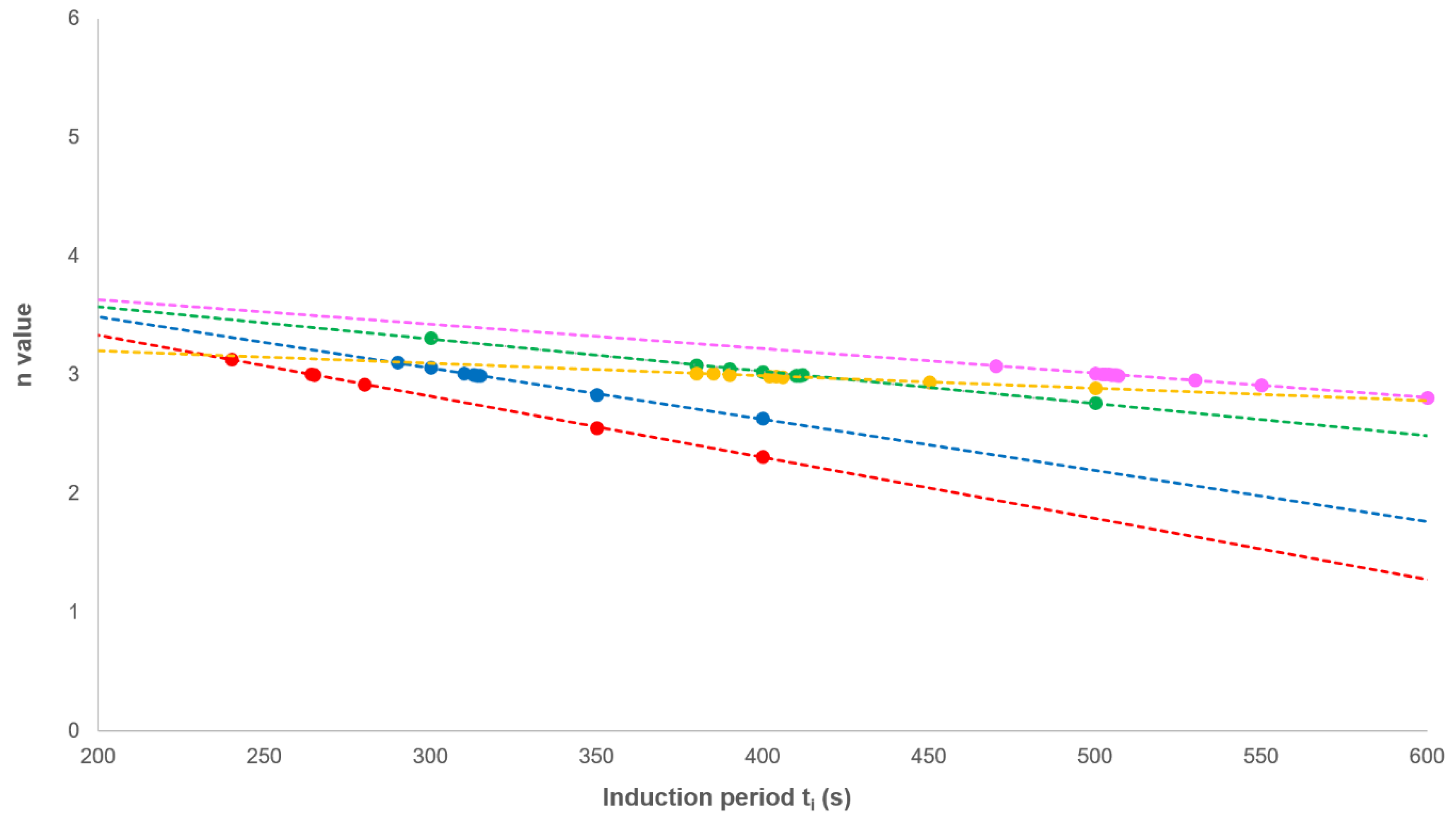
3.1. Analysis of Secondary Crystallisation
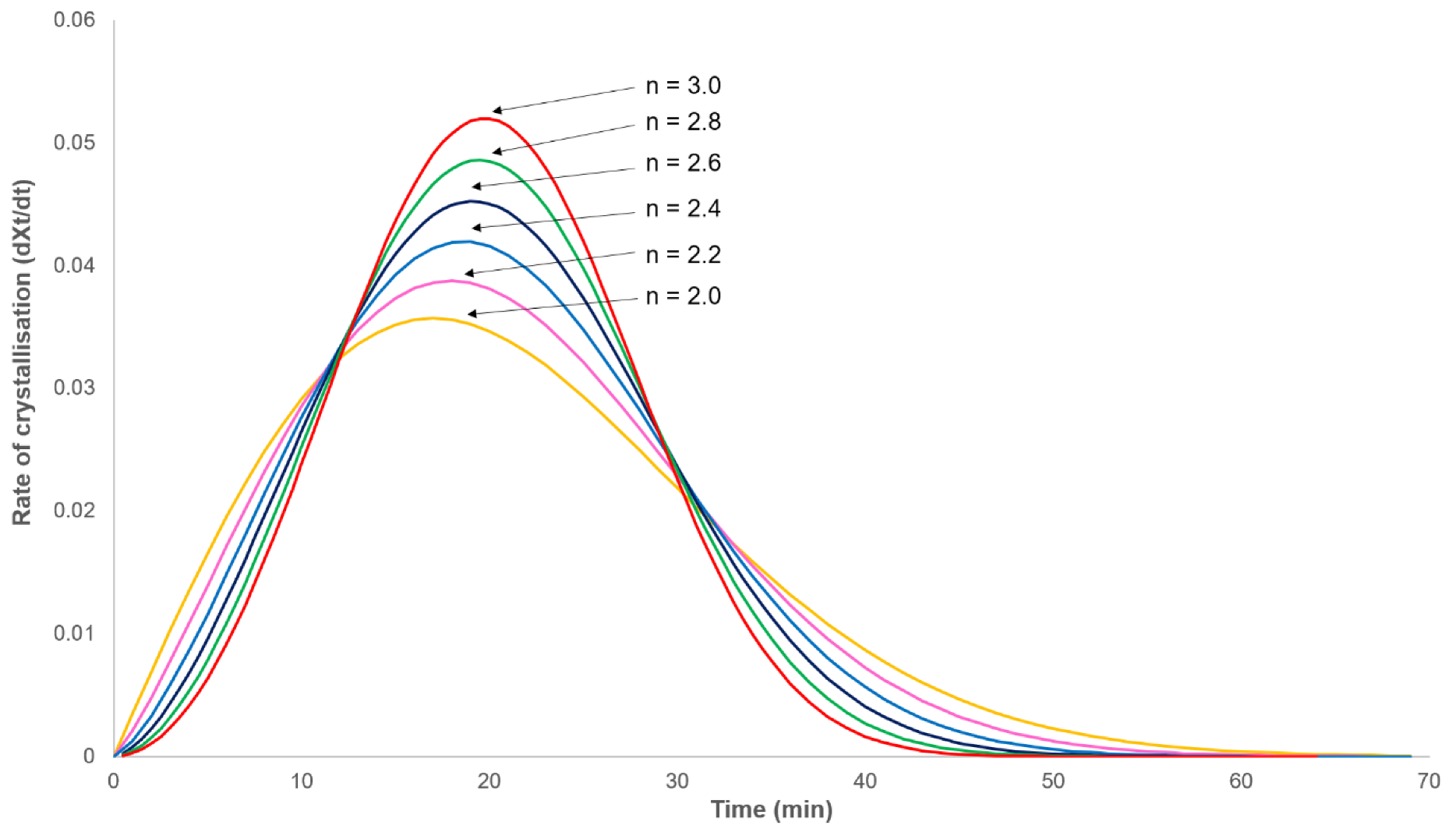
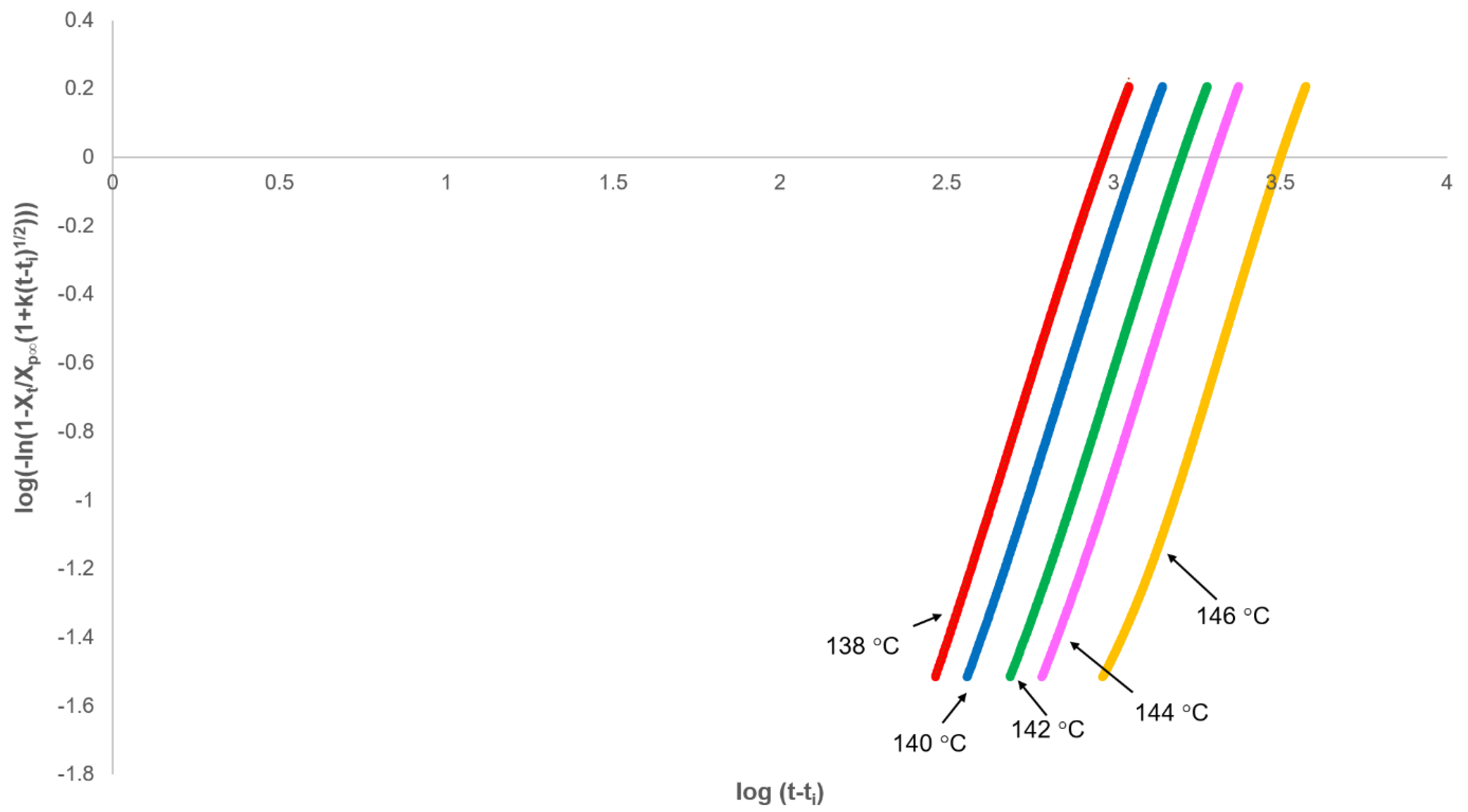
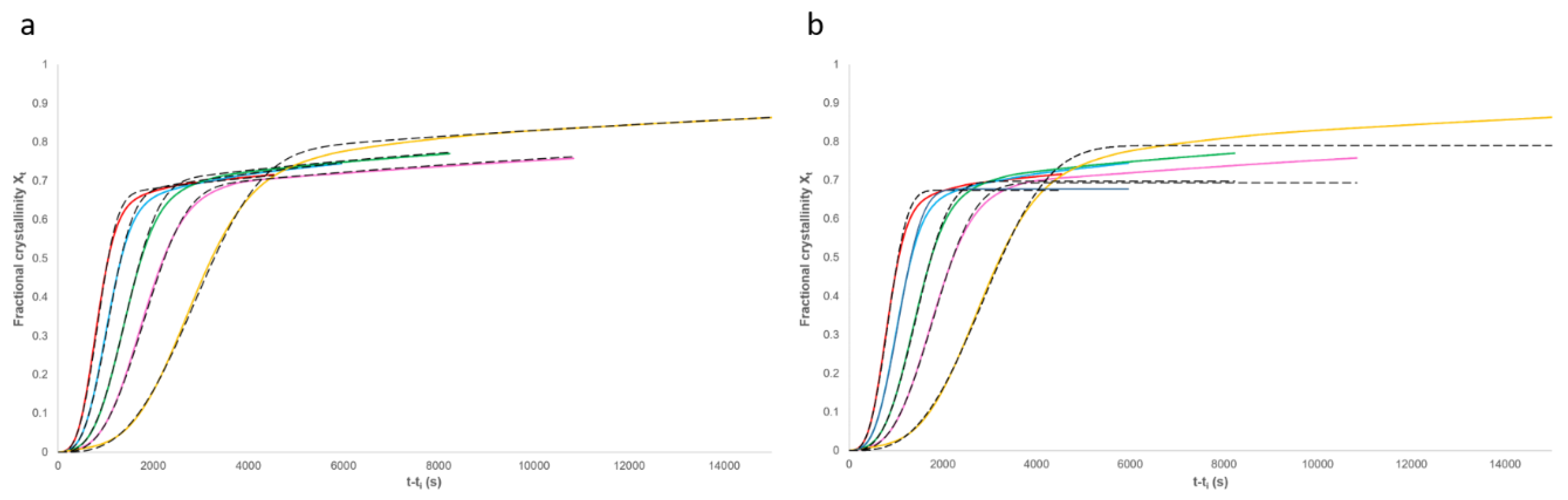
3.2. Analysis of Primary Crystallisation
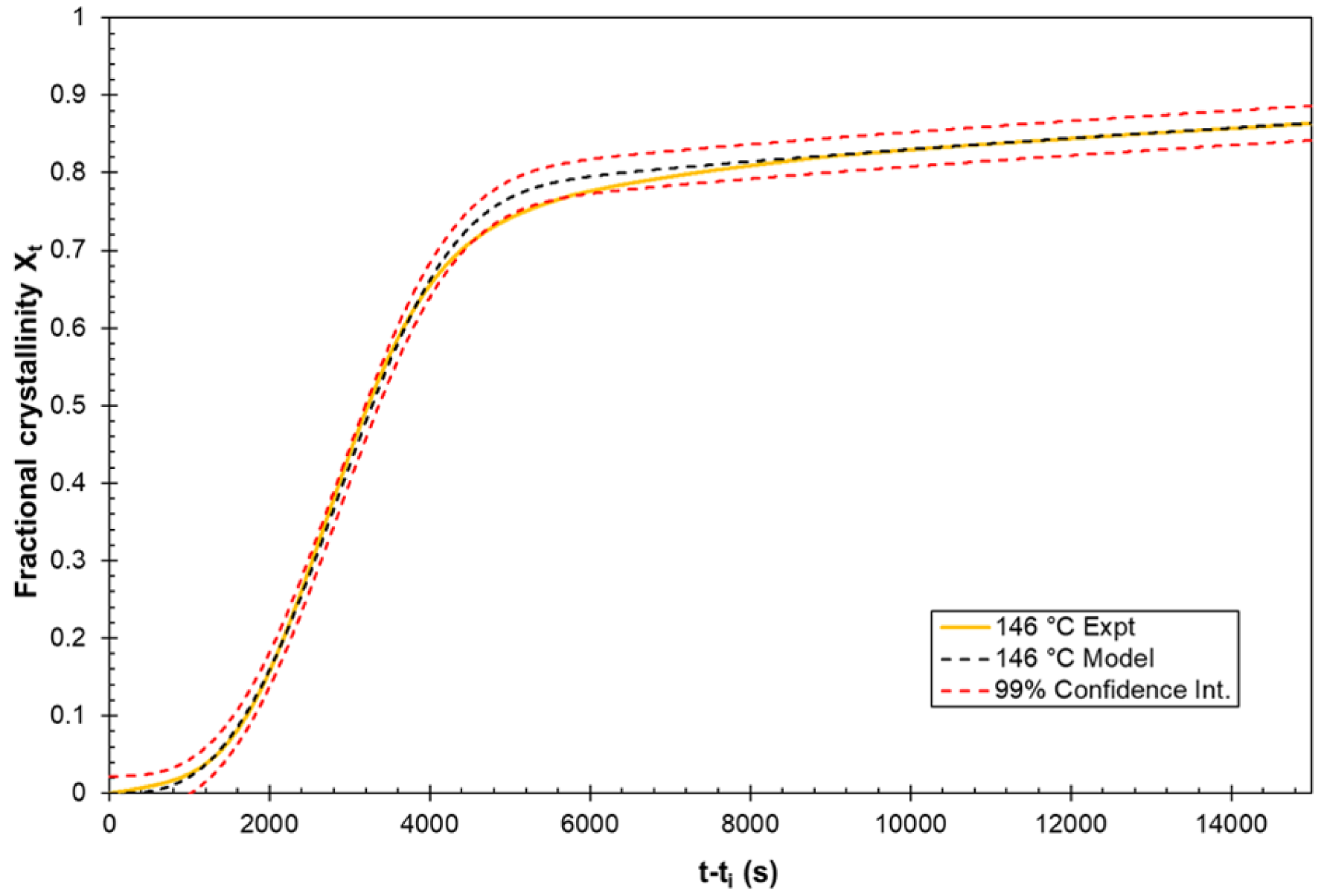
3.3. Applicability of the Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Wang, S.; Daelemans, L.; Fiorio, R.; Gou, M.; D’hooge, D.R.; De Clerck, K.; Cardon, L. Improving Mechanical Properties for Extrusion-Based Additive Manufacturing of Poly(Lactic Acid) by Annealing and Blending with Poly(3-Hydroxybutyrate). Polymers 2019, 11, 1529. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 1996, 49, 1–14. [Google Scholar] [CrossRef]
- Ward, A.C.; Rowley, B.I.; Dawes, E.A. Effect of Oxygen and Nitrogen limitation on poly-beta-hydroxybutyrate biosynthesis in ammonium-grown azobacter-beijerinckii. J. Gen. Microbiol. 1977, 102, 61–68. [Google Scholar] [CrossRef]
- Doi, Y. Microbial Polyesters; VCH Publishers: New York, NY, USA, 1990. [Google Scholar]
- Holmes, P.A. Biologically produced (R)-3-hydroxalkanoate polymers and copolymers. In Developments in Crystalline Polymers—2; Bassett, D.C., Ed.; Elsevier Applied Science Publishers: Essex, UK, 1988. [Google Scholar]
- Dekoning, G.J.M.; Lemstra, P.J. Crystallisation phenoma in bacterial poly(R)-3-hydroxybutyrate. 2. Embrittlement and rejuvenation. Polymer 1993, 34, 4089–4094. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Robbins, K.E.; Kelly, C.A. Secondary crystallisation and degradation in PHB-co-HV: An assessment of long-term stability. Polym. J. 2018, 50, 365–373. [Google Scholar] [CrossRef]
- Tanaka, T.; Iwata, T. Physical Properties, Structure Analysis, and Enzymatic Degradation of Poly (R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate Films and Fibers. In Degradable Polymers and Materials: Principles and Practice; Khemani, K., Scholz, C., Eds.; Amer Chemical Soc.: Washington, DC, USA, 2012; Volume 1114, pp. 171–185. [Google Scholar]
- Kemnitzer, J.E.; Gross, R.A.; McCarthy, S.P.; Liggat, J.; Blundell, D.J.; Cox, M. Crystallisation behavior of predominantly syndiotactic poly(beta-hydroxybutyrate). J. Environ. Polym. Degrad. 1995, 3, 37–47. [Google Scholar] [CrossRef]
- Peng, S.W.; An, Y.X.; Chen, C.; Fei, B.; Zhuang, Y.G.; Dong, L.S. Isothermal crystallization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Eur. Polym. J. 2003, 39, 1475–1480. [Google Scholar] [CrossRef]
- Qian, J.; Zhu, L.Y.; Zhang, J.W.; Whitehouse, R.S. Comparison of different nucleating agents on crystallization of poly(3-hydroxybutyrate-co-3-hydroxyvalerates). J. Polym. Sci. Part. B Polym. Phys. 2007, 45, 1564–1577. [Google Scholar] [CrossRef]
- Owen, A.J.; Heinzel, J.; Skrbic, Z.; Divjakovic, V. Crystallization and melting behavior of PHB and PHB/HV copolymer. Polymer 1992, 33, 1563–1567. [Google Scholar] [CrossRef]
- An, Y.; Dong, L.; Li, L.; Mo, Z.; Feng, Z. Isothermal crystallization kinetics and melting behavior of poly(β-hydroxybutyrate)/poly(vinyl acetate) blends. Eur. Polym. J. 1999, 35, 365–369. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change I—General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Lee, B.; Shin, T.J.; Lee, S.W.; Yoon, J.; Kim, J.; Ree, M. Secondary crystallization behavior of poly(ethylene isophthalate-co-terephthalate): Time-resolved small-angle X-ray scattering and calorimetry studies. Macromolecules 2004, 37, 4174–4184. [Google Scholar] [CrossRef]
- Velisaris, C.N.; Seferis, J.C. Crystallisation kinetics of polyetherethekertone (PEEK) matrices. Polym. Eng. Sci. 1986, 26, 1574–1581. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Jenkins, M.J.; Hay, J.N. Annealing of poly (ethylene terephthalate). Eur. Polym. J. 2014, 50, 235–242. [Google Scholar] [CrossRef]
- Phillipson, K.; Jenkins, M.J.; Hay, J.N. The ageing of poly(epsilon-caprolactone). Polym. Int. 2015, 64, 1695–1705. [Google Scholar] [CrossRef]
- Hillier, I.H. Modified Avrami equation for bulk crystallisation kinetics of spherulitic polymers. J. Polym. Sci. Part A Gen. Pap. 1965, 3, 3067–3078. [Google Scholar] [CrossRef]
- Gordon, M.; Hillier, I.H. Mechanism of secondary crystallisation of polymethylene. Philos. Mag. 1965, 11, 31–41. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Hay, J.N.; Jenkins, M.J. The effect of secondary crystallization on crystallization kinetics—Polyethylene terephthalate revisited. Eur. Polym. J. 2016, 81, 216–223. [Google Scholar] [CrossRef]
- Hsiao, B.S.; Chang, I.Y.; Sauer, B.B. Isothermal crystallization kinetics of poly(ether ketone ketone) and its carbon-fibre-reinforced compisites. Polymer 1991, 32, 2799–2805. [Google Scholar] [CrossRef]
- Choupin, T.; Fayolle, B.; Regnier, G.; Paris, C.; Cinquin, J.; Brule, B. Isothermal crystallization kinetic modeling of poly(etherketoneketone) (PEKK) copolymer. Polymer 2017, 111, 73–82. [Google Scholar] [CrossRef]
- Choupin, T.; Fayolle, B.; Regnier, G.; Paris, C.; Cinquin, J.; Brule, B. A more reliable DSC-based methodology to study crystallization kinetics: Application to poly(ether ketone ketone) (PEKK) copolymers. Polymer 2018, 155, 109–115. [Google Scholar] [CrossRef]
- Phillipson, K.; Jenkins, M.J.; Hay, J.N. The effect of a secondary process on crystallization kinetics—Poly (epsilon-caprolactone) revisited. Eur. Polym. J. 2016, 84, 708–714. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Hay, J.N.; Jenkins, M.J. The effect of secondary crystallization on melting. Eur. Polym. J. 2013, 49, 2697–2703. [Google Scholar] [CrossRef]
- Marsh, J.J.; Turner, R.P.; Carter, J.; Jenkins, M.J. Thermal diffusivity and secondary crystallisation kinetics in poly(lactic acid). Polymer 2019, 179, 121595. [Google Scholar] [CrossRef]
- Aziz, A.A.; Samsudin, S.A.; Hay, J.N.; Jenkins, M.J. The effect of a secondary process on polymer crystallization kinetics-3. Co-poly (lactic acid). Eur. Polym. J. 2017, 94, 311–321. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic—Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Avella, M.; Calandrelli, L.; Immirzi, B.; Malinconico, M.; Martuscelli, E.; Pascucci, B.; Sadocco, P. Novel synthesis blends between bacterial polyesters and acrylic rubber: A study on enzymatic biodegradation. J. Environ. Polym. Degrad. 1995, 3, 49–60. [Google Scholar] [CrossRef]
- Thellen, C.; Coyne, M.; Froio, D.; Auerbach, M.; Wirsen, C.; Ratto, J.A. A Processing, Characterization and Marine Biodegradation Study of Melt-Extruded Polyhydroxyalkanoate (PHA) Films. J. Polym. Environ. 2008, 16, 1–11. [Google Scholar] [CrossRef]
- Rosa, D.D.; Calil, M.R.; Guedes, C.D.F.; Rodrigues, T.C. Biodegradability of thermally aged PHB, PHB-V, and PCL in soil compostage. J. Polym. Environ. 2004, 12, 239–245. [Google Scholar] [CrossRef]
- Smirnov, N. Table for estimating the goodness of fit of empirical distributions. Ann. Math. Stat. 1948, 19, 279–281. [Google Scholar] [CrossRef]
- Kuiper, N.H. Tests concerning random points on a circle. Proc. Koninklijke Nederlandse Akademie van Wetenschappen Ser. A 1960, 63, 38–47. [Google Scholar] [CrossRef]
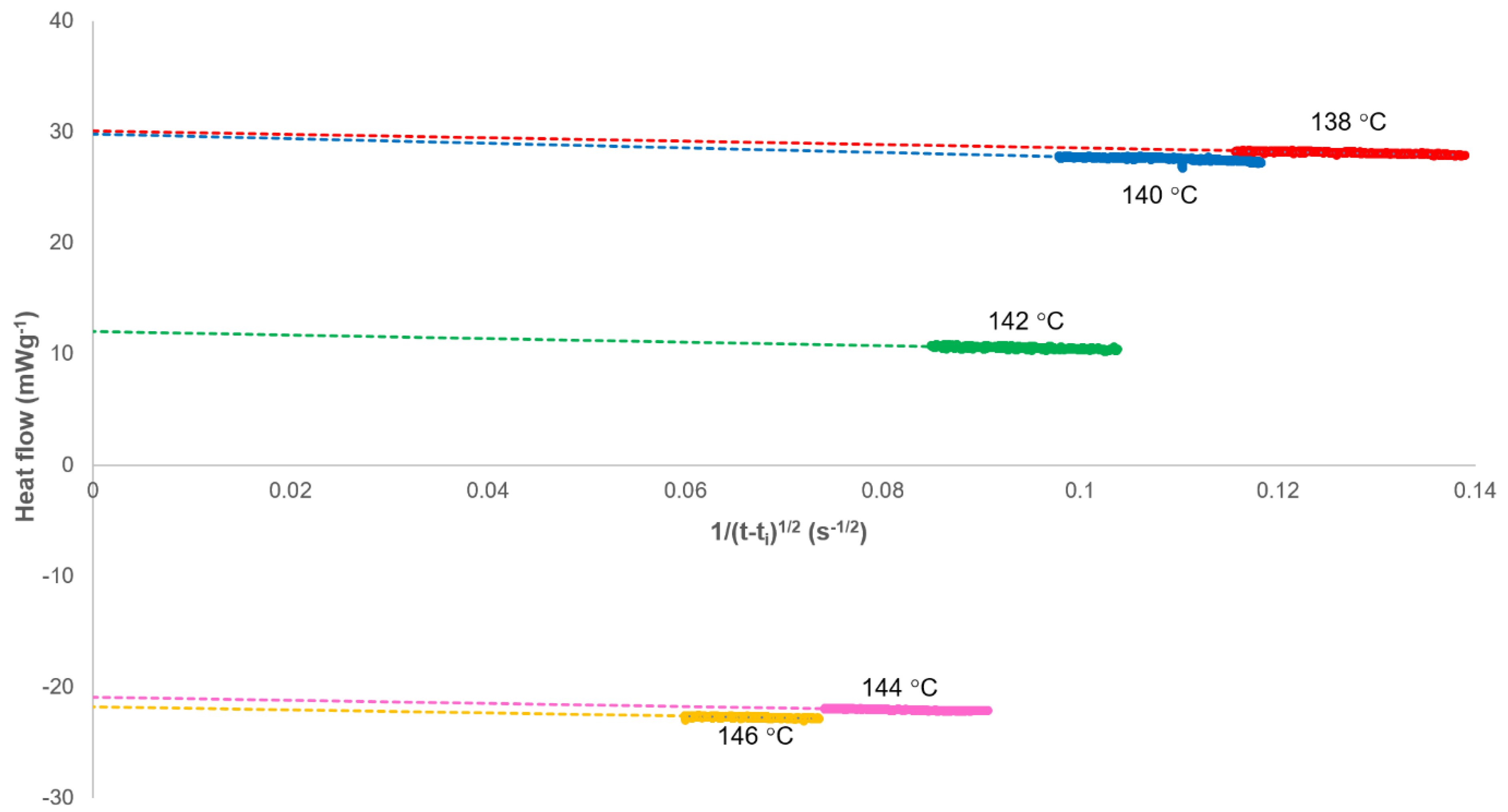
| Crystallisation Temperature/°C | Primary Limit Xp,∞ | Secondary Rate Constant ks/s−1/2 × 103 | ||
|---|---|---|---|---|
| Average | SD | Average | SD | |
| 138 | 0.57 | 0.06 | 4.21 | 2.28 |
| 140 | 0.60 | 0.04 | 3.49 | 1.66 |
| 142 | 0.61 | 0.02 | 2.81 | 1.05 |
| 144 | 0.61 | 0.10 | 2.65 | 1.21 |
| 146 | 0.57 | 0.10 | 4.07 | 1.69 |
| Crystallisation Temperature/°C | Avrami n Value | −log(Zp) | Primary Half-Life/s | ||
|---|---|---|---|---|---|
| Average | SD | Average | SD | ||
| 138 | 3.00 | 8.80 | 0.12 | 761 | 70 |
| 140 | 3.00 | 9.19 | 0.06 | 1027 | 46 |
| 142 | 3.00 | 9.50 | 0.10 | 1300 | 98 |
| 144 | 3.00 | 9.94 | 0.03 | 1824 | 42 |
| 146 | 3.00 | 10.41 | 0.10 | 2613 | 210 |
| Test Statistic | Temperature/°C | ||||
|---|---|---|---|---|---|
| 138 | 140 | 142 | 144 | 146 | |
| R2 | 0.999 | 0.999 | 0.999 | 1.000 | 0.999 |
| Standard deviation (σ) | 0.00591 | 0.00623 | 0.00604 | 0.00251 | 0.00740 |
| Kolmogoro–Smirnov (K–S) | 0.0213 | 0.0214 | 0.0237 | 0.0101 | 0.0263 |
| Kuiper’s | 0.0331 | 0.0293 | 0.0263 | 0.0211 | 0.0410 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly, C.A.; Hay, J.N.; Turner, R.P.; Jenkins, M.J. The Effect of a Secondary Process on the Analysis of Isothermal Crystallisation Kinetics by Differential Scanning Calorimetry. Polymers 2020, 12, 19. https://doi.org/10.3390/polym12010019
Kelly CA, Hay JN, Turner RP, Jenkins MJ. The Effect of a Secondary Process on the Analysis of Isothermal Crystallisation Kinetics by Differential Scanning Calorimetry. Polymers. 2020; 12(1):19. https://doi.org/10.3390/polym12010019
Chicago/Turabian StyleKelly, Catherine A., James N. Hay, Richard P. Turner, and Mike J. Jenkins. 2020. "The Effect of a Secondary Process on the Analysis of Isothermal Crystallisation Kinetics by Differential Scanning Calorimetry" Polymers 12, no. 1: 19. https://doi.org/10.3390/polym12010019
APA StyleKelly, C. A., Hay, J. N., Turner, R. P., & Jenkins, M. J. (2020). The Effect of a Secondary Process on the Analysis of Isothermal Crystallisation Kinetics by Differential Scanning Calorimetry. Polymers, 12(1), 19. https://doi.org/10.3390/polym12010019