1. Introduction
The technology of dissolution and leaching is widely used in uranium mining and metallurgy, and a large amount of low-concentration uranium-containing radioactive wastewater can be generated from these industrial processes, which could pose a serious potential risk to the environment and the health of those involved [
1]. Traditional methods such as reverse osmosis, ion exchange, and evaporation have the demerits of complexity, high energy consumption, and cumbersome operation [
2,
3]. The adsorption method, which possesses the characteristics of a wide source of materials, low cost, high selectivity, fast treatment rate, and large capacity would provide a possibility for the efficient uptake of uranium-bearing wastewater [
4]. However, adsorbents are usually difficult to regenerate and have poor reusability. Recently, “smart” hydrogels have attracted much interest in the treatment of wastewater contaminated with heavy metals. These “smart” hydrogels are characterized by their high expansion capacity, biocompatibility, reversibility, and maneuverability compared with traditional methods [
5,
6]. The intelligent responsiveness of hydrogels means that they are “smart” soft materials with three-dimensional cross-linked network structures that can change volume/shape or phase in sensitive response to environmental stimulation including temperature, pH, magnetic field, electric field, light, and chemical substances [
7,
8,
9,
10,
11]. The response characteristics of “smart” hydrogels are important parameters for their wide application in the fields of drug control release and separation, heavy metal adsorption, and biomedical treatments [
5,
12,
13].
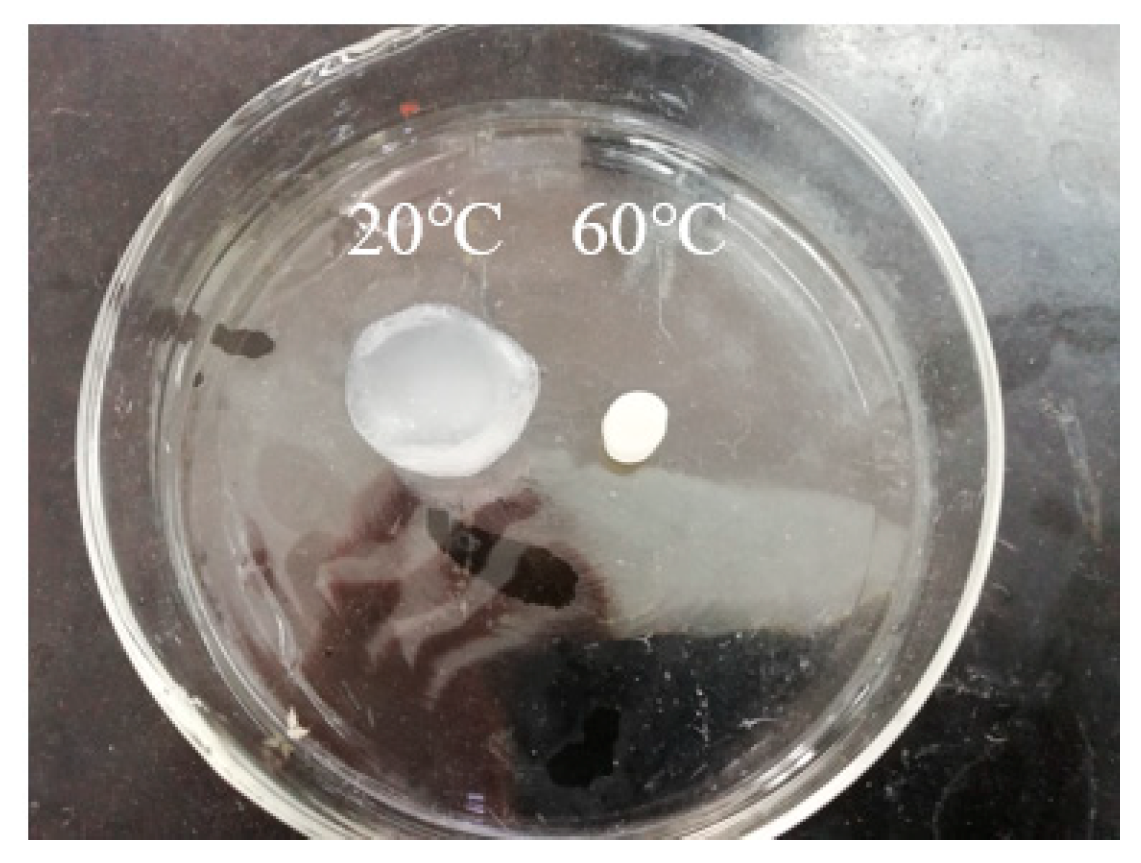
Poly(N-isopropylacrylamide) (PNIPAM) is the most representative temperature-sensitive polymer. It undergoes an abrupt reversible phase transition near the lower critical solution temperature (LCST), exhibiting a dramatic volume change from the swollen network to the shrunken state by expelling free water [
14,
15]. Even though the PNIPAM hydrogel shows intelligent thermal sensitivity, which is beneficial for the treatment of wastewater containing heavy metals, it exhibits a low capacity of adsorption for metal ions. The presence of functional groups within polymer networks helps in binding heavy metals via the formation of complex structures [
16]. Ju et al. [
17] prepared poly(N-isopropylacrylamide-co-benzo-18-crown-6-acrylamide) (P(NIPAM-co-BCAm)) hydrogel by crosslinking the ether group with NIPAM. The P(NIPAM-co-BACm) hydrogel adsorbed Pb
2+ at a temperature lower than the LCST and desorbed Pb
2+ at a temperature higher than the LCST. The calic-conjugated thermo-responsive poly(N-isopropylacrylamide-co-tetra(5-hexenyloxy)-p-tert-butylcalix [4]arene) (P (NIPAM-co-HBCalix)) has also been manufactured for capturing Ni
2+, and its adsorption capacity was maintained above 90% after five cycles of adsorption-desorption [
18].
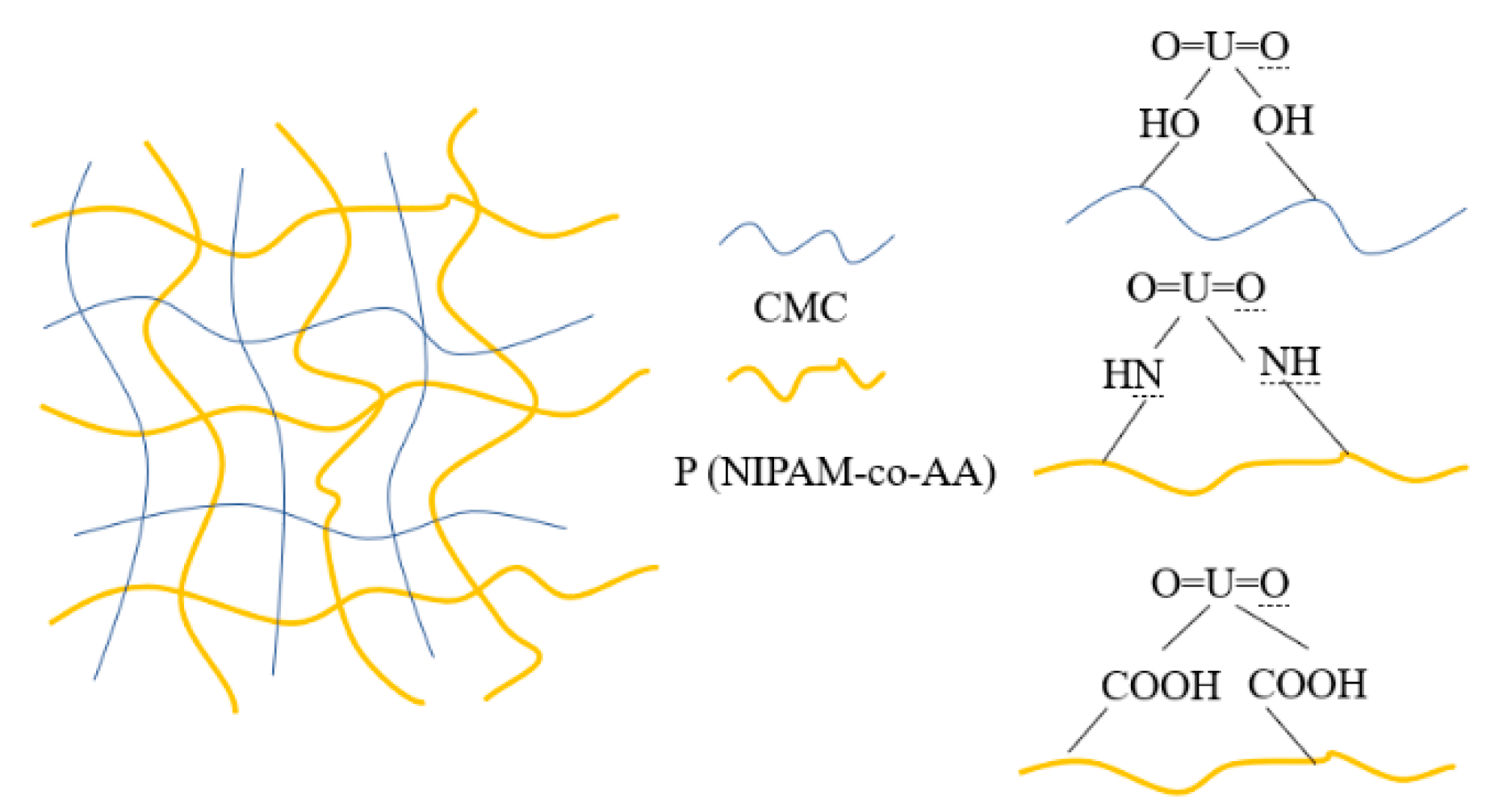
Carboxymethyl cellulose (CMC) is a degradation and biocompatibility biomass material containing a large amount of carboxyl oxygen and lone pair electrons in coordinated bonds that form chelates with metal ions [
19,
20,
21]. Chen et al. [
22] produced a bio-based adsorbent consisting of polyacrylamide, polyacrylic acid, and CMC (CMC-PAMA) by the thermal crosslinking of sodium carboxymethylcellulose, polyacrylic acid, and polyacrylamide with good stability and recyclability, whose static adsorption capacities for methylene blue (MB) and Pb(II) were, respectively, 1611.44 mg·g
−1 and 840.11 mg·g
−1. Sun et al. [
23] applied biomaterials (CMC/gelatin/starch) to stabilize FeS nanoparticles for the removal of Hg(II). The experiments showed that the three biomaterial-stabilized nanoparticles could improve the adsorption efficiency significantly. Among those CMC-based polymers, the interactions of the OH group and metal ions would contribute to improve the stability and insolubility of water, which is conducive to the separation of metal ions from water after adsorption [
16].
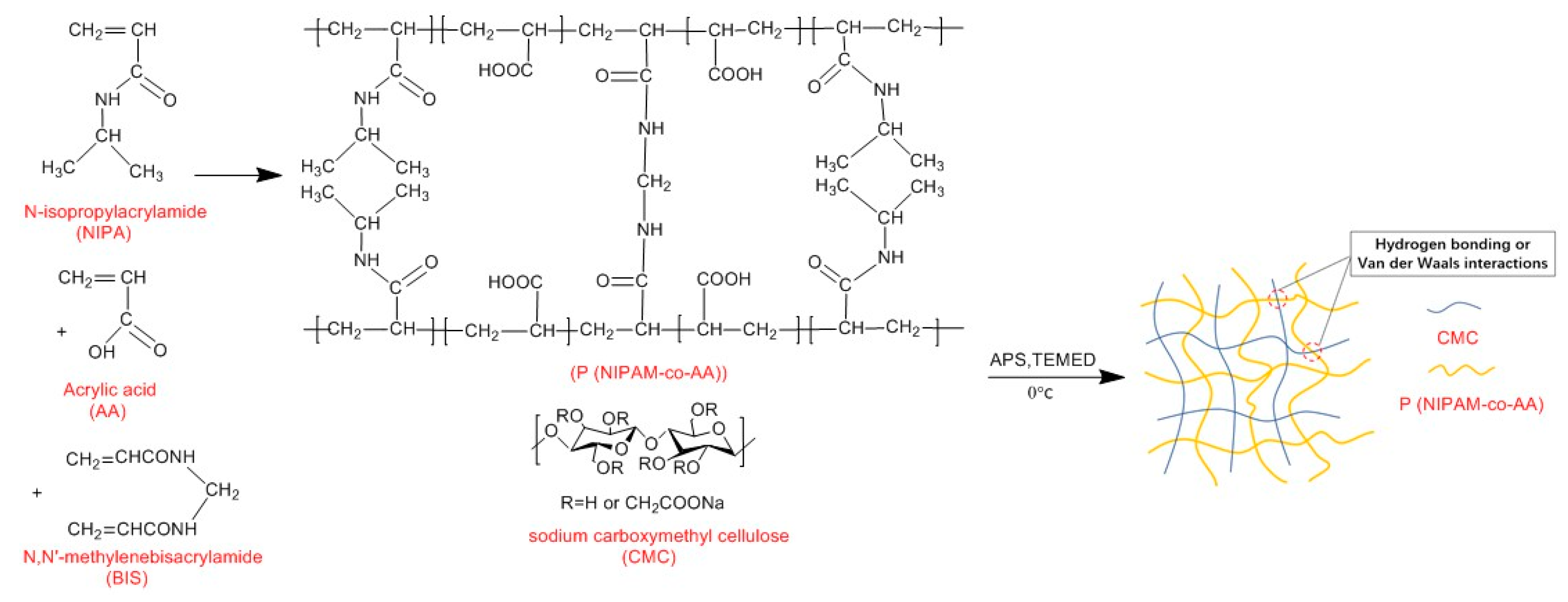
Our experiments aimed to use N-isopropylacrylamide (NIPAM) as a matrix to prepare a temperature sensitive intelligent hydrogel. During gel preparation, polymerization parameters such as polymerization temperature, the ratio of crosslinker to monomer, and the type of polymer composition could contribute to the desirable properties of thermo-sensitive hydrogels [
24]. Even though the polymers of NIPAM, CMC, and acrylic acid (AA) in Na-montmorillonite (MMT) have been fabricated [
25], the principal element of preparation of thermo-sensitive hydrogel and the influence mechanism are still unclear. Moreover, temperature-sensitive hydrogels prepared by pre-experiment were fragile after the adsorption of water, which did not meet the reusability demands of uranium removal. Therefore, we have attempted to optimize the synthesis condition for the CMC/P (NIPAM-co-AA) hydrogel through L16 (4
5) orthogonal experiments and determine the dominant factor in the polymerization. The chemical structure, thermostability, and morphology have been represented by a series of characterizations. The behaviors of composite hydrogel adsorption of U(VI), including adsorption kinetics, isotherms, thermodynamics, and reusability performance, were systematically investigated.
2. Materials and Methods
2.1. Materials
The main materials for the preparation of thermo-sensitive hydrogels of CMC/P (NIPAM-co-AA) include NIPAM, AA, CMC, N,N-methylene bis acrylamide (BIS), ammonium persulphate (APS), and N,N,N′,N′-tetramethylethylenediamine (TEMED). The sources of these reagents/materials are shown in
Table 1. Among them, N-isopropyl acrylamide was recrystallized three times in n-hexane; the rest of the materials were of analytical purity.
2.2. Preparation of CMC/P (NIPAM-co-AA) Hydrogels by Orthogonal Experiments
The L16 (45) orthogonal experiments were designed for the optimization of the synthesis condition. Intelligent hydrogels were made by free radical polymerization. Recrystallization NIPAM, AA, BIS (crosslinking agent), and CMC (0.6 g of each) were added in sequence to three flasks containing 10 mL of deionized water under the nitrogen gas condition, which was well mixed using an electric mixer. After half an hour of continuously admitting the nitrogen gas to the reaction flasks, the reaction was initiated in the presence of APS (initiator) and TEMED (catalyst), then the mixture was quickly transferred to a cylindrical sealed tube and placed in a water bath for 24 h at a temperature of 0, 25, 35 and 70 °C to prepare the hydrogel.
After polymerization, the composite hydrogel was washed in deionized water over a 48 h period to remove the unreacted polymer monomer, changing the water every 12 h. Finally, the hydrogel was sliced then vacuum freeze-dried over 24 h. The addition amounts of each polymer monomer are shown in
Table 2 and
Table 3.
2.3. Index Test of Orthogonal Experiments
2.3.1. Swelling Behavior of Composite Hydrogel
The water absorption rate (SR) of the CMC/P-(NIPAM-co-AA) hydrogel was determined by the tea-bag method [
26]. Composite hydrogel samples were accurately weighed after drying through vacuum freezing in the freeze dryer (FD5-series, SIM). The mass of the dried hydrogel samples were recorded as
Wg. The dry gel was placed in empty tea-bags of 5 cm × 6 cm, which was made of plant fiber. The samples in the tea-bags were then immersed in a buffer solution of pH = 2 for 24 h. The data was recorded to obtain the mass of the hydrogel after absorbing water as
Ws. The water adsorption ratio (
SR) of the composite hydrogel was calculated as follows:
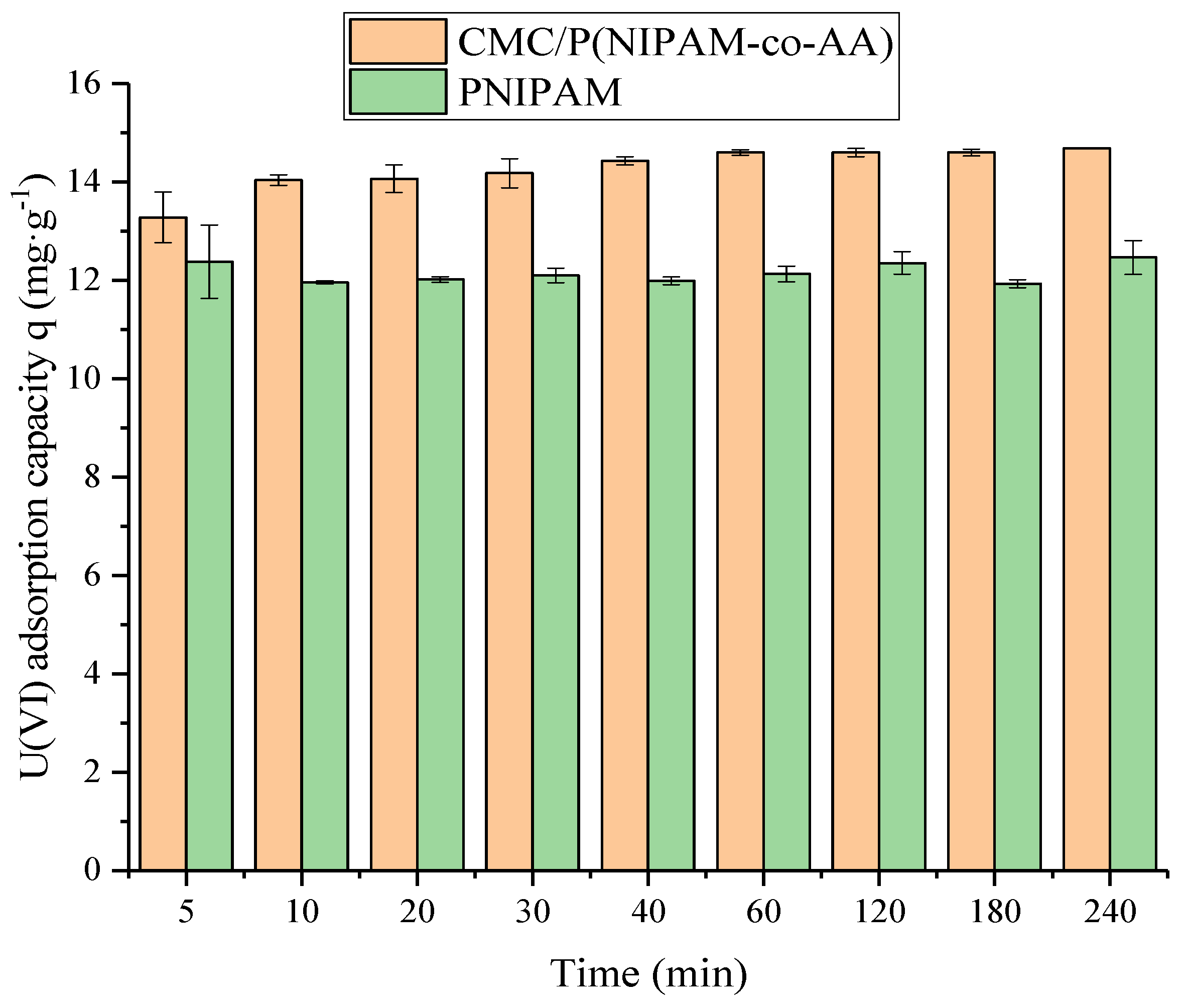
2.3.2. Adsorption Capacity of Uranium
The adsorption capacity of uranium was determined by immersing the 10 mg hydrogel in 30 mg L
−1 U(VI) solutions at pH = 6 and a temperature of 298 K for 2 h. The adsorption uptake amount of U(VI) by the hydrogel (
qe, mg·g
−1) and the removal rate (
η, %) were determined by Equations (2) and (3), respectively, as follow:
where
C0 (mg·L
−1) and
Ct (mg·L
−1) represent the initial concentration and equilibrated concentration of U(VI), and m (g) and V (mL) are the mass of the hydrogel and the volume of the solution, respectively.
2.3.3. Study on the Structure and Thermal Stability of Gel
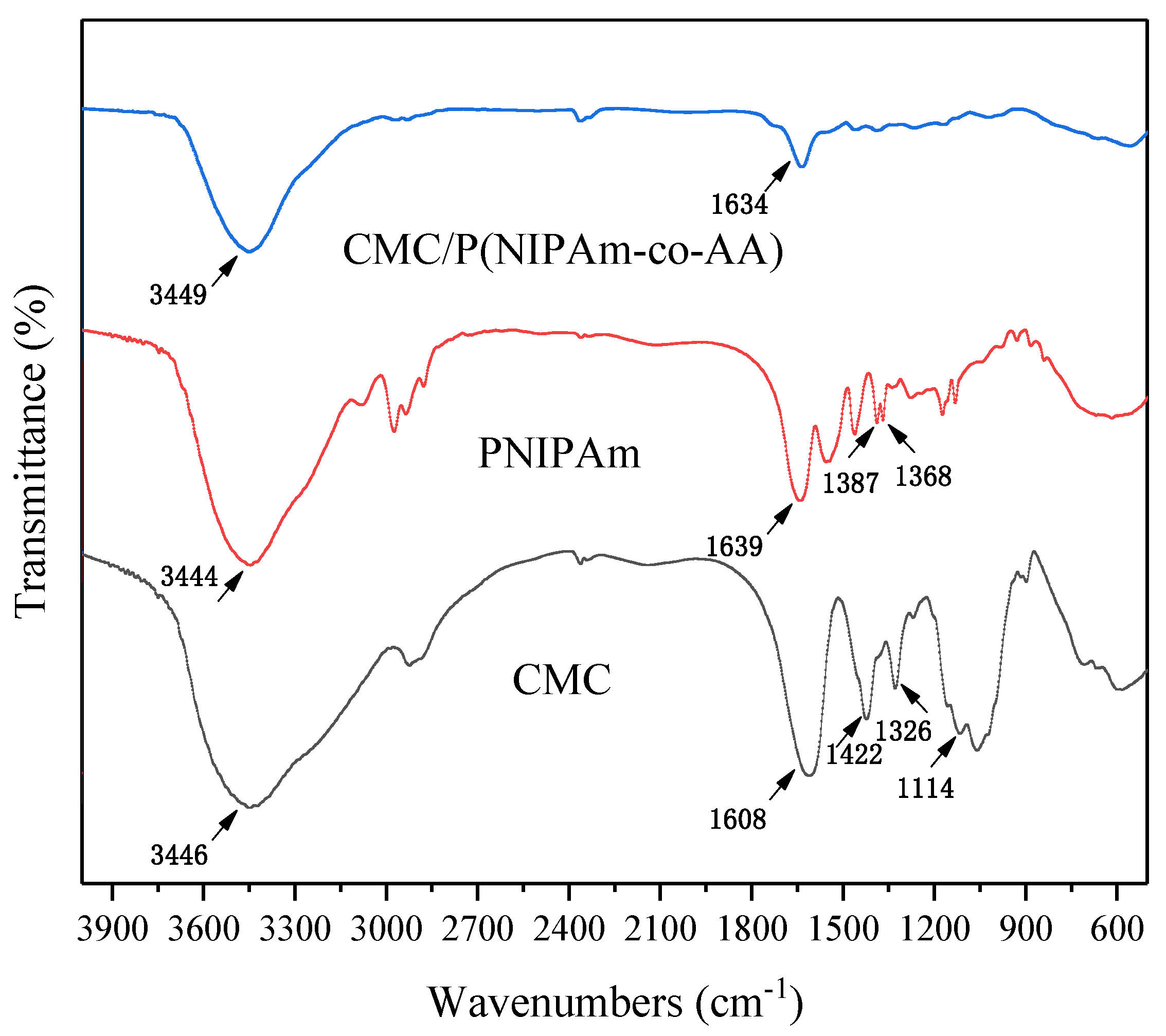
The structure of hybrid hydrogels was confirmed by the Fourier-transform infrared (FTIR) spectra of a Nicolet-460 spectrometer (Nicolet, Madison, WI, USA) from the wavenumber range of 400 to 4000 cm−1. The thermal stability of the gels was performed by thermogravimetric analysis (TGA) in the temperature range of 30–600 °C with dried gel on a DSC 200-F3 (Netzsch, Bavaria, Germany).
2.4. Batch Sorption Experiments
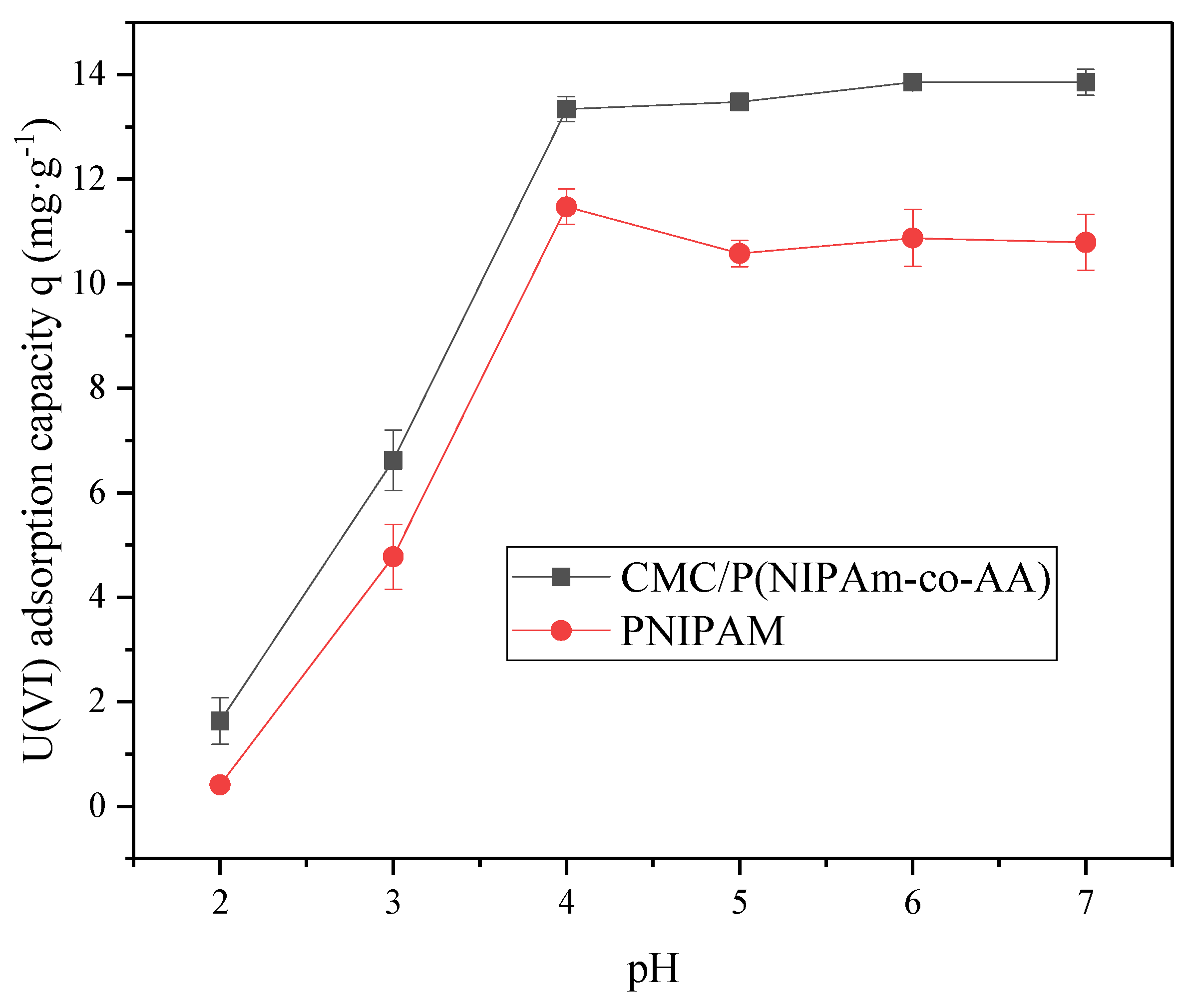
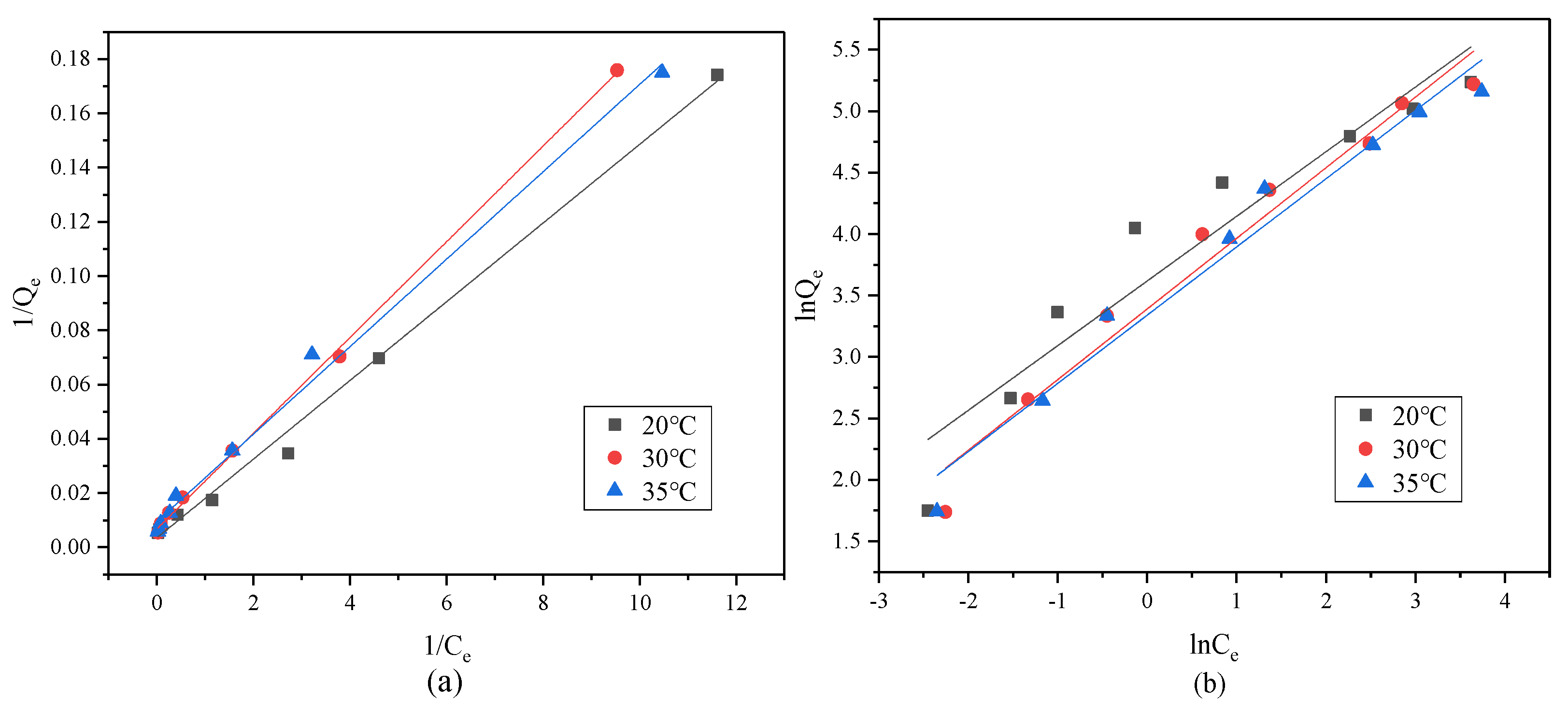
The evaluation of the adsorption properties of the CMC/P (NIPAM-co-AA) hydrogel for the uptake of U(VI) was carried out. Different concentrations of the U(VI) solution (30 mL of each) were shaken on a shaking table at 150 rpm so that proper reaction could take place with a constant temperature and pH value. The pH value was regulated by HCl and NaOH solutions. The solid-liquid separation was then done by filtration. The uranium concentration in the remaining solution was determined by spectrophotometry (WFJ 2000, Unic, Shanghai, China).
2.5. Mechanism Analysis of Adsorption on U(VI)
2.5.1. Characterization
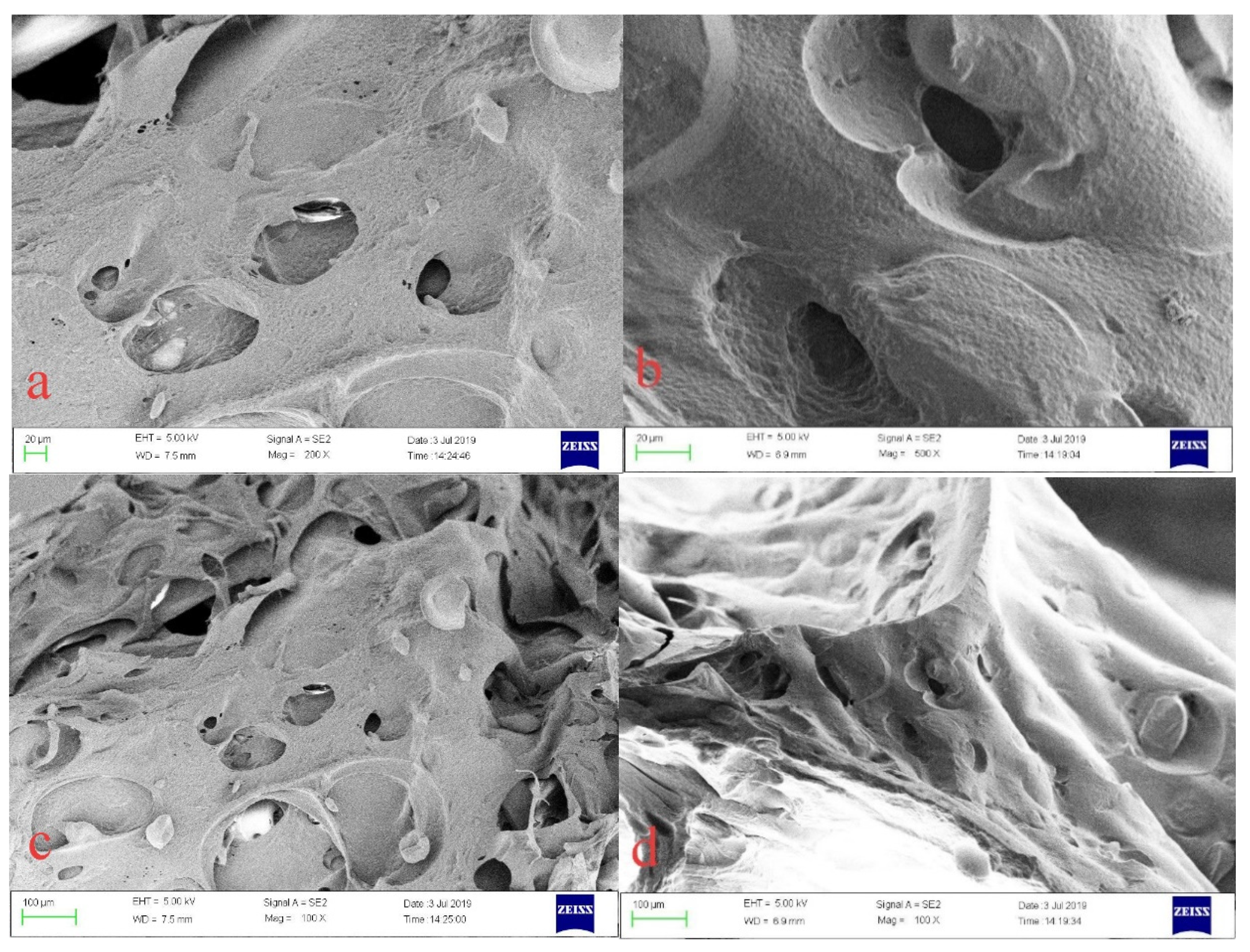
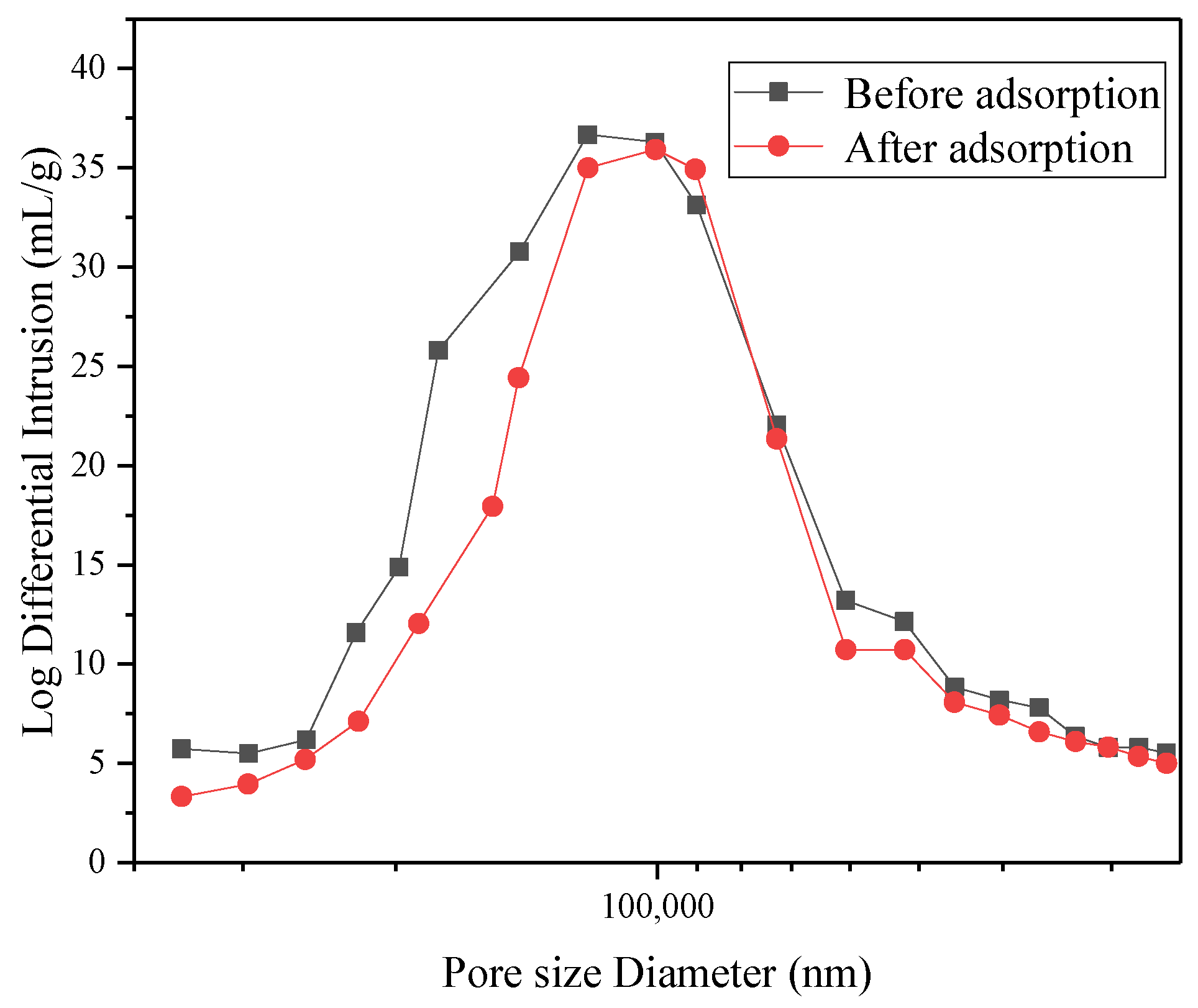
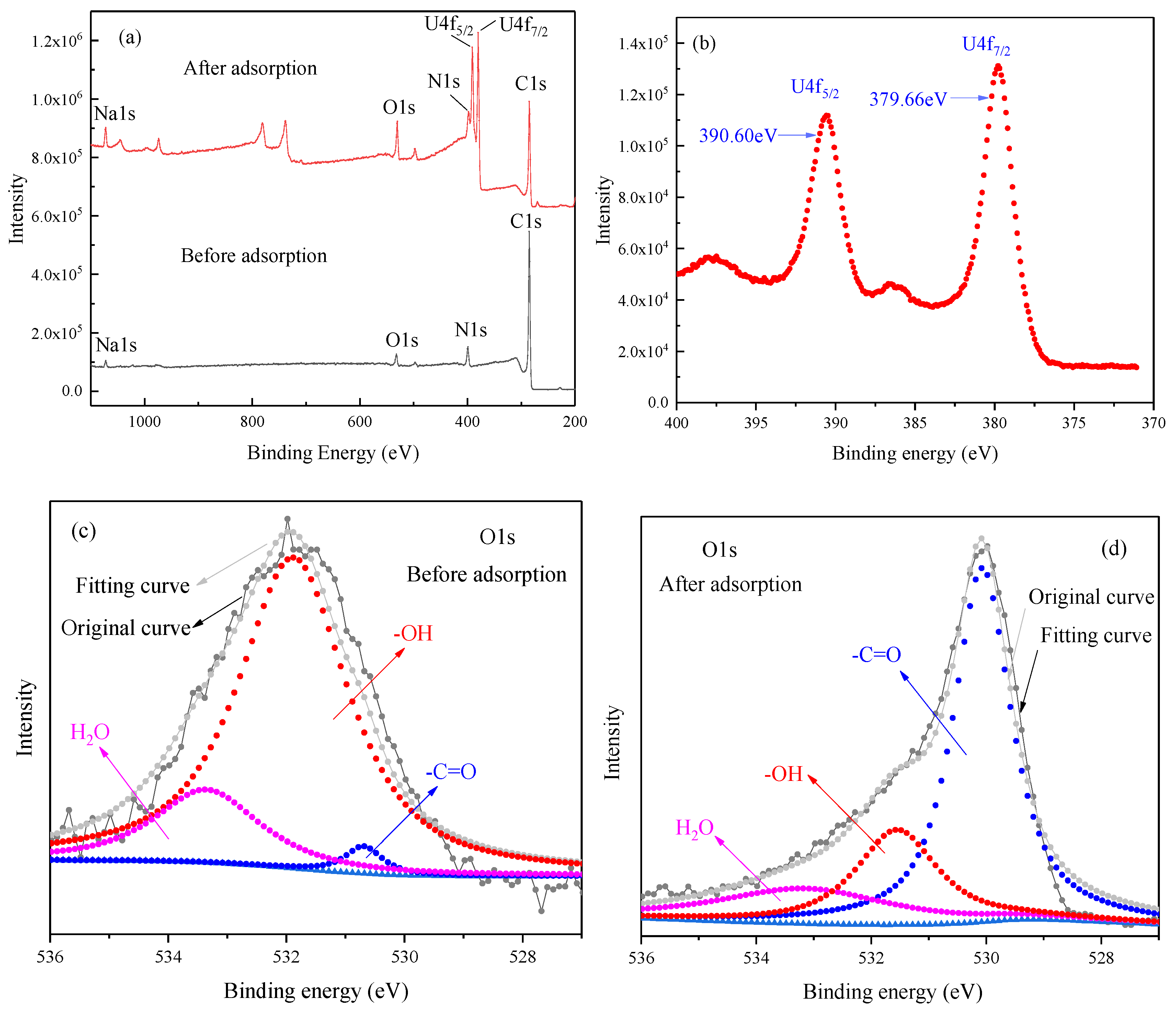
The CMC/P (NIPAM-co-AA) hydrogel, before and after the adsorption of U(VI), was characterized by scanning electron microscopy (SEM), mercury intrusion porosimetry (MIP), and X-ray photoelectron spectroscopy (XPS). The SEM images were obtained by a JSM-7500F SEM instrument (JEOL, Akishima, Japan) after quenching in liquid nitrogen. The pore size of the hydrogel before and after U(VI)-loading were examined by MIP AutoPore IV (Micromeritics, Norcross, GA, USA). XPS spectra were acquired on an Escalab 250Xi (Thermo Fisher Scientific, Waltham, MA, USA).
2.5.2. Chemical Modification of the Hydrogel
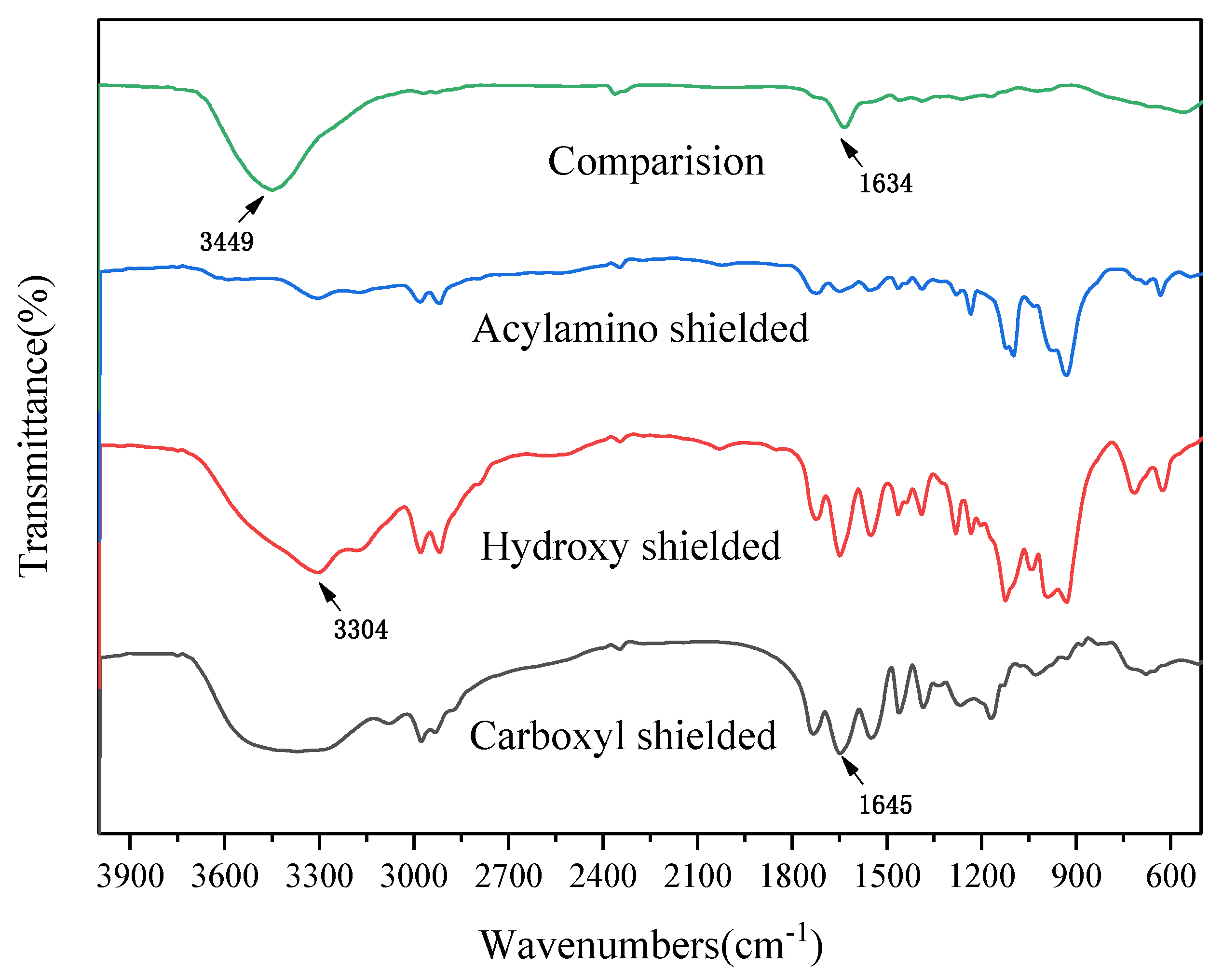
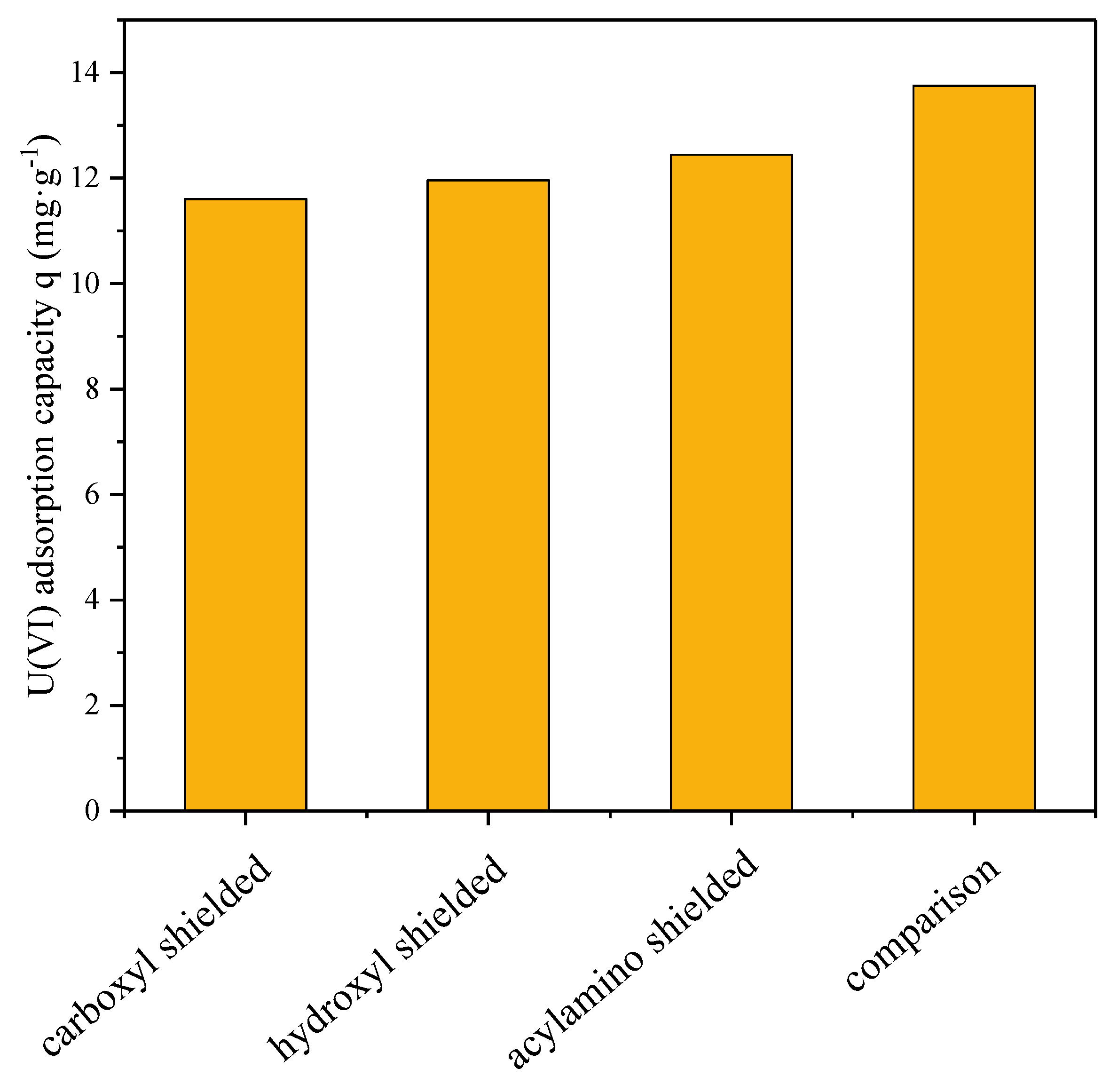
In order to examine the functional groups that play a major role in the adsorption of U(VI), the surface of the hydrogel was treated in series with methyl alcohol and concentrated hydrochloric acid to shield the carboxyl group, and then with formaldehyde to mask the hydroxyl group [
27,
28].
Ten milligrams of hydrogel was mixed with 20 mL of methyl alcohol and 0.2 mL of concentrated hydrochloric acid at 20 °C in a reaction flask and was then shaken on a shaking table for 8 h. Ten milligrams of hydrogel was immersed in 10 mL of formaldehyde under the same condition for 8 h. Ten milligrams of the hydrogel was added into the mixture of 20 mL of formic acid and 10 mL of formaldehyde to shield the amide group. Then, after treatment, the hydrogels were all washed three times with distilled water and were added to 5 mg L−1 U(VI) solution for 1 h of adsorption reaction. The remaining U(VI) solution was measured by spectrophotometry (WFJ 2000, Unic, Shanghai, China), as mentioned above.
2.6. Desorption and Recycling Experiment
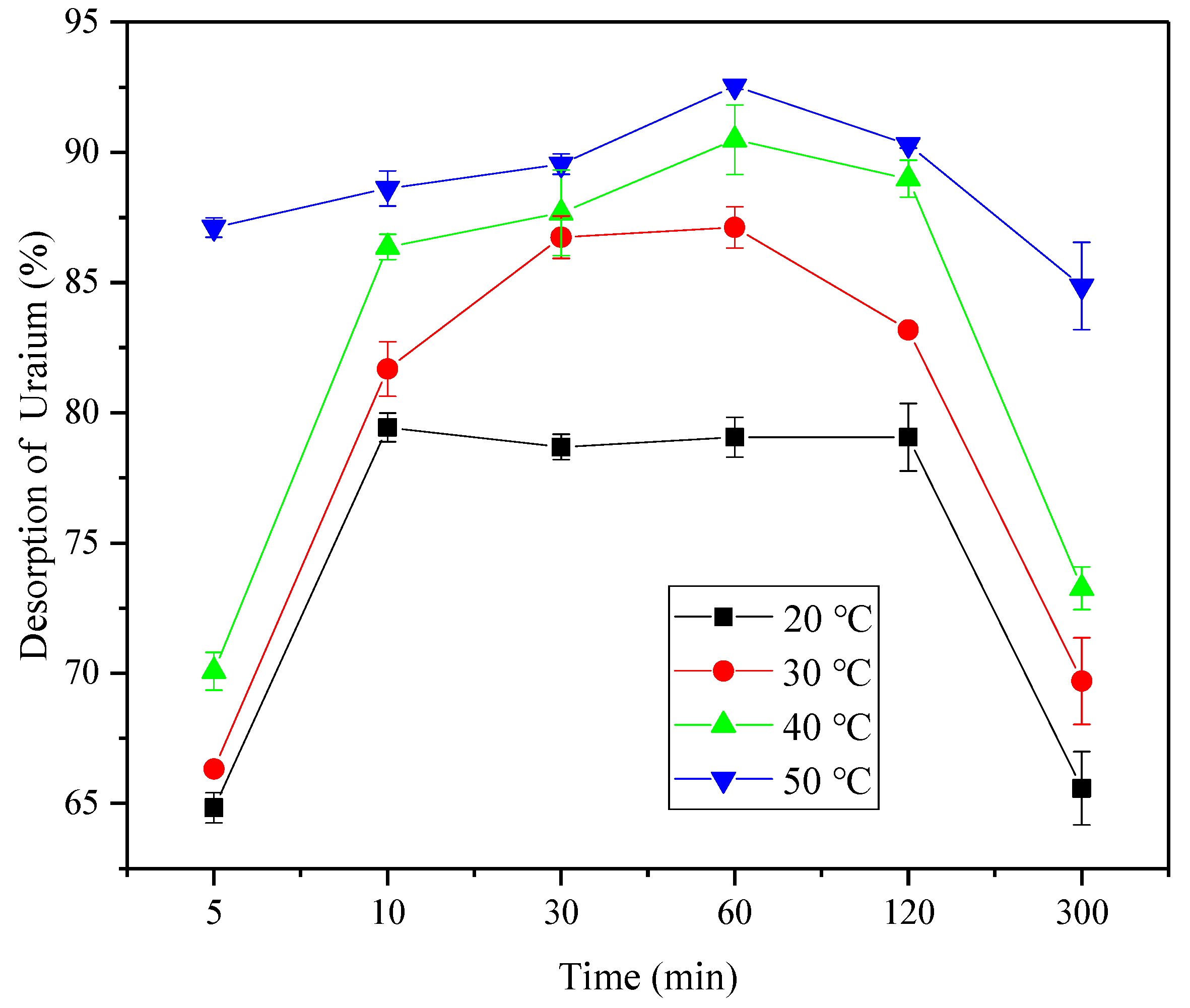
The hydrogel was placed under optimal adsorption conditions to achieve adsorption equilibrium and was washed three times with distilled water. The desorption of U(VI) from the hydrogel was monitored by placing the hydrogel in 0.1 mol L−1 of HNO3 solution at different temperatures, where it was left for between 10 min and 5 h.
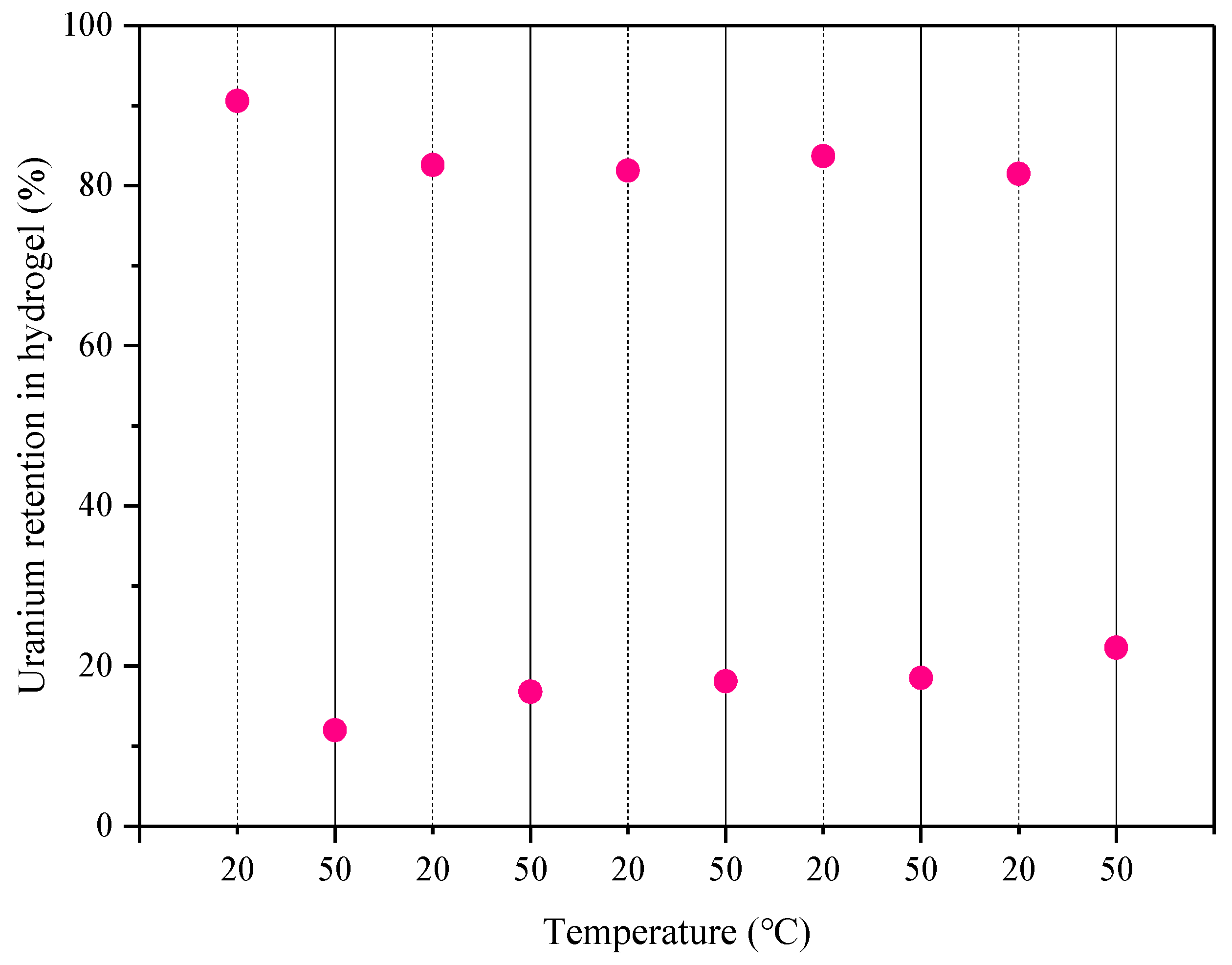
The regeneration ability of the hydrogel was evaluated by conducting a heating cycle of adsorption-desorption. The alternated adsorption-desorption cycles were repeated five times at 20 and 50 °C. The remaining U(VI) concentration in the solution was determined by spectrophotometry.