Electrical Field-Assisted Gene Delivery from Polyelectrolyte Multilayers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasmid DNA Preparation
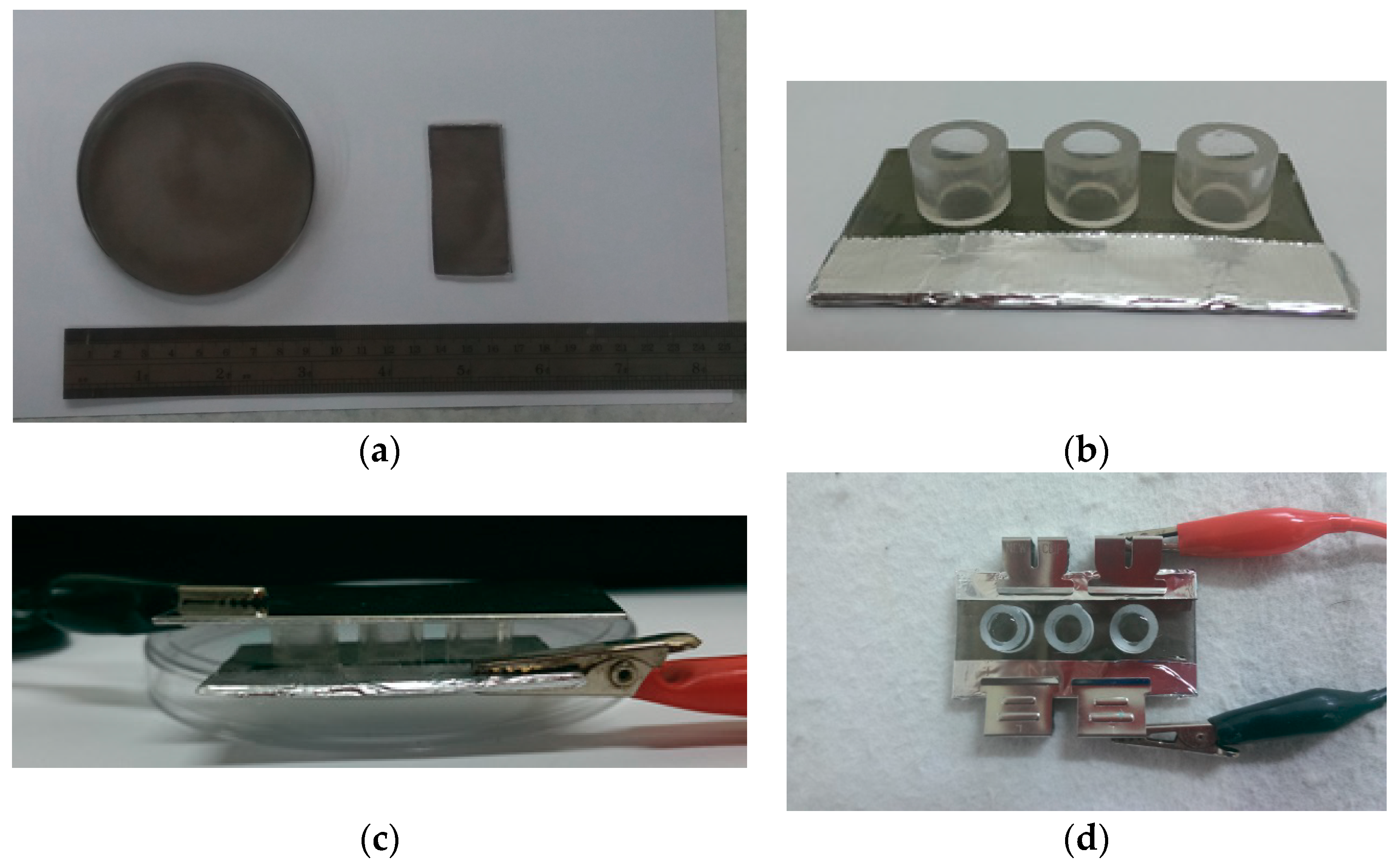
2.3. The Preparation of Polypyrrole (PPy) Films
2.4. Layer-by-Layer Assembly of PEMs
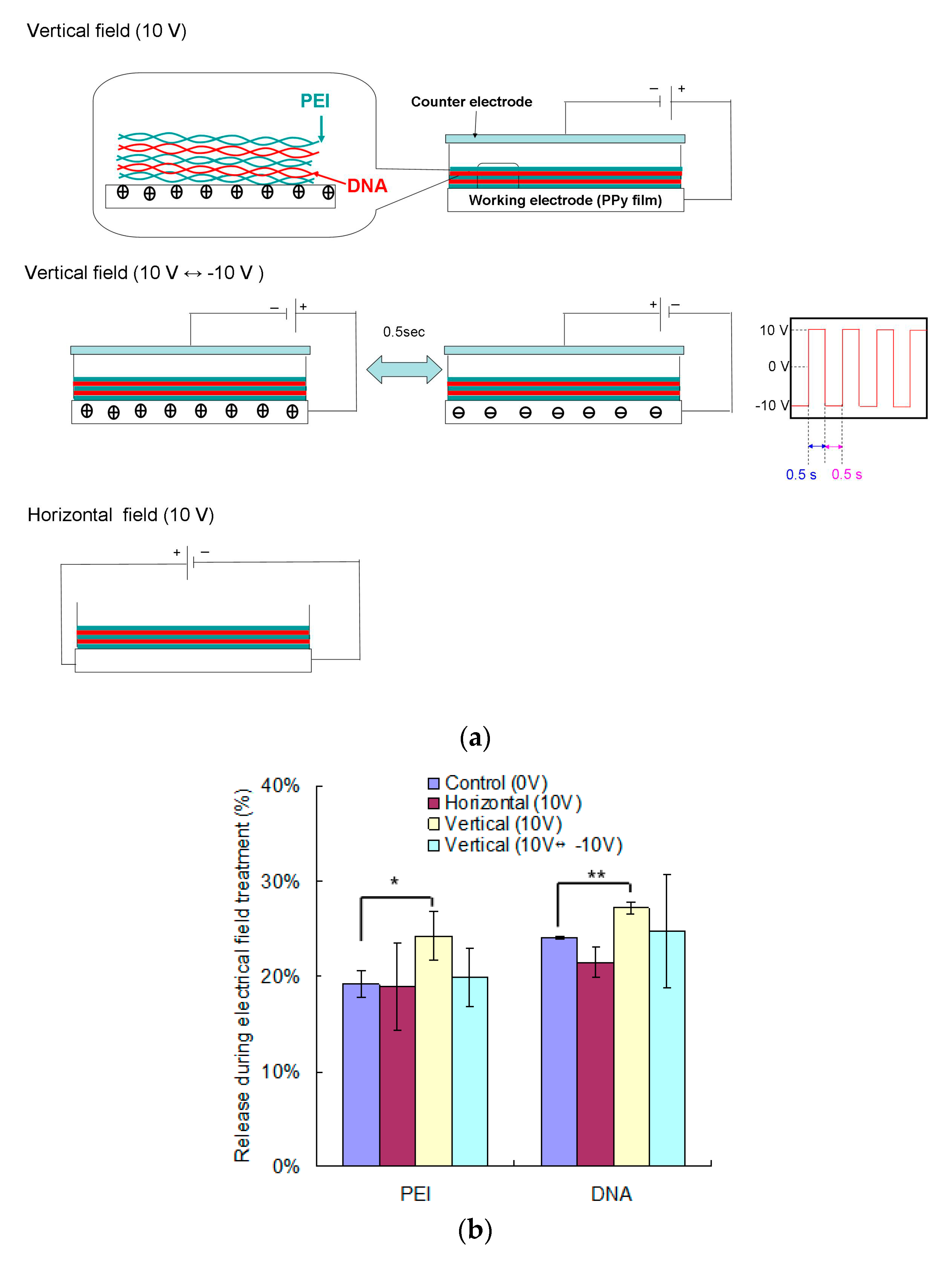
2.5. The Pretreatment of Electrical Fields to Promote DNA and PEI Release from PEMs
2.6. Surface Morphologies of PEMs
2.7. Characteristics of Polyplexes Formed by PEMs-Released DNA and PEI
2.8. Cell Transfection
2.9. Statistical Analysis
3. Results and Discussion
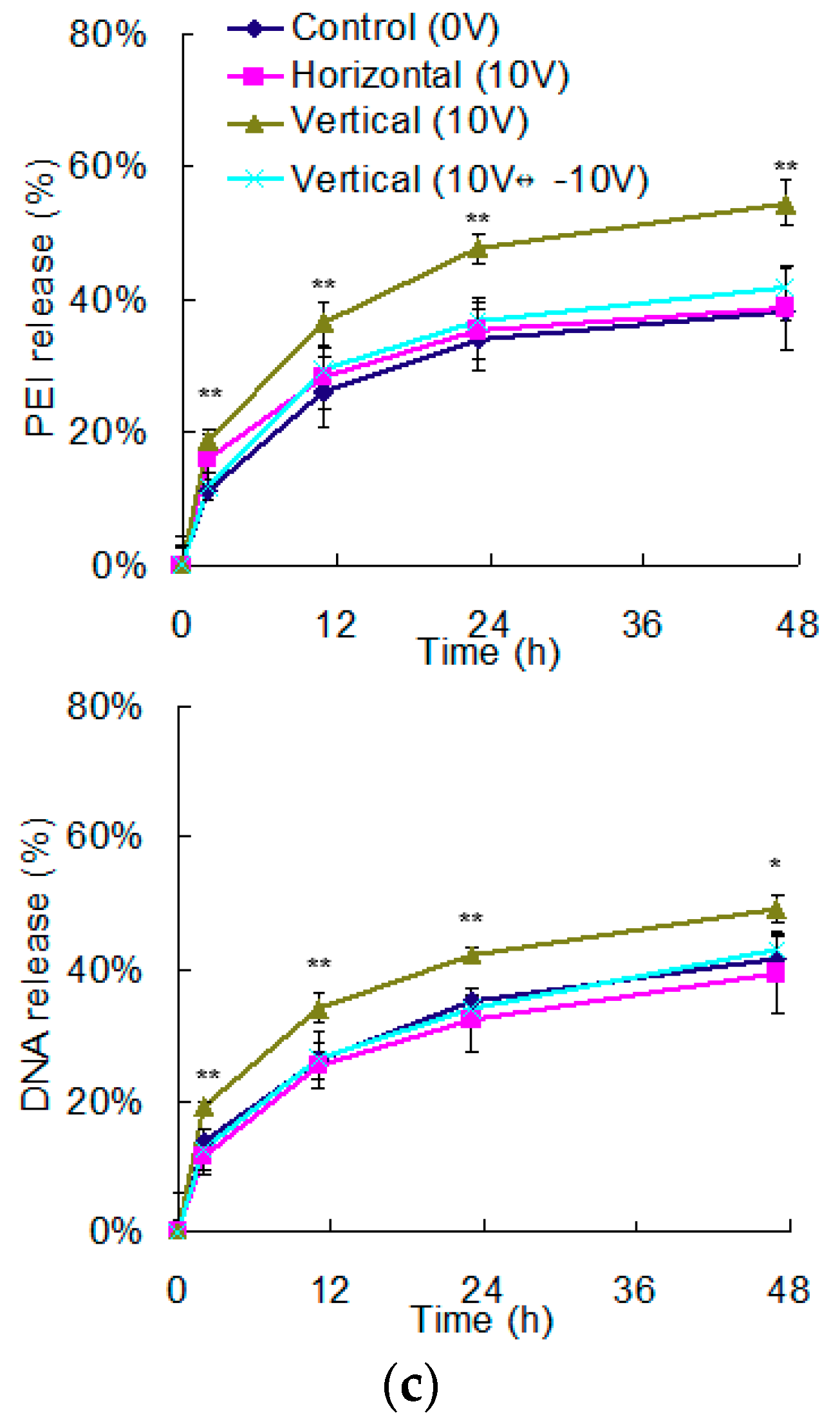
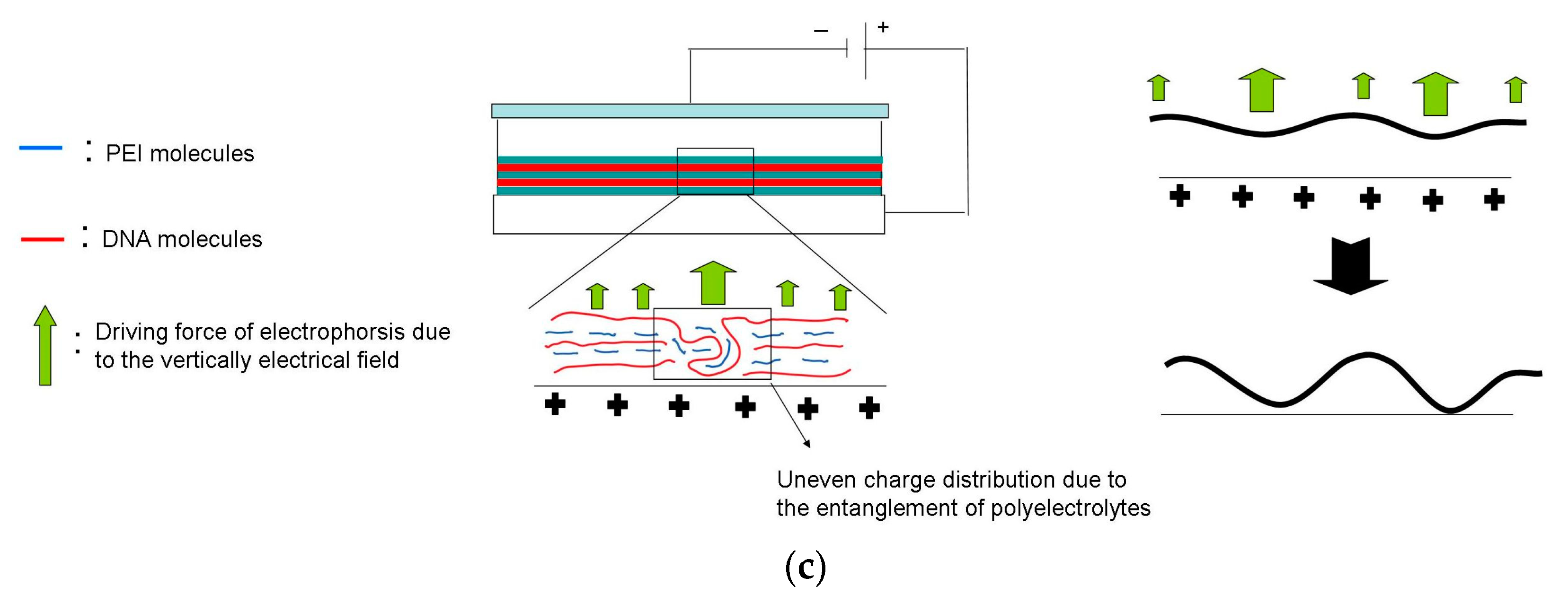
3.1. The Effects of Electrical Field on the Dissociation of LbL Multilayers
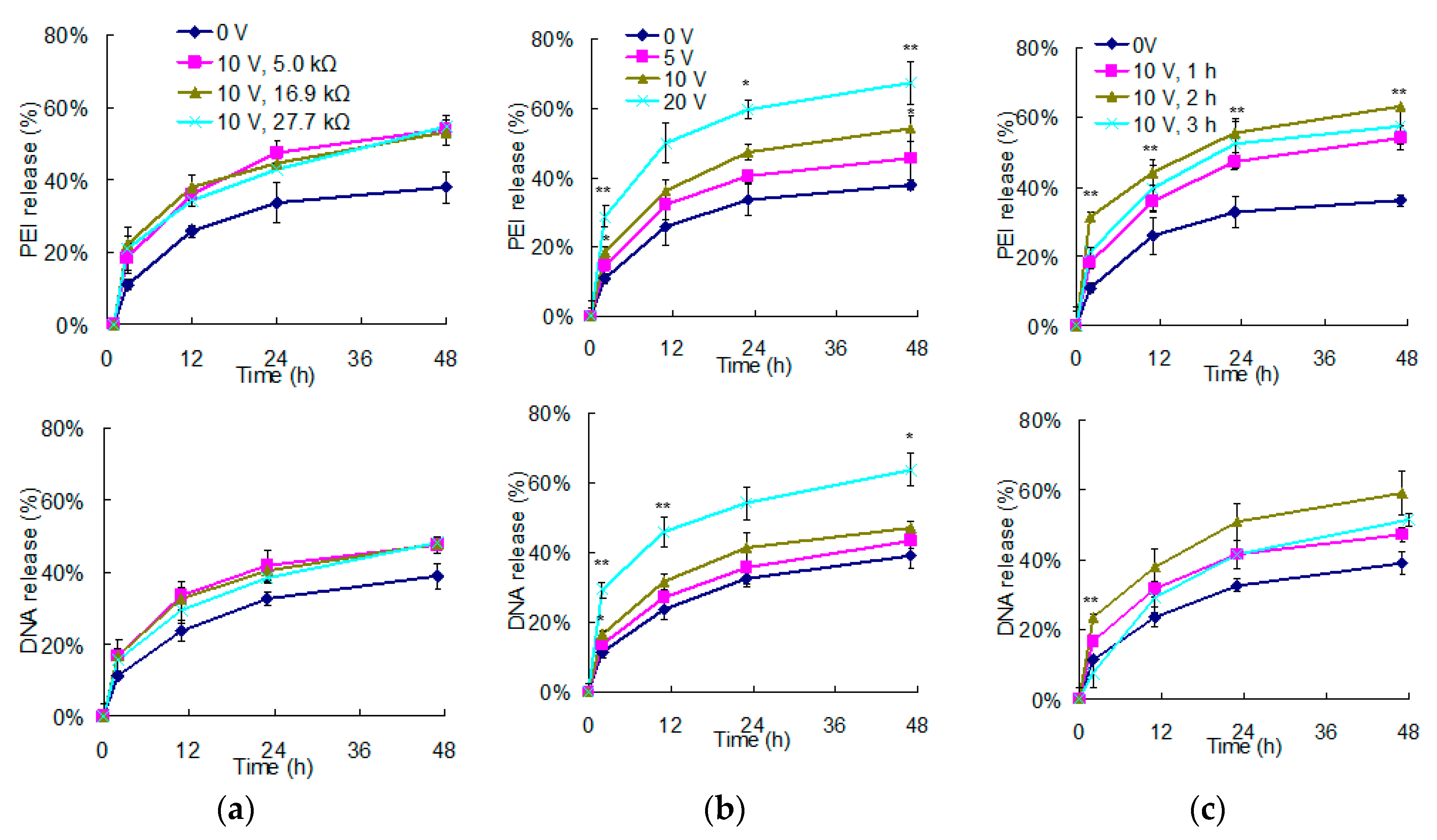
3.2. The Regulation of Polyelectrolyte Delivery through the Parameters of Vertically Electrical Fields
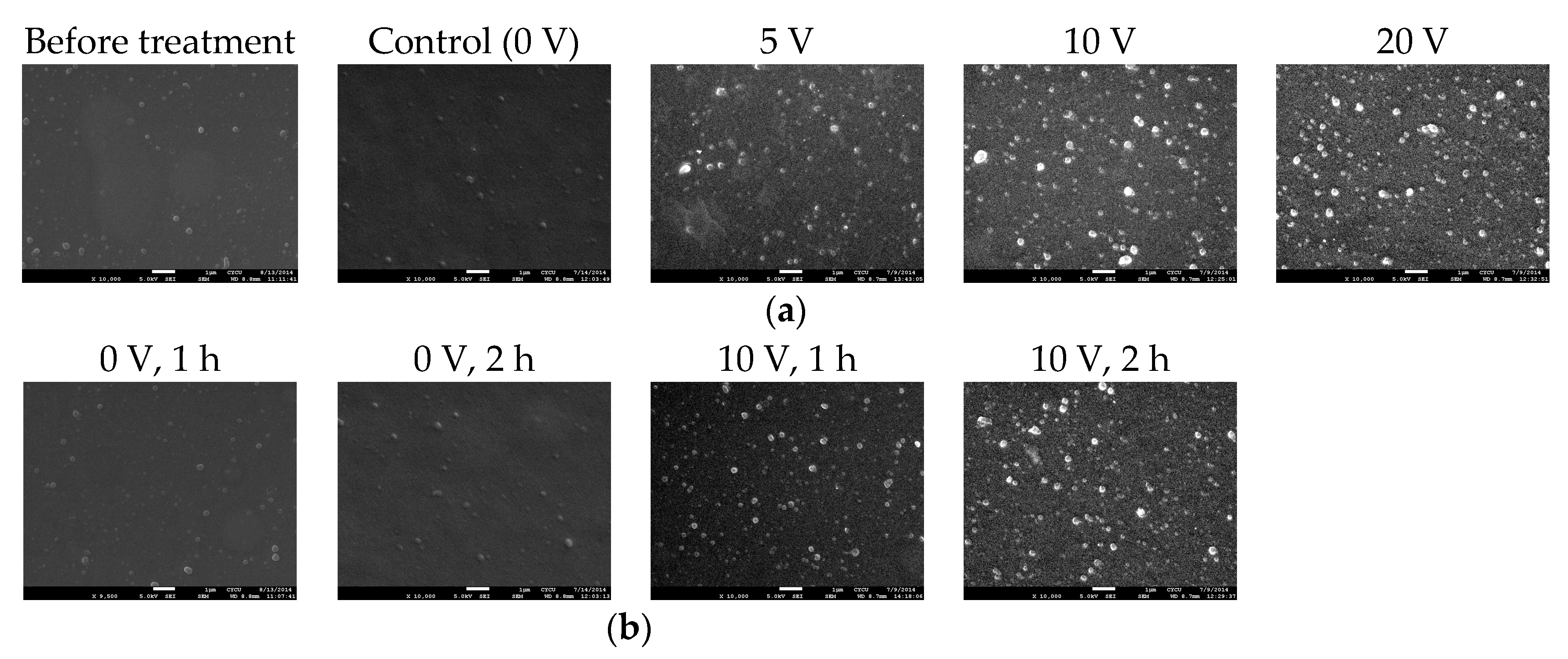
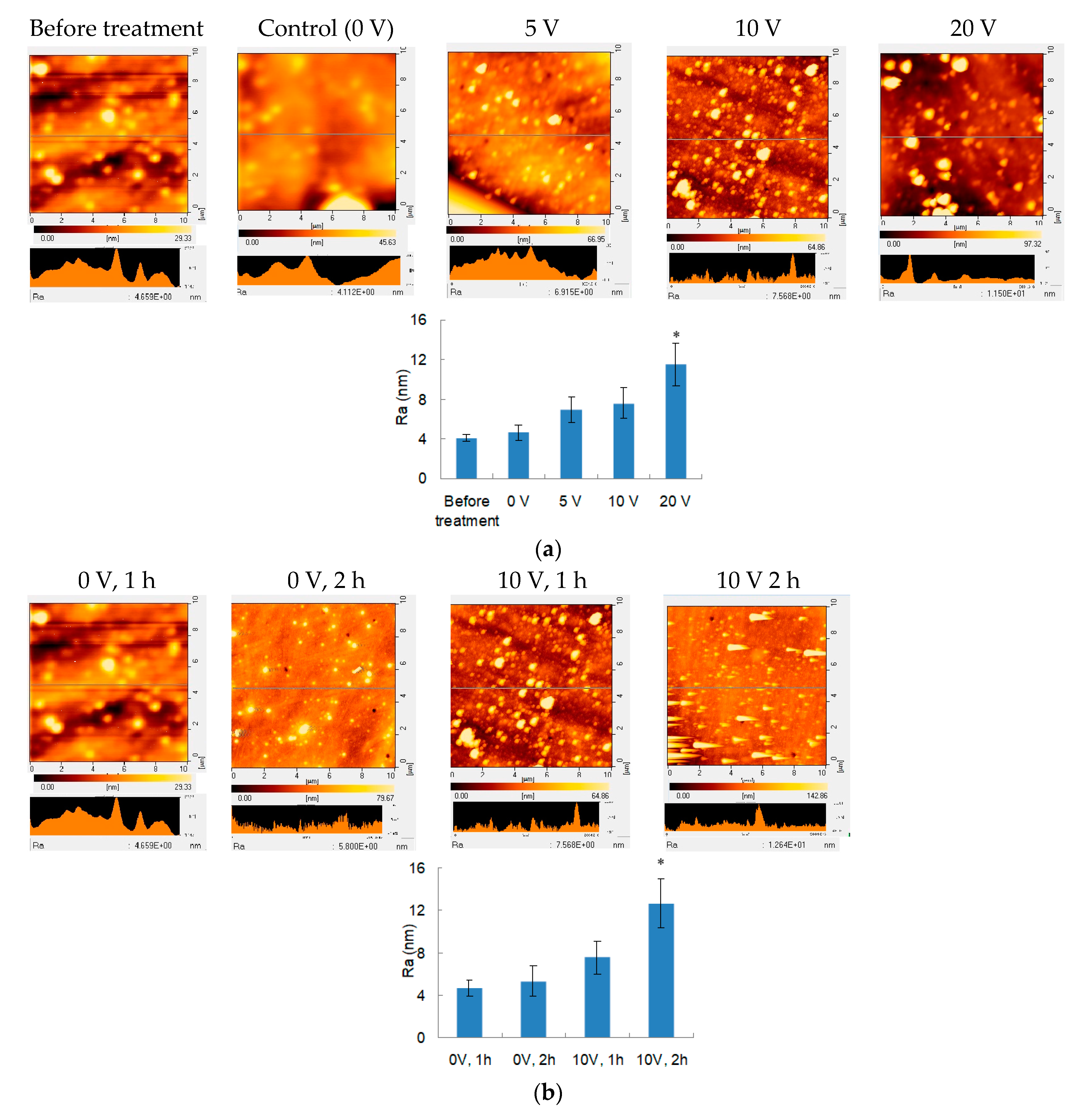
3.3. Surface Morphology of Electrical Field Treated PEMs
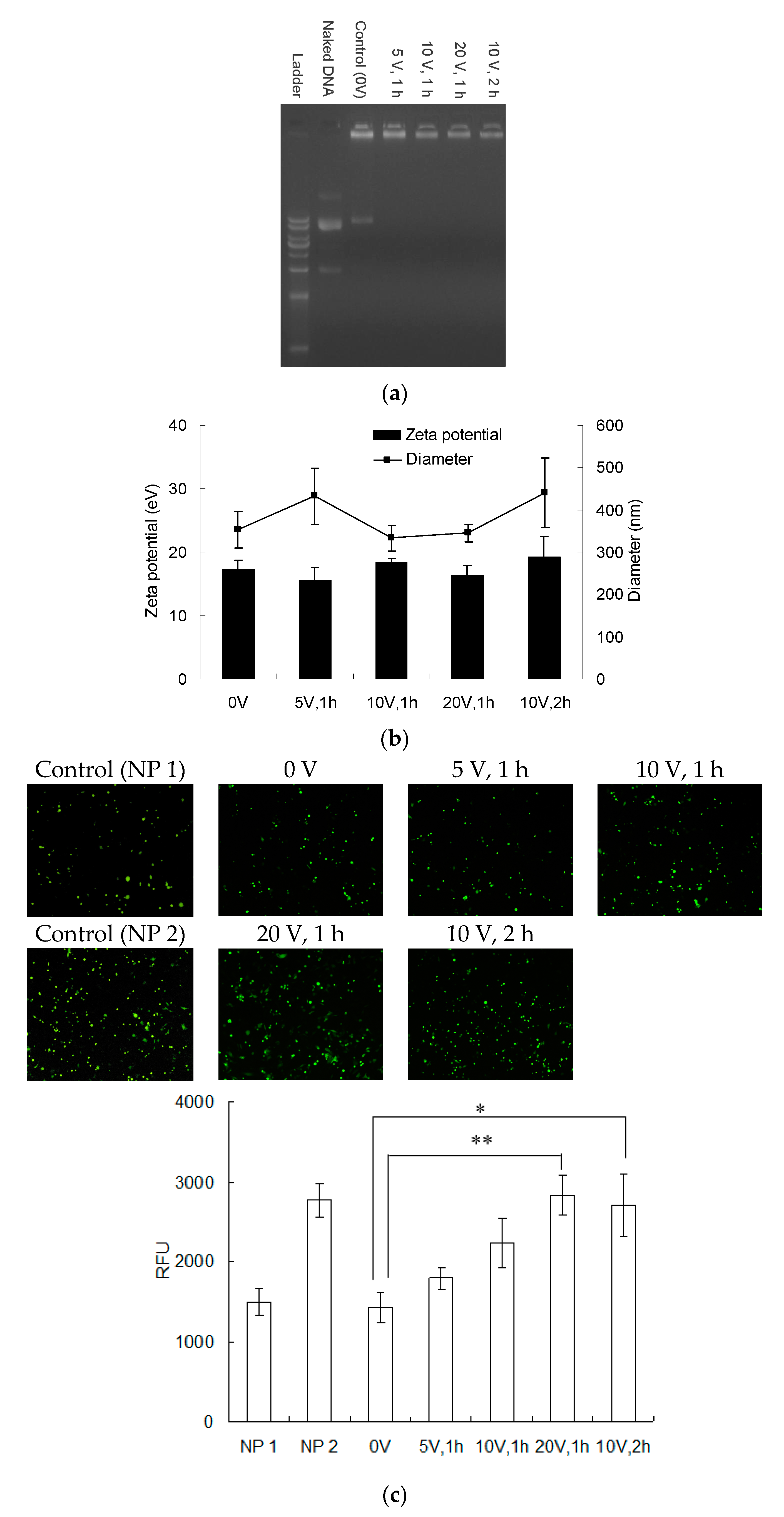
3.4. The Release of Polyelectrolyte from PEMs for Transfection Application
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Xu, X.Y.; Chen, Y.F.; Tan, Q.G.; Chen, Z.J.; Li, Y.; Wu, W.G.; Wang, X.F.; Liu, Y.B. Construction of multilayer films with bactericidal and long-term antitumor drug release properties as a non-vascular stent coating for therapy in obstruction. J. Mater. Chem. B 2019, 7, 4963–4972. [Google Scholar] [CrossRef]
- Chang, H.; Ren, K.F.; Zhang, H.; Wang, J.L.; Wang, B.L.; Ji, J. The (prs/hgf-pdna) multilayer films for gene-eluting stent coating: Gene-protecting, anticoagulation, antibacterial properties, and in vivo antirestenosis evaluation. J. Biomed. Mater. Res. Part B 2015, 103, 430–439. [Google Scholar] [CrossRef]
- Manju, S.; Kunnatheeri, S. Layer-by-layer modification of poly (methyl methacrylate) intra ocular lens: Drug delivery applications. Pharm. Dev. Technol. 2010, 15, 379–385. [Google Scholar] [CrossRef]
- de Avila, E.D.; Castro, A.G.B.; Tagit, O.; Krom, B.P.; Lowik, D.; van Well, A.A.; Bannenberg, L.J.; Vergani, C.E.; van den Beucken, J. Anti-bacterial efficacy via drug-delivery system from layer-by-layer coating for percutaneous dental implant components. Appl. Surf. Sci. 2019, 488, 194–204. [Google Scholar] [CrossRef]
- Gronowicz, G.; Jacobs, E.; Peng, T.; Zhu, L.; Hurley, M.; Kuhn, L.T. Calvarial bone regeneration is enhanced by sequential delivery of fgf-2 and bmp-2 from layer-by-layer coatings with a biomimetic calcium phosphate barrier layer. Tissue Eng. Part A 2017, 23, 1490–1501. [Google Scholar] [CrossRef]
- Hao, W.P.; Han, J.; Chu, Y.; Huang, L.; Zhuang, Y.; Sun, J.; Li, X.R.; Zhao, Y.N.; Chen, Y.Y.; Dai, J.W. Collagen/heparin bi-affinity multilayer modified collagen scaffolds for controlled bfgf release to improve angiogenesis in vivo. Macromol. Biosci. 2018, 18, 1800086. [Google Scholar] [CrossRef]
- Herron, M.; Schurr, M.J.; Murphy, C.J.; McAnulty, J.F.; Czuprynski, C.J.; Abbott, N.L. Interfacial stacks of polymeric nanofilms on soft biological surfaces that release multiple agents. ACS Appl. Mater. Interfaces 2016, 8, 26541–26551. [Google Scholar] [CrossRef]
- Agarwal, A.; Nelson, T.B.; Kierski, P.R.; Schurr, M.J.; Murphy, C.J.; Czuprynski, C.J.; McAnulty, J.F.; Abbott, N.L. Polymeric multilayers that localize the release of chlorhexidine from biologic wound dressings. Biomaterials 2012, 33, 6783–6792. [Google Scholar] [CrossRef]
- Palama, I.E.; Arcadio, V.; D’Amone, S.; Biasiucci, M.; Gigli, G.; Cortese, B. Therapeutic pcl scaffold for reparation of resected osteosarcoma defect. Sci. Rep. 2017, 7, 12672. [Google Scholar] [CrossRef]
- Anouz, R.; Repanas, A.; Schwarz, E.; Groth, T. Novel surface coatings using oxidized glycosaminoglycans as delivery systems of bone morphogenetic protein 2 (bmp-2) for bone regeneration. Macromol. Biosci. 2018, 18, e1800283. [Google Scholar] [CrossRef]
- Hu, W.W.; Chen, Y.J.; Ruaan, R.C.; Chen, W.Y.; Cheng, Y.C.; Chien, C.C. The regulation of DNA adsorption and release through chitosan multilayers. Carbohydr. Polym. 2014, 99, 394–402. [Google Scholar] [CrossRef]
- Koenig, O.; Neumann, B.; Schlensak, C.; Wendel, H.P.; Nolte, A. Hyaluronic acid/poly(ethylenimine) polyelectrolyte multilayer coatings for sirna-mediated local gene silencing. PLoS ONE 2019, 14, e0212584. [Google Scholar] [CrossRef]
- Hu, W.W.; Tsou, S.L. The effect of alginate on DNA delivery from layer-by-layer assembled films. Carbohydr. Polym. 2014, 101, 240–248. [Google Scholar] [CrossRef]
- Peng, N.; Yu, H.; Wang, Z.Y.; Zhang, Y.; Deng, K.; Li, J.M.; Lu, L.L.; Zou, T.; Liu, Y.; Huang, S.W. Dendrimer-grafted bioreducible polycation/DNA multilayered films with low cytotoxicity and high transfection ability. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 98, 737–745. [Google Scholar] [CrossRef]
- Xie, L.X.; Ding, X.; Budry, R.; Mao, G.Z. Layer-by-layer DNA films incorporating highly transfecting bioreducible poly(amido amine) and polyethylenimine for sequential gene delivery. Int. J. Nanomed. 2018, 13, 4943–4960. [Google Scholar] [CrossRef]
- Hu, W.W.; Chen, Y.J. In situ transfection using layer-by-layer assembled chitosan/DNA multilayers. Int. J. Nanotechnol. 2017, 14, 1031–1044. [Google Scholar] [CrossRef]
- Jewell, C.M.; Zhang, J.T.; Fredin, N.J.; Lynn, D.M. Multilayered polyelectrolyte films promote the direct and localized delivery of DNA to cells. J. Control. Release 2005, 106, 214–223. [Google Scholar] [CrossRef]
- Khan, Z.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Ndesendo, V.M.K.; Pillay, V. A novel multilayered multidisk oral tablet for chronotherapeutic drug delivery. Biomed Res. Int. 2013, 2013, 16. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, J.T.; Lynn, D.M. Polyelectrolyte multilayers fabricated from ′charge-shifting′ anionic polymers: A new approach to controlled film disruption and the release of cationic agents from surfaces. Soft Matter 2008, 4, 1688–1695. [Google Scholar] [CrossRef]
- Nolan, C.M.; Serpe, M.J.; Lyon, L.A. Thermally modulated insulin release from microgel thin films. Biomacromolecules 2004, 5, 1940–1946. [Google Scholar] [CrossRef]
- Aytar, B.S.; Prausnitz, M.R.; Lynn, D.M. Rapid release of plasmid DNA from surfaces coated with polyelectrolyte multilayers promoted by the application of electrochemical potentials. ACS Appl. Mater. Interfaces 2012, 4, 2726–2734. [Google Scholar] [CrossRef]
- Bleiziffer, O.; Eriksson, E.; Yao, F.; Horch, R.E.; Kneser, U. Gene transfer strategies in tissue engineering. J. Cell. Mol. Med. 2007, 11, 206–223. [Google Scholar] [CrossRef]
- Hu, W.W.; Zheng, Y.R. Electrophoretic deposition to promote layer-by-layer assembly for in situ gene delivery application. Colloids Surf. B Biointerfaces 2015, 133, 171–178. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Du, M.; Li, Y.; Chen, Y. Enhanced gene transfection efficiency by low-dose 25 kda polyethylenimine by the assistance of 1.8 kda polyethylenimine. Drug Deliv. 2018, 25, 1740–1745. [Google Scholar] [CrossRef]
- Hu, W.W.; Syu, W.J.; Chen, W.Y.; Ruaan, R.C.; Cheng, Y.C.; Chien, C.C.; Li, C.; Chung, C.A.; Tsao, C.W. Use of biotinylated chitosan for substrate-mediated gene delivery. Bioconjug. Chem. 2012, 23, 1587–1599. [Google Scholar] [CrossRef]
- Hu, W.W.; Yeh, C.C.; Tsai, C.W. The conjugation of indolicidin to polyethylenimine for enhanced gene delivery with reduced cytotoxicity. J. Mater. Chem. B 2018, 6, 5781–5794. [Google Scholar] [CrossRef]
- Kovacevic, D.; van der Burgh, S.; de Keizer, A.; Stuart, M.A.C. Kinetics of formation and dissolution of weak polyelectrolyte multilayers: Role of salt and free polyions. Langmuir 2002, 18, 5607–5612. [Google Scholar] [CrossRef]
- Chremos, A.; Douglas, J.F. Influence of branching on the configurational and dynamical properties of entangled polymer melts. Polymers 2019, 11, 1045. [Google Scholar] [CrossRef]
- Vazquez, E.; Dewitt, D.M.; Hammond, P.T.; Lynn, D.M. Construction of hydrolytically-degradable thin films via layer-by-layer deposition of degradable polyelectrolytes. J. Am. Chem. Soc. 2002, 124, 13992–13993. [Google Scholar] [CrossRef]
- Boulmedais, F.; Tang, C.S.; Keller, B.; Voros, J. Controlled electrodissolution of polyelectrolyte multilayers: A platform technology towards the surface-initiated delivery of drugs. Adv. Funct. Mater. 2006, 16, 63–70. [Google Scholar] [CrossRef]
- Dieguez, L.; Darwish, N.; Graf, N.; Voros, J.; Zambelli, T. Electrochemical tuning of the stability of pll/DNA multilayers. Soft Matter 2009, 5, 2415–2421. [Google Scholar] [CrossRef]
- Li, C.; Hsu, Y.T.; Hu, W.W. The regulation of osteogenesis using electroactive polypyrrole films. Polymers 2016, 8, 258. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-C.; Guo, S.-L.; Chung, K.-D.; Hu, W.-W. Electrical Field-Assisted Gene Delivery from Polyelectrolyte Multilayers. Polymers 2020, 12, 133. https://doi.org/10.3390/polym12010133
Cheng Y-C, Guo S-L, Chung K-D, Hu W-W. Electrical Field-Assisted Gene Delivery from Polyelectrolyte Multilayers. Polymers. 2020; 12(1):133. https://doi.org/10.3390/polym12010133
Chicago/Turabian StyleCheng, Yu-Che, Shu-Lin Guo, Kun-Da Chung, and Wei-Wen Hu. 2020. "Electrical Field-Assisted Gene Delivery from Polyelectrolyte Multilayers" Polymers 12, no. 1: 133. https://doi.org/10.3390/polym12010133
APA StyleCheng, Y.-C., Guo, S.-L., Chung, K.-D., & Hu, W.-W. (2020). Electrical Field-Assisted Gene Delivery from Polyelectrolyte Multilayers. Polymers, 12(1), 133. https://doi.org/10.3390/polym12010133






