1. Introduction
Many myopia patients choose to wear contact lenses for convenience and appearance in addition to correct vision. Feeling comfortable becomes a critical factor for contact lens wearers due to an extensive wearing period. Once contact lenses are worn, some tear film components such as proteins are immediately deposited on the surface of the lens. When tear proteins accumulate on the contact lens, immune reactions can be triggered resulting in discomfort, red eyes, or even eye conditions such as conjunctivitis [
1,
2]. Therefore, removing deposited proteins from the lens or preventing proteins from being adsorbed becomes a critical function for contact lens care solutions along with other functions such as rinsing, inhibiting microbial growth, and storing contact lenses.
It has been shown that the in vitro friction coefficient of contact lenses is corresponded to an in vivo comfort degree [
3,
4], thus modifying the materials or the surface of contact lenses has been investigated for reducing friction coefficient of contact lenses [
5]. Non-functionalized polyvinyl alcohol (PVA) is added to Nelfilcon-A lenses during the manufacturing process to provide comfort. Non-functionalized PVA from Nelfilcon-A lenses has been proved to enhance comfort feeling, and even help to reduce dryness for some contact lens wearers [
6,
7]. Another good way for providing lubrication is by adding lubricants to contact lens care solutions. Lubricants in the care solution might be kept on the surface of the lens during the storage time, thus wearers may feel comfortable when wearing contact lenses the next day. Hyaluronan (HA) is a commonly used lubricant in contact lens solutions because of its high hydrophilic properties and viscoelastic nature [
8].
Poly-gamma-glutamic acid (γ-PGA) is a naturally occurring polymer and it has been applied in the medicine, cosmetics, and sanitary industry [
9,
10]. Our previous study has shown that γ-PGA can be a lubricant in the contact lens care solution, especially it could reduce the high friction coefficient caused by lysozymes [
11,
12]. However, the potential mechanism is still unclear. Therefore, we investigated the bio-tribological effects of γ-PGA on lysozyme-ionic contact lens in this study. One of the ionic contact lenses, Etafilcon-A, was used. Different normal loads and velocities were tested to investigate the effects of γ-PGA in the lysozyme-ionic contact lens system. In addition, the viscosity property and the ability to remove adsorbed lysozyme of γ-PGA were investigated to understand the potential mechanism of γ-PGA as a lubricant. Our results demonstrated that the interactions between γ-PGA and lysozyme were distinct under the conditions of different normal loads and velocities, and provided a bio-tribological prove of γ-PGA with functions of removing adsorbed lysozyme and providing lubrication.
2. Materials and Methods
2.1. Chemicals, Reagents, and Contact Lens
To prepare 100 mL of γ-PGA-containing contact lens care solution, 0.015 g CaCl2 (Sigma, St. Louis, MO, USA), 0.15 g KCl (Sigma, St. Louis, MI, USA), 0.45 g NaCl (Sigma, St. Louis, MI, USA), 1.8 g Na2HPO4 (Sigma, St. Louis, MI, USA), and 0.5 g ethylenediaminetetraacetic acid (EDTA, Sigma) were added into 100 mL of distilled water. 1.5 g of γ-PGA (Vedan Enterprise Corporation, Taichung City, Taiwan) and 0.05 g poloxamer-407 (Wei Ming Pharmaceutical Mfg. Co., Ltd., Taipei City, Taiwan) were then added into the solution, and the solution was passed through a 0.22 μm filter (EMD Millipore Corporation, Billerica, MA, USA). 40 μL of 25 mg/mL epigallocatechin gallate (EGCG, Sigma), 0.1 mL of hyaluronic acid (HA, Maxigen Biotech Inc., Taoyuan City, Taiwan), and 2.5 mL of 5% chlorine dioxide (ClO2, Sigma) were added into the filtered solution. For comparing the friction coefficient of different solutions, 1 DAY ACUVUE MOIST contact lens (Etafilcon-A, Johnson & Johnson, New Brunswick, NJ, USA) was used. Lysozyme powder (Sigma, St. Louis, MI, USA) was dissolved in either phosphate-buffered saline (PBS) or γ-PGA-containing contact lens care solution for the final concentration as 1.9 mg/mL.
2.2. In Vitro Contact Lens Friction Testing System
A Nano Tribometer (NTR3, Anton Paar, Graz, Austria) was used for testing the friction coefficient of different solutions. The testing was conducted under a linear reciprocating movement with an amplitude of 2 mm and the contact lens was sliding against a ruby ball with a radius of 2 mm. Contact lenses were removed from the package, and the excess liquid from the lens was removed by lens tissue. The contact lens was mounted onto a semi-spherical sample holder with a plastic base that matches the internal curvature of the lens. The lens was clamped with the upper part of the holder. Three magnetic pegs are embedded in the clamping upper part and in the support lower part of the contact lens holder. Two types of tests were performed in this study. For the load variations, the speed was set to 0.2 cm/s while the load varied between 0.4 and 5 miniNewton (mN). For the speed variations, the load was set to 1 mN while the speed varied between 0.03 and 1 cm/s. There were 50 sequences for each test and 30 cycles for each sequence. For example, 1 sequence represented when the lens was sliding at 0.2 cm/s under 0.4 mN for 30 cycles. The next sequence would be the lens was sliding at 0.2 cm/s for 30 cycles under 0.5 mN if it was for the load variations. All tests were performed at room temperature and the contact lenses were completely immersed in the tested solutions during the entire time.
2.3. Lysozyme Concentration Measurement
The Bio-Rad DC protein assay (Bio-Rad, Hercules, CA, USA) was used and the lowest limit of this assay was 5 μg/mL. The preparation was done according to the manufacturer’s instructions. The standard curve was established by preparing 0, 0.05, 0.1, 0.2, 0.4, 0.8, 1.6, and 3.2 mg/mL of lysosomal solutions. After the reaction was completed, each sample was read by an Enzyme-Linked ImmunoSorbent Assay reader with a wavelength of 750 nm, and the optical density value was obtained. The lysozyme concentration in the sample could be obtained by using the standard curve.
2.4. Lysozyme Deposition Analysis
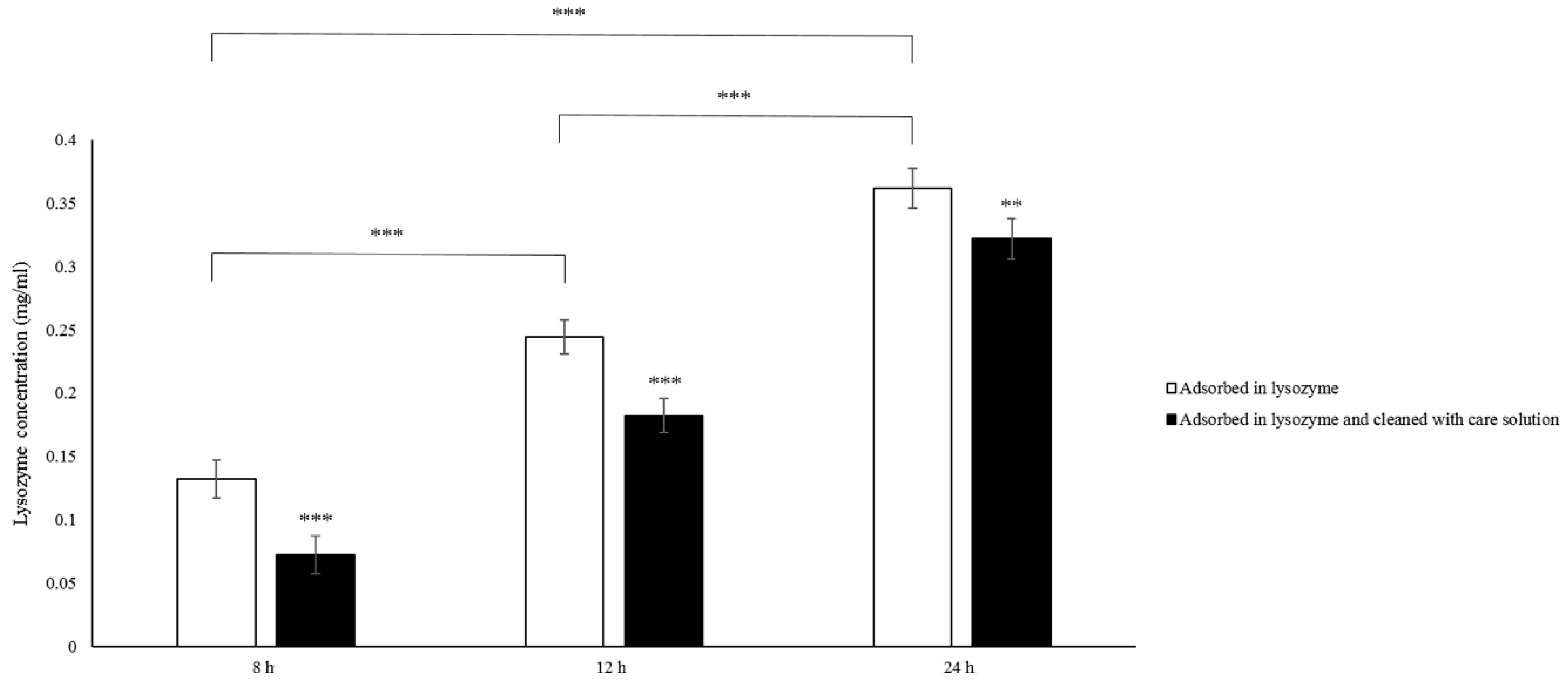
Etafilcon-A contact lenses were placed in 1.9 mg/mL lysozyme solution (original solution) at 37 °C for 8, 16, or 24 h. The lens was then transferred to γ-PGA-containing contact lens care solution, and was on the shaker for 30 min and then removed. Each condition was repeated four times. Lysozyme concentration in the original solution and in the care solution was measured after the lens was removed, thus lysozyme deposition concentration on each lens was calculated as below:
2.5. Viscosity Analysis
The viscosity of solutions was measured by DV-III Ultra programmable rheometer (Brookfield, Middleboro, MA, USA). The speed was 6 rpm (revolution per minute), and the temperature was at 25 °C. The viscosity of γ-PGA-containing care solution with and without lysozyme was measured, and each sample was repeated 3 times.
2.6. Statistical Analysis
Differences in lysozyme deposition analysis and viscosity analysis between different conditions were assessed by the student’s t-test to make an allowance of comparisons. A value of p < 0.05 was considered significant.
4. Discussion
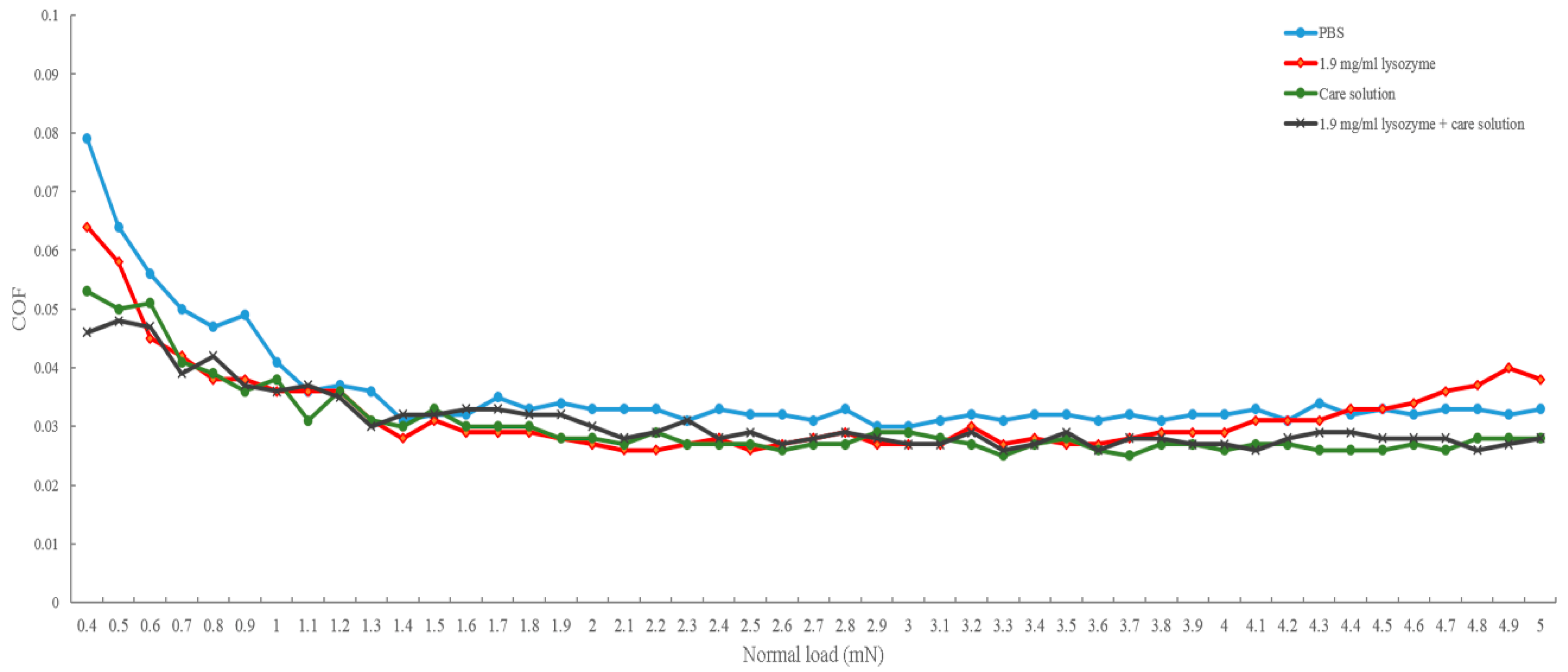
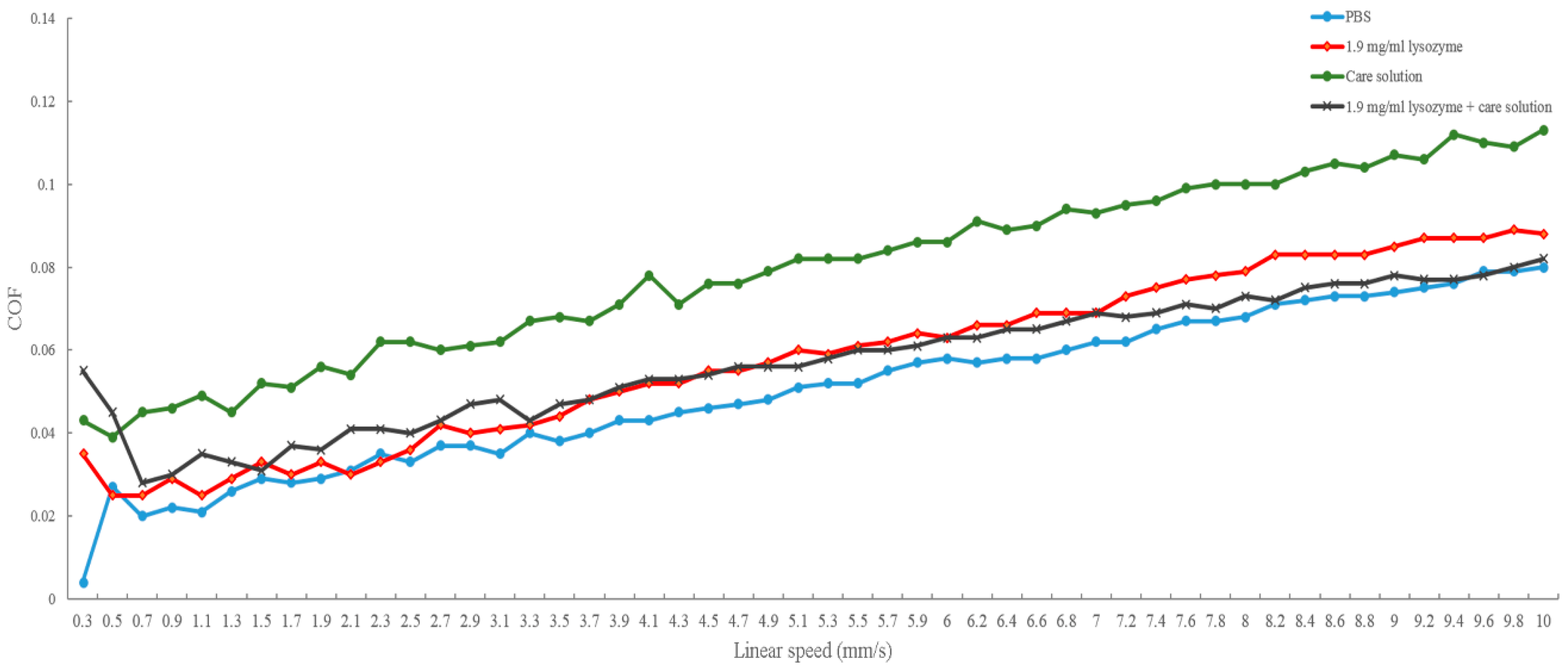
In this study, we investigated the bio-tribological effect of γ-PGA-containing care solution in the lysozyme-ionic contact lens system. When the normal load varied from 0.4 to 5 mN, the friction coefficient of PBS was the highest until the normal load was larger than 4.4 mN and then the friction coefficient of lysozyme was the highest. When the solution contained γ-PGA, the friction coefficient was the lowest overall. When the velocities varied from 0.03 and 1 cm/s, the friction coefficient of γ-PGA-containing care solution was the highest. The friction coefficient of lysozyme and the combination of lysozyme and γ-PGA-containing care solution was similar, but the friction coefficient of lysozyme became higher when the velocity was faster than 0.7 cm/s. The lysozyme deposition result demonstrated that the γ-PGA-containing care solution could effectively remove the adsorbed lysozyme from Etafilcon-A contact lenses. In addition, the viscosity analysis showed that adding lysozyme would increase the viscosity of the γ-PGA-containing care solution.
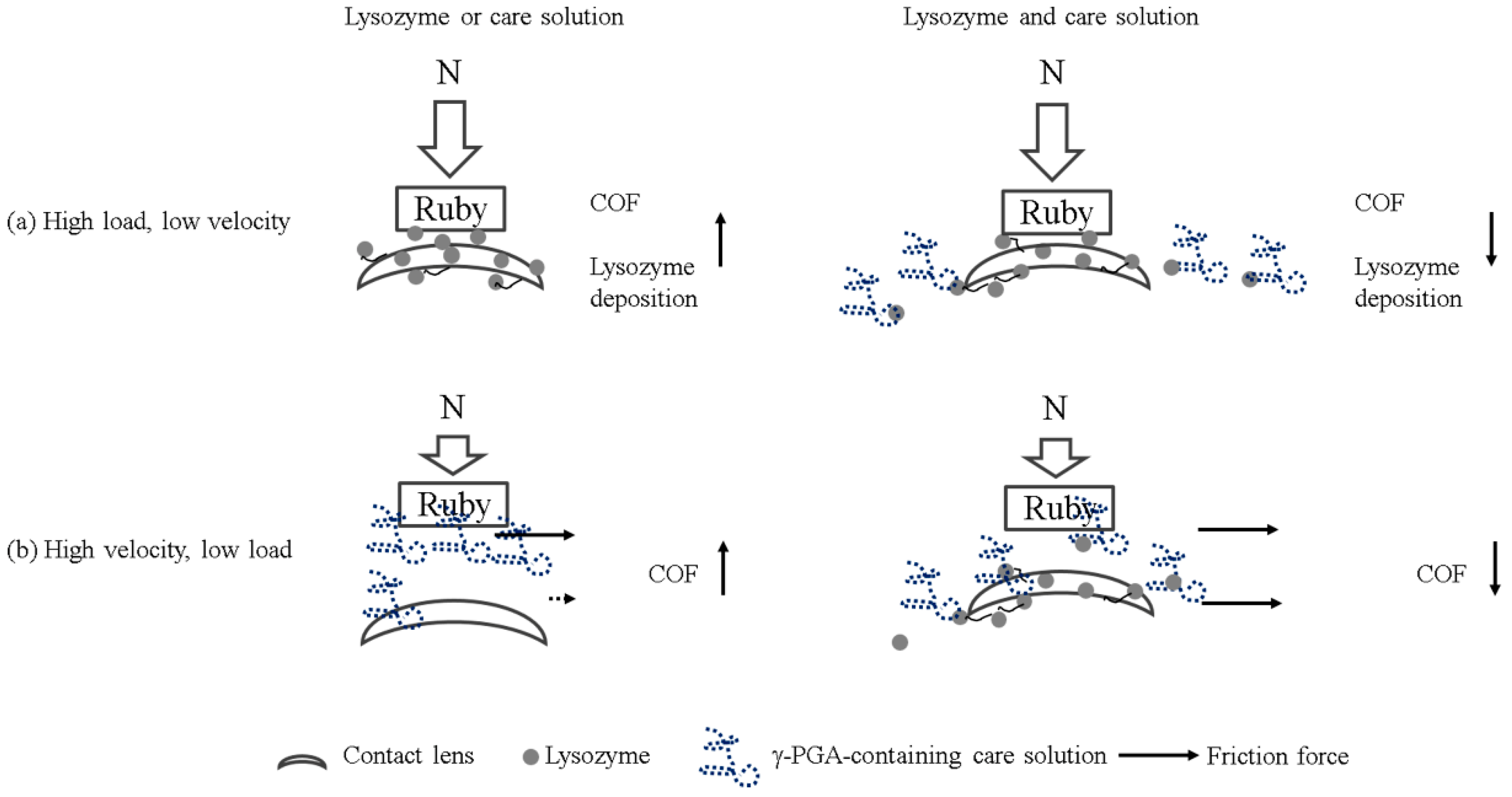
Multiple lubrication regimes have been shown to exist during the blinking cycle when contact lenses are in the eye [
13]. When the load is high and the blinking speed is slow, boundary lubrication occurs between the contact lens and the eyelid. Under boundary lubrication, the lubricant between two sliding surfaces forms a thin layer. Whether the lubricant can be adsorbed on the surface determines the friction coefficient, rather than the viscosity of the lubricant [
14]. Therefore, the result of load variations could be explained by boundary lubrication (
Figure 5a). The friction coefficient of the lysozyme was higher than the friction coefficient of γ-PGA-containing care solution and of the combination of lysozyme and γ-PGA-containing care solution (
Figure 2). The lysozyme deposition result demonstrated that the amount of lysozyme deposition was increasing when the incubation time was longer (
Figure 4), thus the amount of lysozyme on the surface of Etafilcon-A might be increased during frictional movement. In addition, the water content of lens material also affects protein deposition [
15,
16]. Etafilcon-A is a high water content material resulting in larger pore sizes, thus the lysozyme would penetrate into the matrix of Etafilcon-A. Therefore, the friction coefficient of lysozyme was higher on the end of load variation testing due to the high amount of adsorbed lysozyme. When γ-PGA was present with lysozyme, γ-PGA might take away lysozyme from the lens surface resulting in a reduction of friction coefficient (
Figure 5a). Lysozyme was positively charged while both γ-PGA and Etafilcon-A were negatively charged [
17,
18], thus γ-PGA could take away lysozyme by forming an ionic bond. However, why lysozyme would bind to γ-PGA instead of Etafilcon-A requires further investigation.
When the load is low and the blinking speed is fast, elastohydrodynamic or hydrodynamic lubrication occurs between the contact lens and the eyelid during blinking [
13]. At high speed, the lubricant with high viscosity would separate two surfaces and shear stress increases resulting in a high friction coefficient. The result of velocity variations might be explained by elastohydrodynamic or hydrodynamic lubrication. The viscosity of 1.9 mg/mL lysozyme was around 0.8 cP [
19], and it was lower than the γ-PGA-containing care solution resulting in a lower friction coefficient (
Figure 3). Interestingly, the viscosity of lysozyme and γ-PGA-containing care solution was higher than the viscosity of the γ-PGA-containing care solution but the friction coefficient was smaller (
Figure 3). This result suggested that the materials of surfaces and the interaction between γ-PGA and contact surfaces should be taken into account. When ruby is in acid solution, hydrogen bonds can be adsorbed on the surface resulting in a positively charged surface [
20]. We then hypothesized that negatively charged γ-PGA would bind to the ruby surface but would be away from negatively charged pHEMA surface. The adhesion affinity of γ-PGA to two surfaces might result in different friction forces, resulting in an increase of friction coefficient (
Figure 5b). In contrast, the presence of lysozyme may interact with γ-PGA resulting in similar friction force between the γ-PGA/ruby surface and γ-PGA/pHEMA surface. Therefore, the friction coefficient of γ-PGA-containing care solution and lysozyme was smaller than the γ-PGA-containing care solution (
Figure 3).
Although we proposed a potential model for the effect of γ-PGA in the lysozyme-ionic contact lens system under load and velocity variations, there are some limitations. We have not yet verified whether the amount of lysozyme deposition was indeed increased on the end of frictional cycles, or whether γ-PGA and lysozyme could form a stronger ionic bond than the bond between lysozyme and Etafilcon-A. In addition, we need to investigate whether γ-PGA would interact differently with ruby and with the Etafilcon-A lens to better understand the bio-tribological mechanism for velocity variations.
The current study demonstrated that γ-PGA can reduce the high friction coefficient caused by lysozyme in load variations. In velocity variations, the combination of γ-PGA and lysozyme showed a lower friction coefficient although the friction coefficient of γ-PGA itself was high. However, whether the in vitro results can be applied for increasing comfort degree in vivo requires further investigation.