A Green, Rapid and Efficient Dual-Sensors for Highly Selective and Sensitive Detection of Cation (Hg2+) and Anion (S2−) Ions Based on CMS/AgNPs Composites
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Apparatus
2.2. Synthesis of CMS/AgNPs Colloidal Solution
2.3. Characterization of CMS/AgNPs Colloidal Solution
2.4. Colorimetric Detection of Hg2+ and S2−
3. Results and Discussion
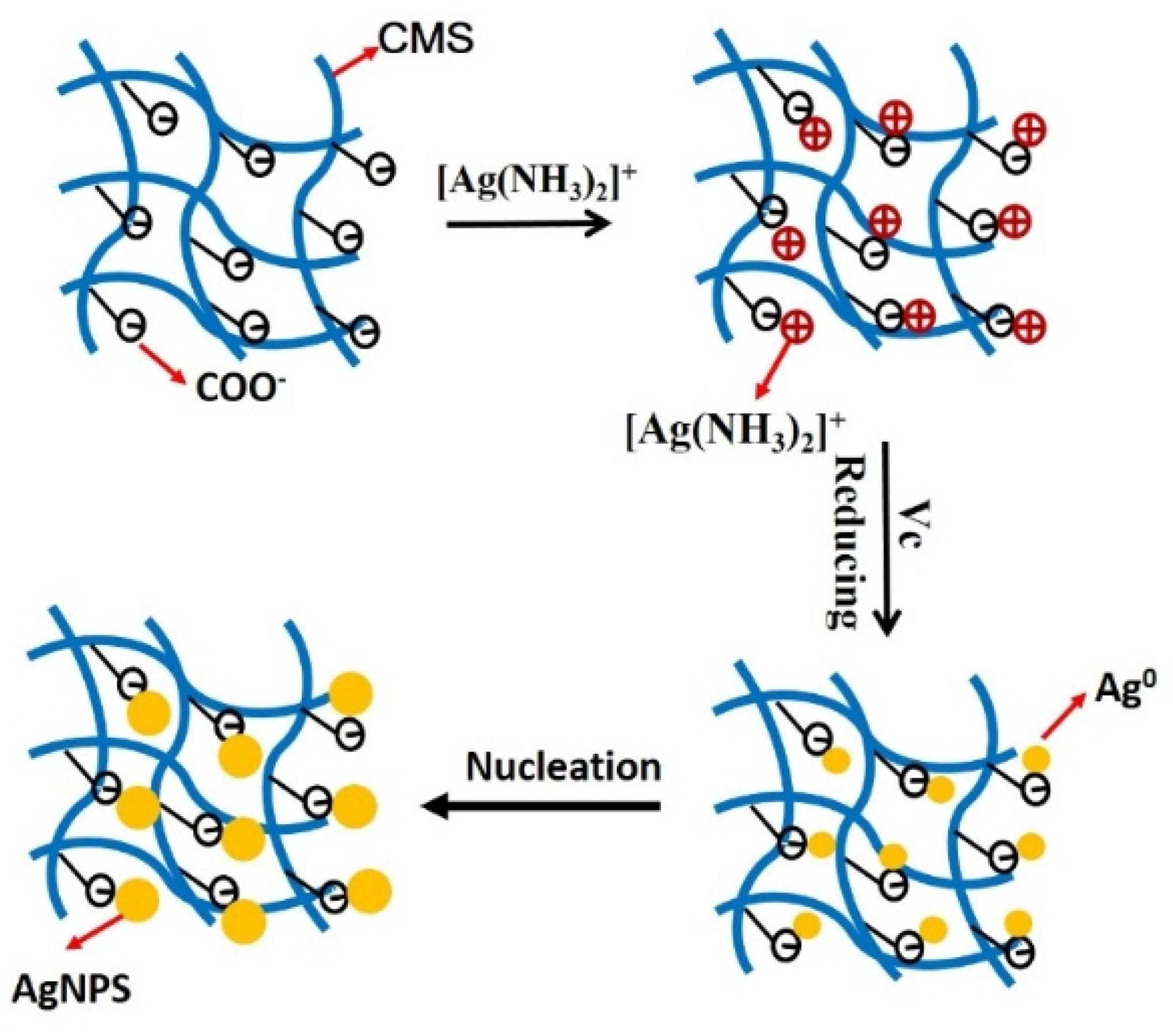
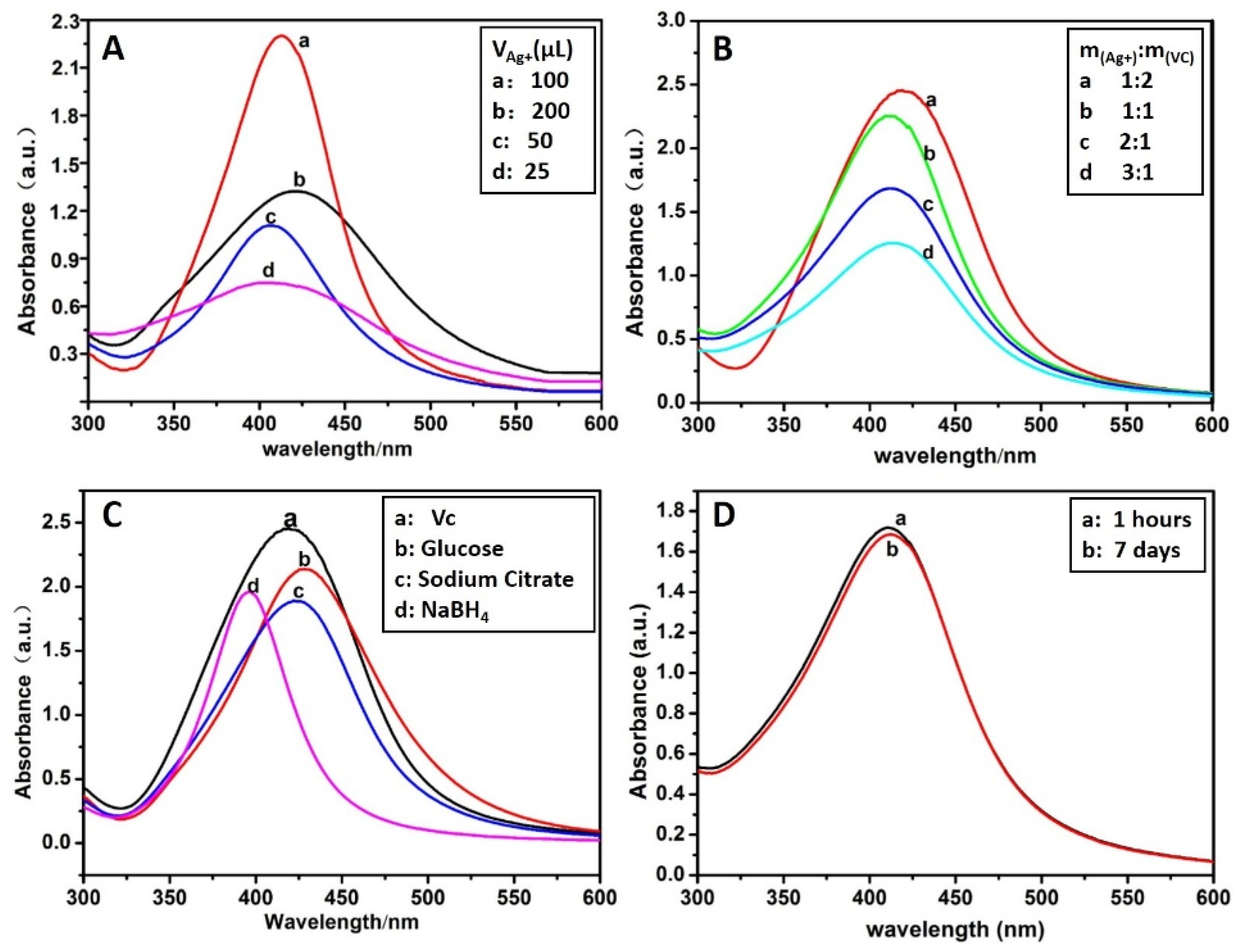
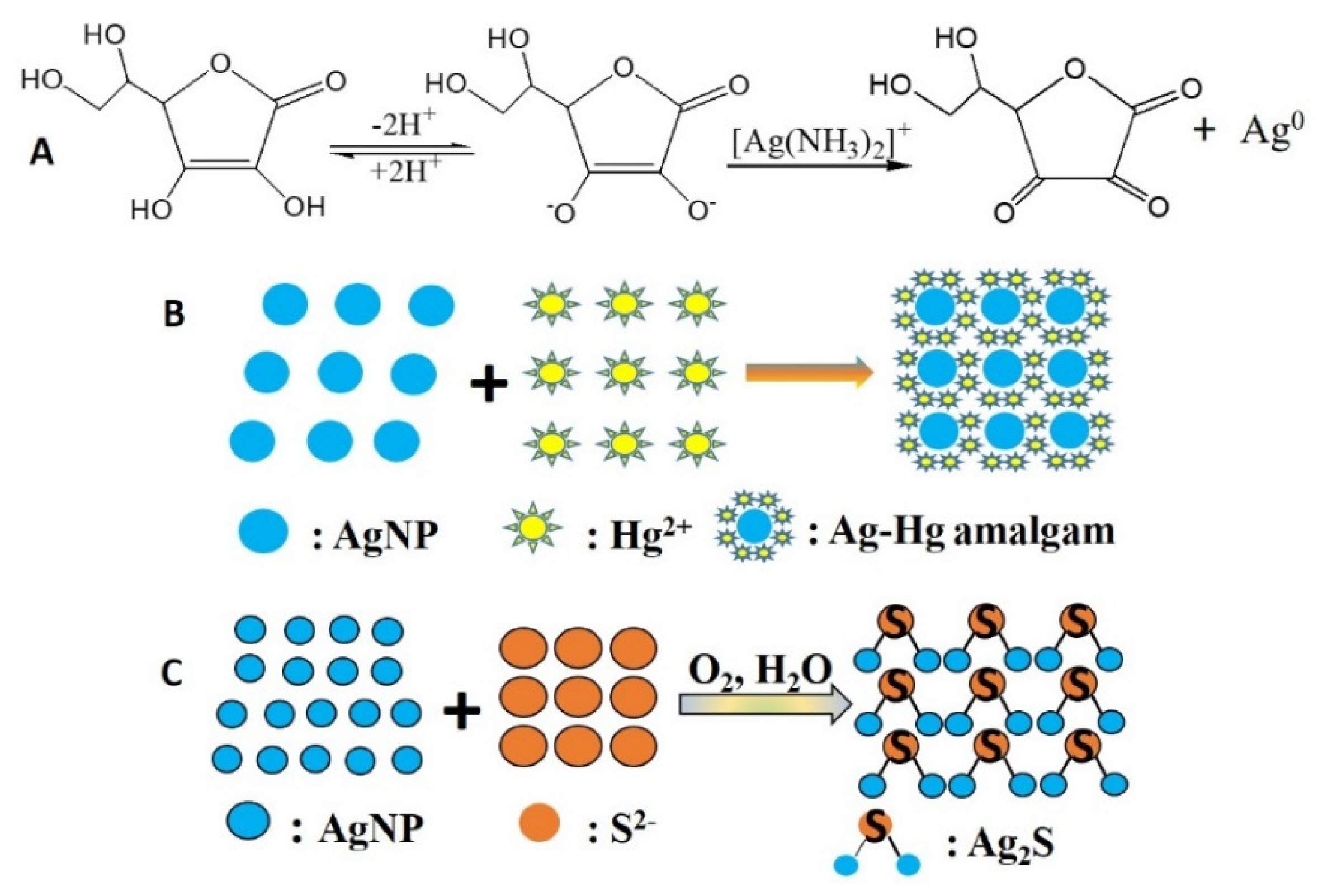
3.1. Preparation of CMS/AgNPs Colloidal Solution
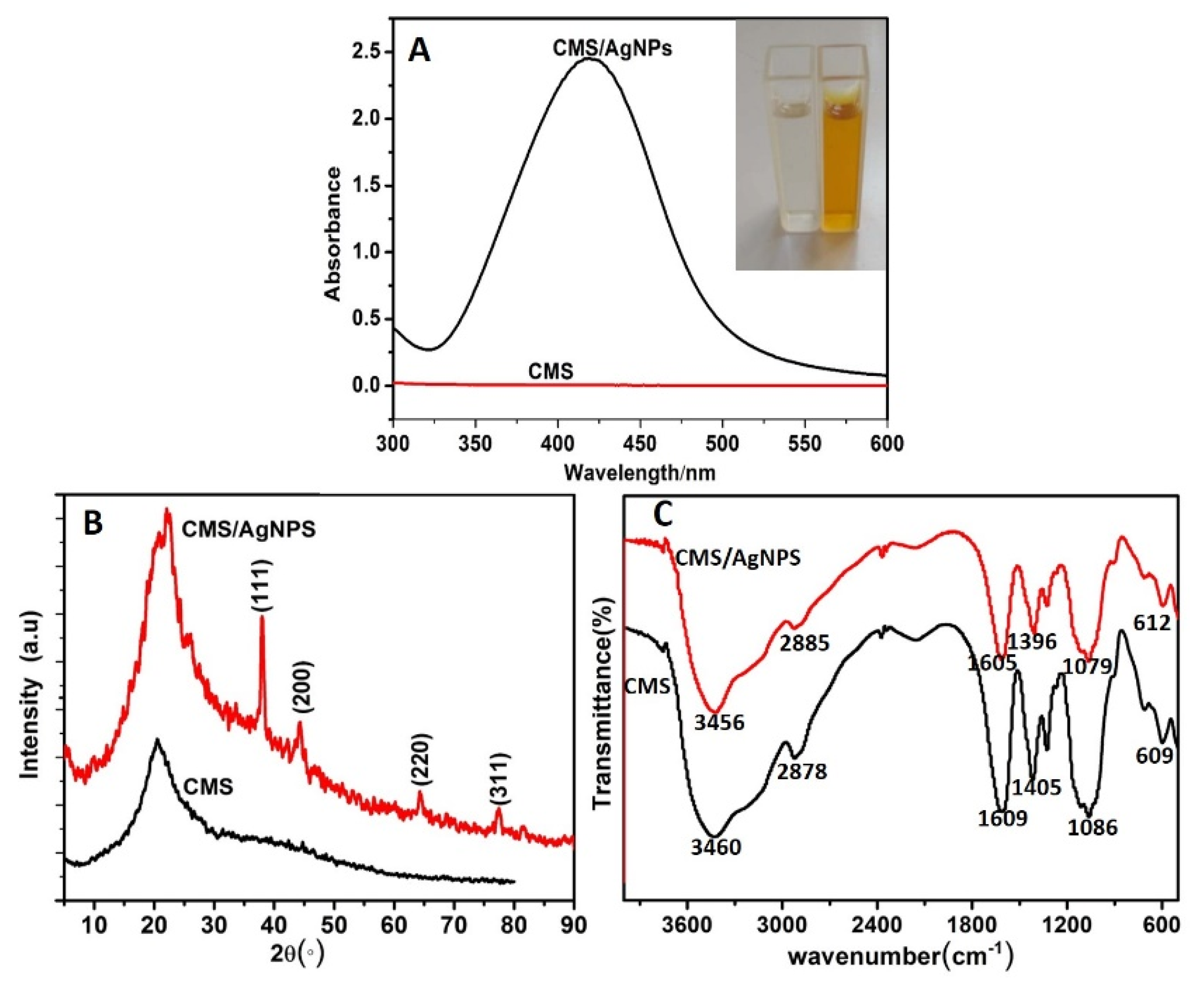
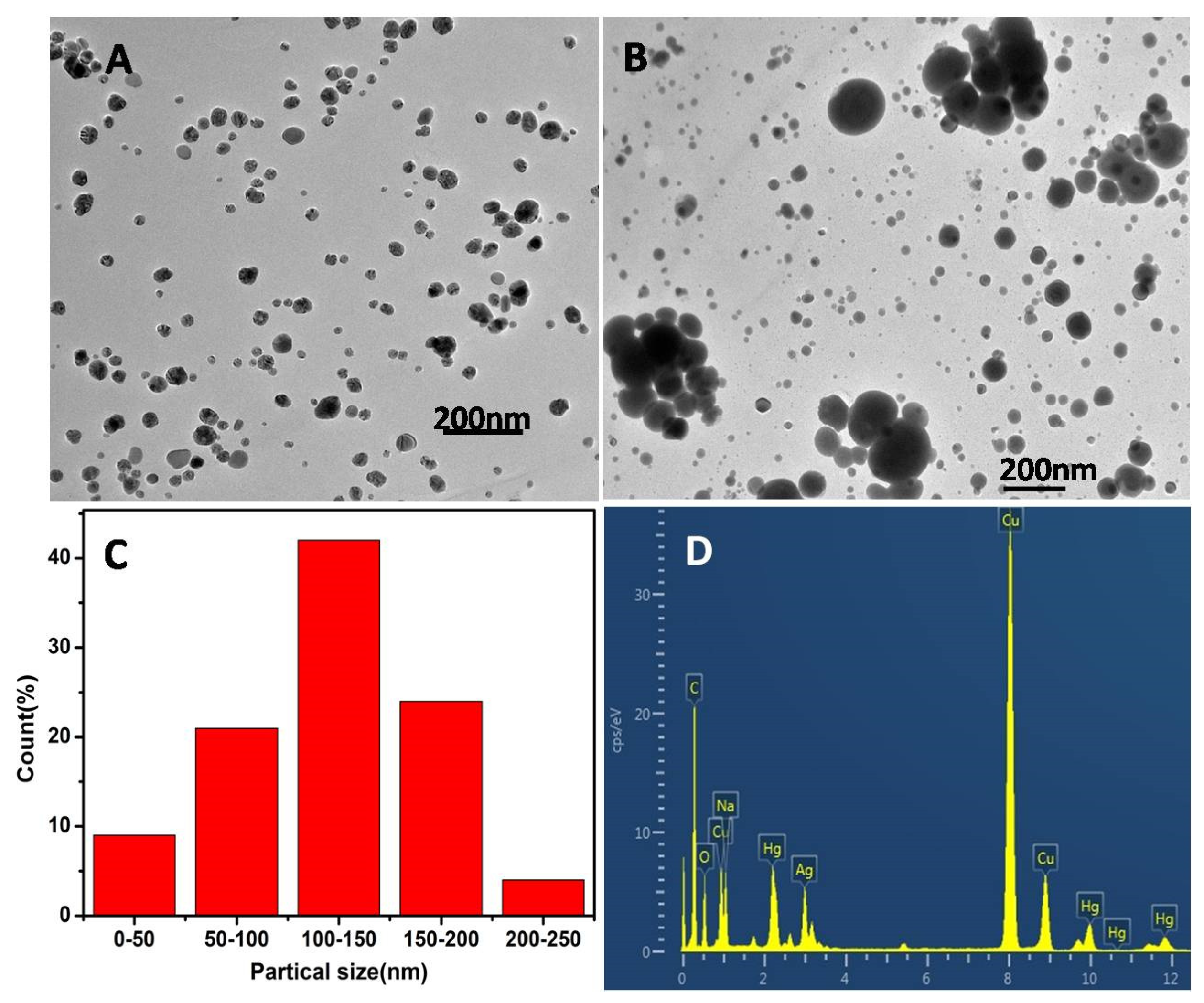
3.2. Characterization Studies of AgNPs
3.3. Mechanism for CMS/AgNPs Composites and the Detection of Hg2+ and S2−
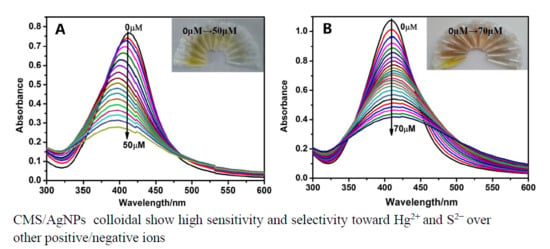
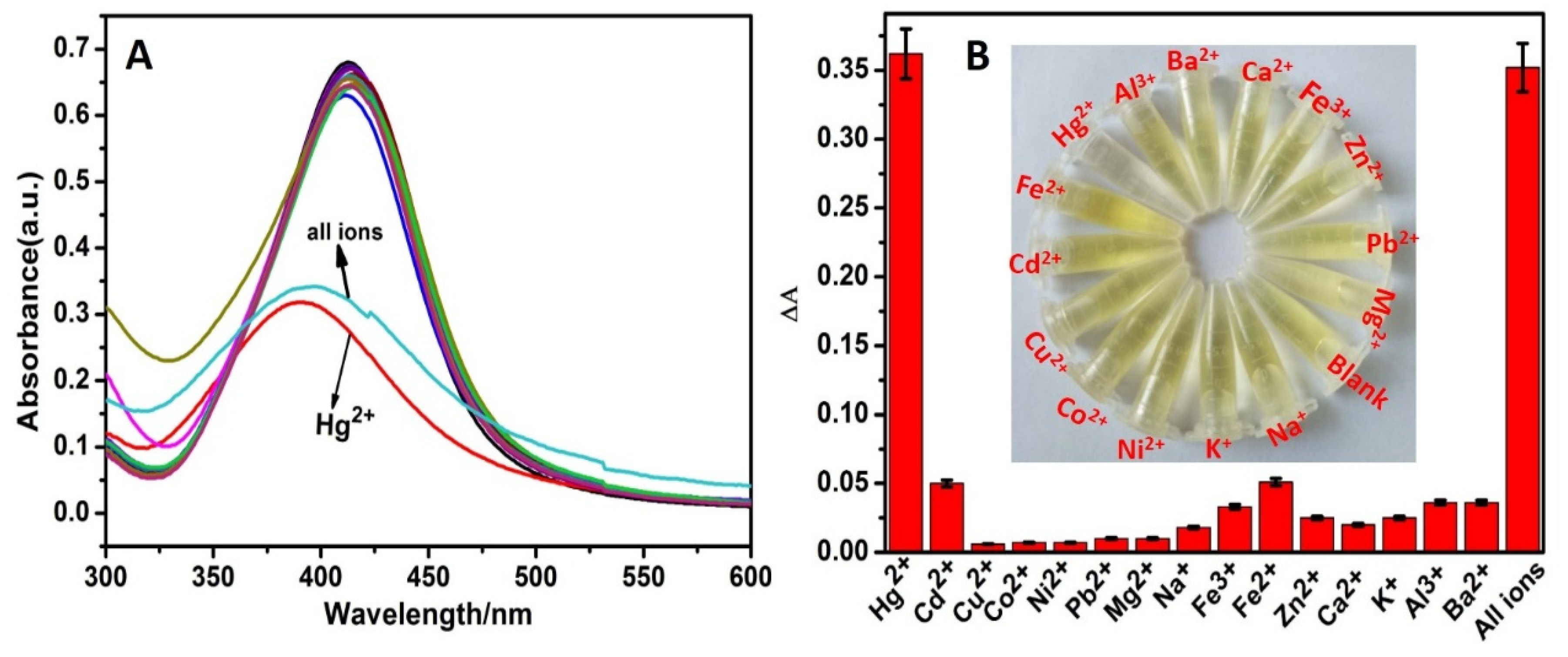
3.4. Detection Selectivity and Sensitivity of Hg2+
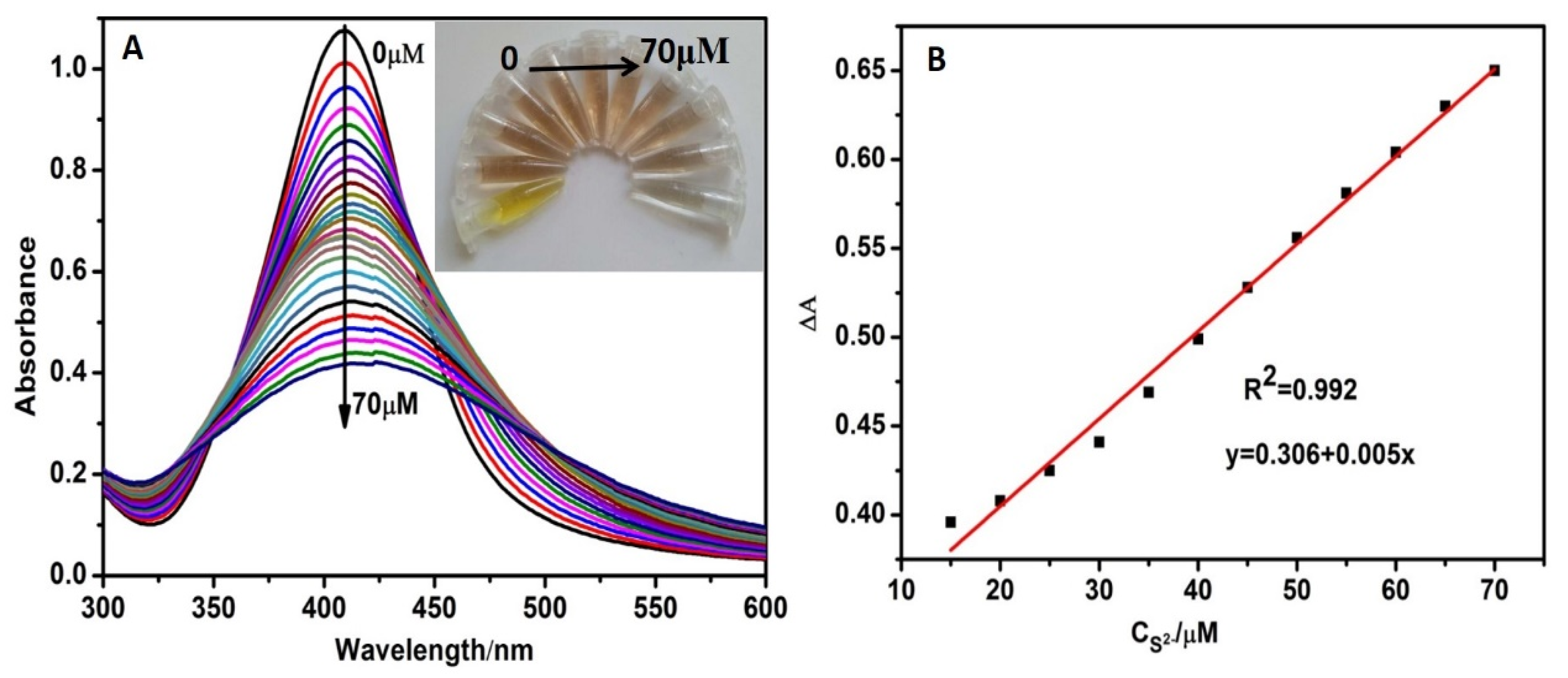
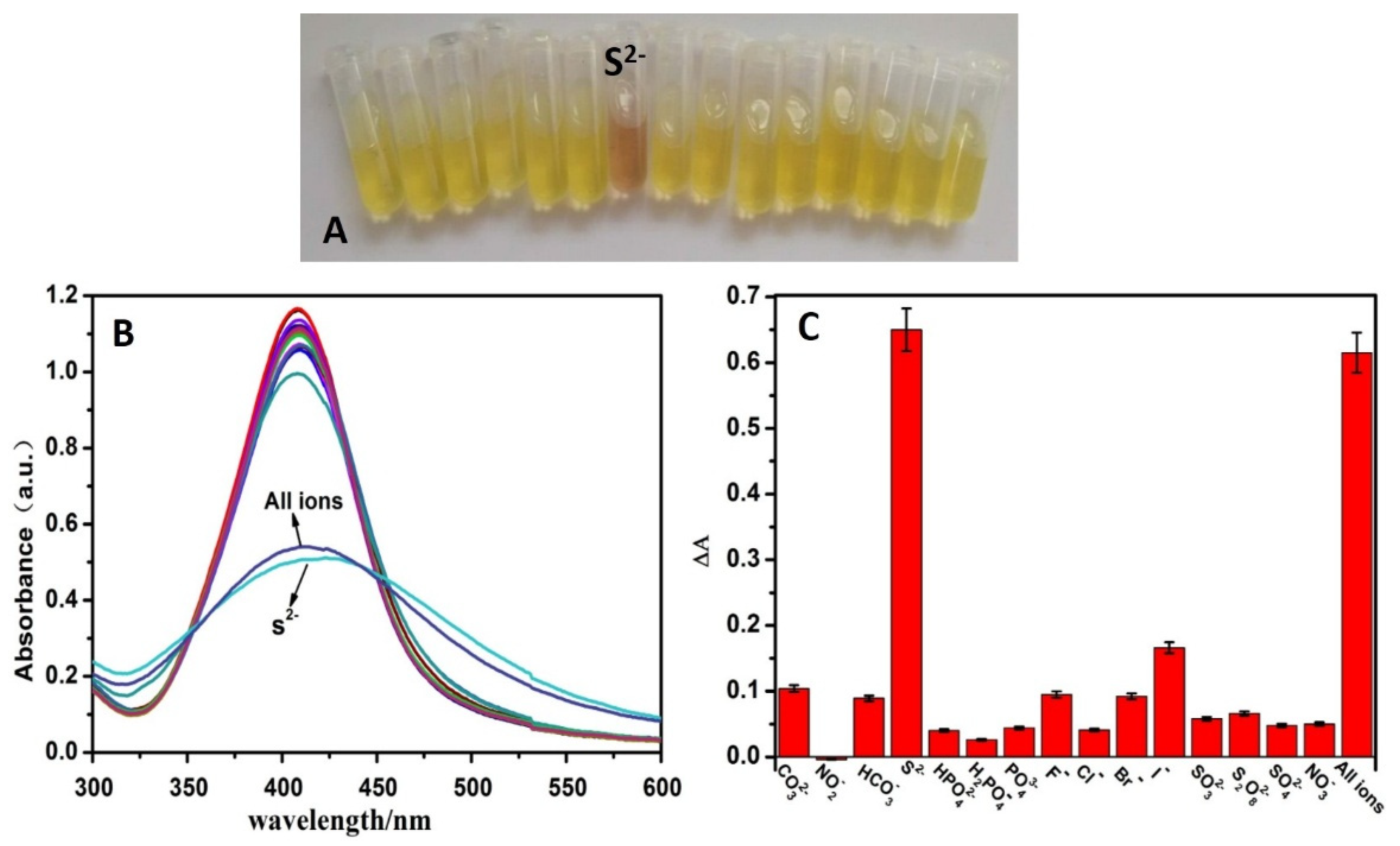
3.5. Sensitivity and Selectivity for S2− Detection
3.6. Detection of Hg2+ and S2− in Tap Water and Lake Water
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, S.; Wu, D. Adapting ecological risk valuation for natural resource damage assessment in water pollution. Environ. Res. 2018, 164, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kahn, M.E.; Liu, Y.; Wang, Z. The consequences of spatially differentiated water pollution regulation in China. J. Environ. Econ. Manag. 2018, 88, 468–485. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Z. Industrial water pollution, water environment treatment, and health risks in China. Environ. Pollut. 2016, 218, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Guo, X.; Lv, C.; Wang, H.; Di, D. Study on the quantification method of water pollution ecological compensation standard based on emergy theory. Ecol. Indic. 2018, 92, 189–194. [Google Scholar] [CrossRef]
- Xun, Y.; Feng, L.; Li, Y.; Dong, H. Mercury accumulation plant Cyrtomium macrophyllum and its potential for phytoremediation of mercury polluted sites. Chemosphere 2017, 189, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Zhang, W.; Sun, G.; Feng, Z.; Hurley, J.P.; Yang, L.; Shang, L.; Feng, X. Mercury risk in poultry in the Wanshan Mercury Mine, China. Environ. Pollut. 2017, 230, 810–816. [Google Scholar] [CrossRef]
- Wells, E.M.; Herbstman, J.B.; Lin, Y.H.; Hibbeln, J.R.; Halden, R.U.; Witter, F.R.; Goldman, L.R. Methyl mercury, but not inorganic mercury, associated with higher blood pressure during pregnancy. Environ. Res. 2017, 154, 247–252. [Google Scholar] [CrossRef]
- Aaseth, J.; Hilt, B.; Bjorklund, G. Mercury exposure and health impacts in dental personnel. Environ. Res. 2018, 164, 65–69. [Google Scholar] [CrossRef]
- Hancock, J.T. Hydrogen sulfide and environmental stresses. Environ. Exp. Bot. 2019, 161, 50–56. [Google Scholar] [CrossRef]
- Godoi, A.F.L.; Grasel, A.M.; Polezer, G.; Brown, A.; Potgieter-Vermaak, S.; Scremim, D.C.; Yamamoto, C.I.; Godoi, R.H.M. Human exposure to hydrogen sulphide concentrations near wastewater treatment plants. Sci. Total Environ. 2018, 610–611, 583–590. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. Gasotransmitter hydrogen sulfide signaling in neuronal health and disease. Biochem. Pharm. 2018, 149, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.L.F.; Da Costa, M.A.P.; Silva, L.O.B.; Dos Santos, W.N.L. Simultaneous determination of mercury and selenium in fish by CVG AFS. Food Chem. 2019, 273, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Karlitepe, F.; Ocalan, T.; Tunalioglu, N. Performance analysis of Real Time PPP for transit of Mercury. Measurement 2018, 129, 358–367. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Yuan, C.; Chen, Y. Speciation analysis of silver sulfide nanoparticles in environmental waters by magnetic solid-phase extraction coupled with ICP-MS. J. Anal. At. Spectrom. 2016, 31, 2285–2292. [Google Scholar] [CrossRef]
- Gan, T.; Lv, Z.; Sun, J.; Shi, Z.; Liu, Y. Preparation of graphene oxide-wrapped carbon sphere@silver spheres for high performance chlorinated phenols sensor. J. Hazard. Mater. 2016, 302, 188–197. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Park, W.H. Green synthesis of silver nanoparticles stabilized with mussel-inspired protein and colorimetric sensing of lead(II) and copper(II) ions. Int. J. Mol. Sci. 2016, 17, 2006. [Google Scholar] [CrossRef]
- Tachapermpon, Y.; Chaneam, S.; Charoenpanich, A.; Sirirak, J.; Wanichacheva, N. Highly Cu2+-sensitive and selective colorimetric and fluorescent probes: Utilizations in batch, flow analysis and living cell imaging. Sens. Actuators B Chem. 2017, 241, 868–878. [Google Scholar] [CrossRef]
- Chavada, V.D.; Bhatt, N.M.; Sanyal, M.; Shrivastav, P.S. Pyrophosphate functionalized silver nanoparticles for colorimetric determination of deferiprone via competitive binding to Fe(III). Microchim. Acta 2017, 184, 4203–4208. [Google Scholar] [CrossRef]
- Ly, N.H.; Joo, S.W. Silver nanoparticle-enhanced resonance raman sensor of chromium(III) in seawater samples. Sensors 2015, 15, 10088–10099. [Google Scholar] [CrossRef]
- Liu, T.; Dong, J.X.; Liu, S.G.; Li, N.; Lin, S.M.; Fan, Y.Z.; Lei, J.L.; Luo, H.Q.; Li, N.B. Carbon quantum dots prepared with polyethyleneimine as both reducing agent and stabilizer for synthesis of Ag/CQDs composite for Hg2+ ions detection. J. Hazard. Mater. 2017, 322 Pt B, 430–436. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, L.; Zeng, L.; Shen, A.; Hu, J. A label-free SERS probe for highly sensitive detection of Hg2+ based on functionalized Au@Ag nanoparticles. Talanta 2017, 162, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.B.A.; Mariappan, M.; Veerappan, A. Nanosilver cotton swabs for highly sensitive and selective colorimetric detection of sulfide ions at nanomolar level. Sens. Actuators B Chem. 2017, 244, 831–836. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, L.; Miao, Y.; Liu, C.; Zhang, C. A colorimetric sensor for the highly selective detection of sulfide and 1,4-dithiothreitol based on the in situ formation of silver nanoparticles using dopamine. Sensors 2017, 17, 626. [Google Scholar] [CrossRef] [PubMed]
- Bothra, S.; Solanki, J.N.; Sahoo, S.K. Functionalized silver nanoparticles as chemosensor for pH, Hg2+ and Fe3+ in aqueous medium. Sens. Actuators B Chem. 2013, 188, 937–943. [Google Scholar] [CrossRef]
- Li, L.; Gui, L.; Li, W. A colorimetric silver nanoparticle-based assay for Hg(II) using lysine as a particle-linking reagent. Microchim. Acta 2015, 182, 1977–1981. [Google Scholar] [CrossRef]
- Shanmugaraj, K.; Ilanchelian, M. Colorimetric determination of sulfide using chitosan-capped silver nanoparticles. Microchim. Acta 2016, 183, 1721–1728. [Google Scholar] [CrossRef]
- Vasileva, P.; Alexandrova, T.; Karadjova, I. Application of starch-stabilized silver nanoparticles as a colorimetric sensor for mercury(II) in 0.005 mol/L nitric acid. J. Chem. 2017, 2017, 6897960. [Google Scholar] [CrossRef]
- Ma, P.; Liang, F.; Yang, Q.; Wang, D.; Sun, Y.; Wang, X.; Gao, D.; Song, D. Highly sensitive SERS probe for mercury(II) using cyclodextrin-protected silver nanoparticles functionalized with methimazole. Microchim. Acta 2014, 181, 975–981. [Google Scholar] [CrossRef]
- Deng, L.; Ouyang, X.; Jin, J.; Ma, C.; Jiang, Y.; Zheng, J.; Li, J.; Li, Y.; Tan, W.; Yang, R. Exploiting the higher specificity of silver amalgamation: Selective detection of mercury(II) by forming Ag/Hg amalgam. Anal. Chem. 2013, 85, 8594–8600. [Google Scholar] [CrossRef]



| Sample | Hg2+ Added | (μM) Found | Recovery (%) | S2− Added | (μM) Found | Recovery (%) |
|---|---|---|---|---|---|---|
| Tap water | 0 | 0 | - | 0 | 0 | - |
| 5.00 | 5.06 | 101.20 | 5.00 | 4.87 | 97.40 | |
| 10.00 | 9.86 | 98.60 | 10.00 | 10.34 | 103.40 | |
| Lake water | 0 | 0 | - | 0 | 0 | - |
| 5.00 | 4.78 | 95.60 | 5.00 | 5.19 | 103.80 | |
| 10.00 | 9.61 | 96.10 | 10.00 | 9.83 | 98.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Ma, L.; Zhang, L.; Zhao, W.; Li, Z.; Li, Q. A Green, Rapid and Efficient Dual-Sensors for Highly Selective and Sensitive Detection of Cation (Hg2+) and Anion (S2−) Ions Based on CMS/AgNPs Composites. Polymers 2020, 12, 113. https://doi.org/10.3390/polym12010113
Xue Y, Ma L, Zhang L, Zhao W, Li Z, Li Q. A Green, Rapid and Efficient Dual-Sensors for Highly Selective and Sensitive Detection of Cation (Hg2+) and Anion (S2−) Ions Based on CMS/AgNPs Composites. Polymers. 2020; 12(1):113. https://doi.org/10.3390/polym12010113
Chicago/Turabian StyleXue, Yun, Lina Ma, Lei Zhang, Wanting Zhao, Zichao Li, and Qun Li. 2020. "A Green, Rapid and Efficient Dual-Sensors for Highly Selective and Sensitive Detection of Cation (Hg2+) and Anion (S2−) Ions Based on CMS/AgNPs Composites" Polymers 12, no. 1: 113. https://doi.org/10.3390/polym12010113
APA StyleXue, Y., Ma, L., Zhang, L., Zhao, W., Li, Z., & Li, Q. (2020). A Green, Rapid and Efficient Dual-Sensors for Highly Selective and Sensitive Detection of Cation (Hg2+) and Anion (S2−) Ions Based on CMS/AgNPs Composites. Polymers, 12(1), 113. https://doi.org/10.3390/polym12010113