Novel Phosphorus-Nitrogen-Containing Ionic Liquid Modified Metal-Organic Framework as an Effective Flame Retardant for Epoxy Resin
Abstract
1. Introduction
2. Experimental
2.1. Materials
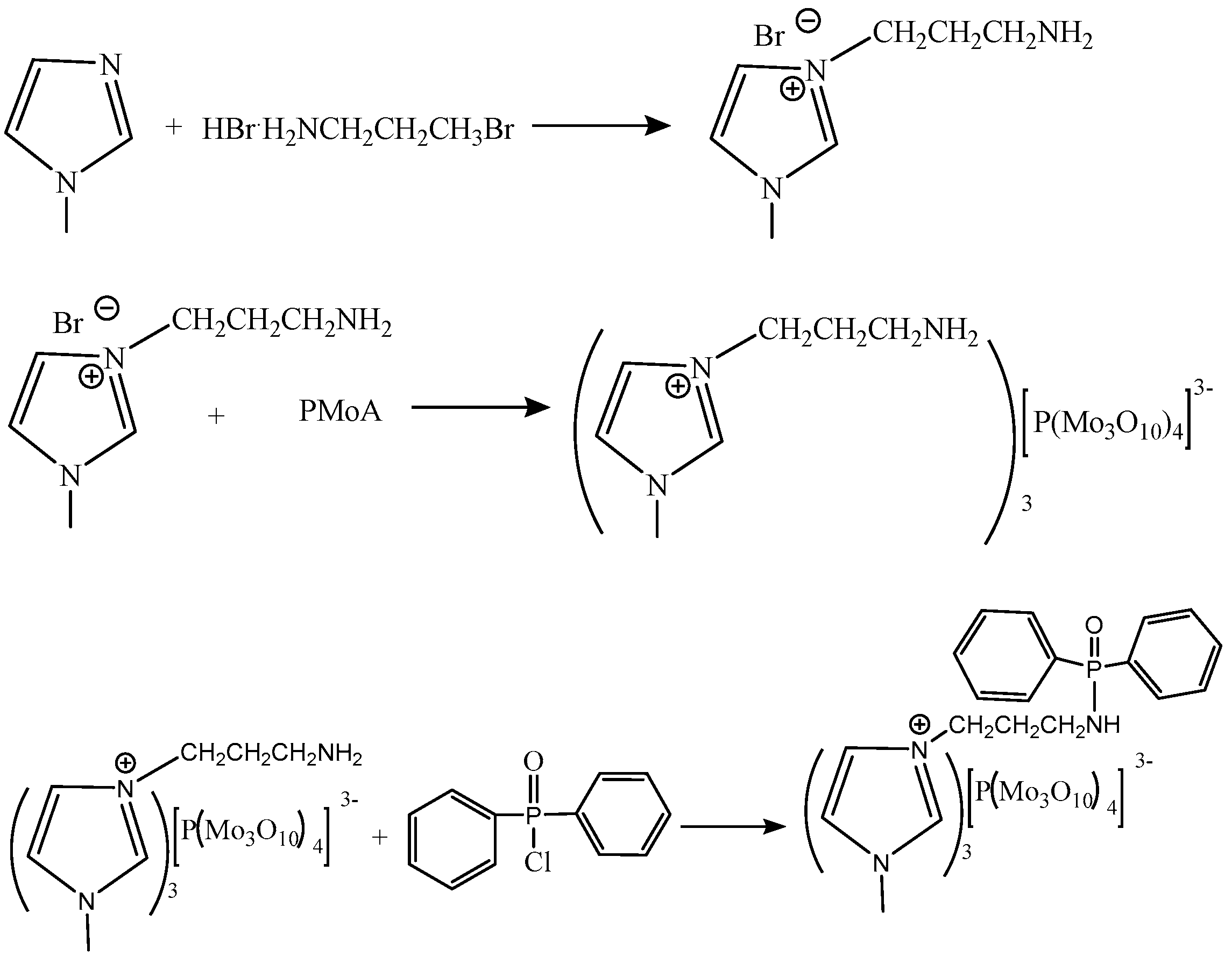
2.2. Synthesis of [NH2C3bim][PMo]
2.3. Synthesis of [DPP-NC3bim][PMo]
2.4. Synthesis of NH2-MIL-101(Al)
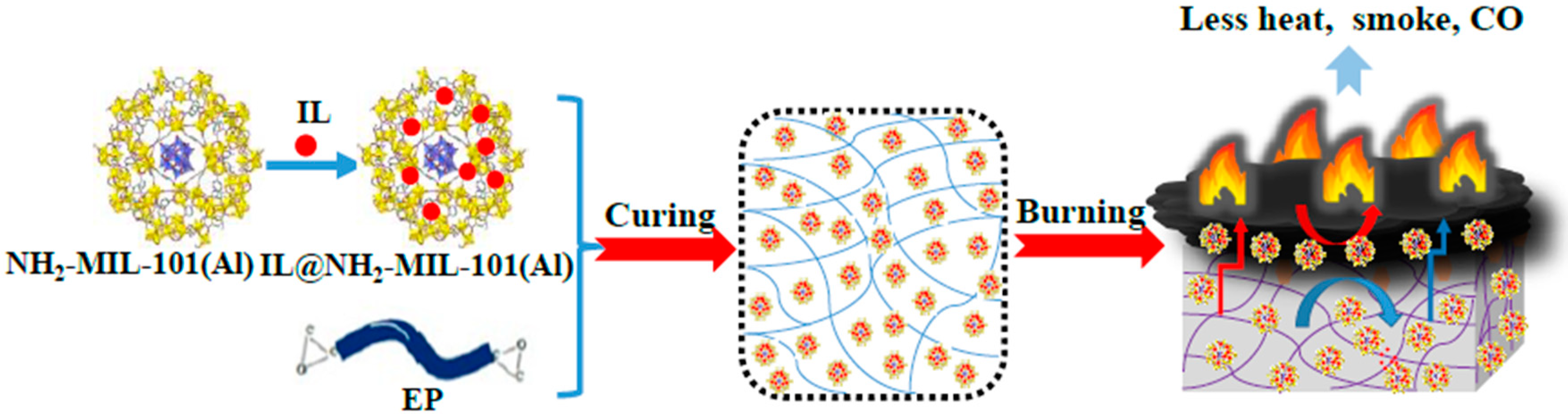
2.5. Synthesis of [DPP-NC3bim][PMo]@NH2-MIL-101(Al)
2.6. Synthesis of EP Composites
2.7. Characterizations
3. Results and Discussion
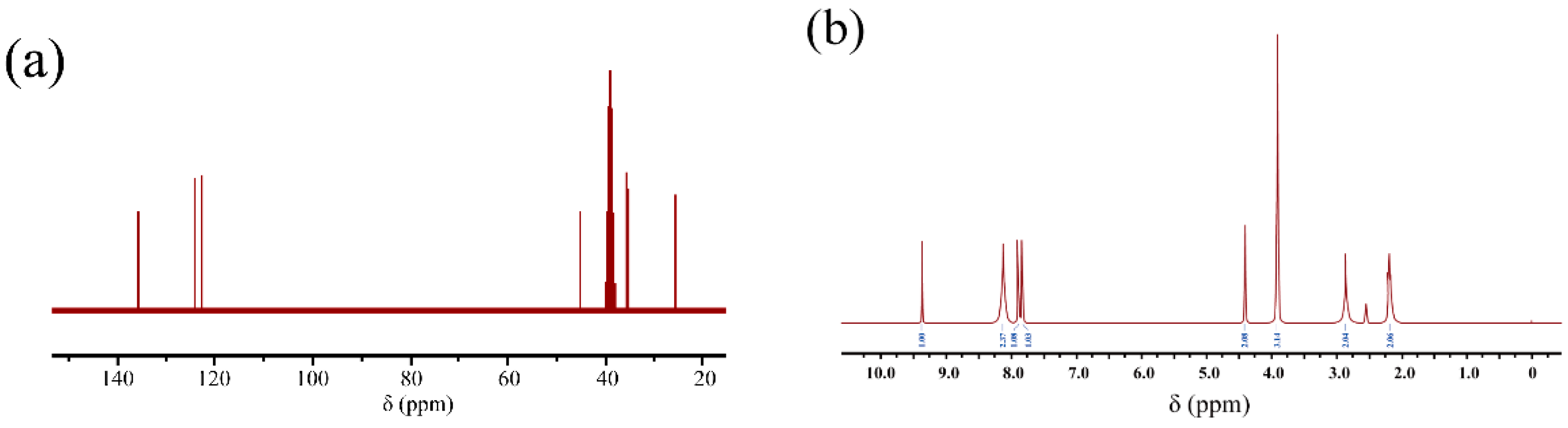
3.1. Characterization of the Materials
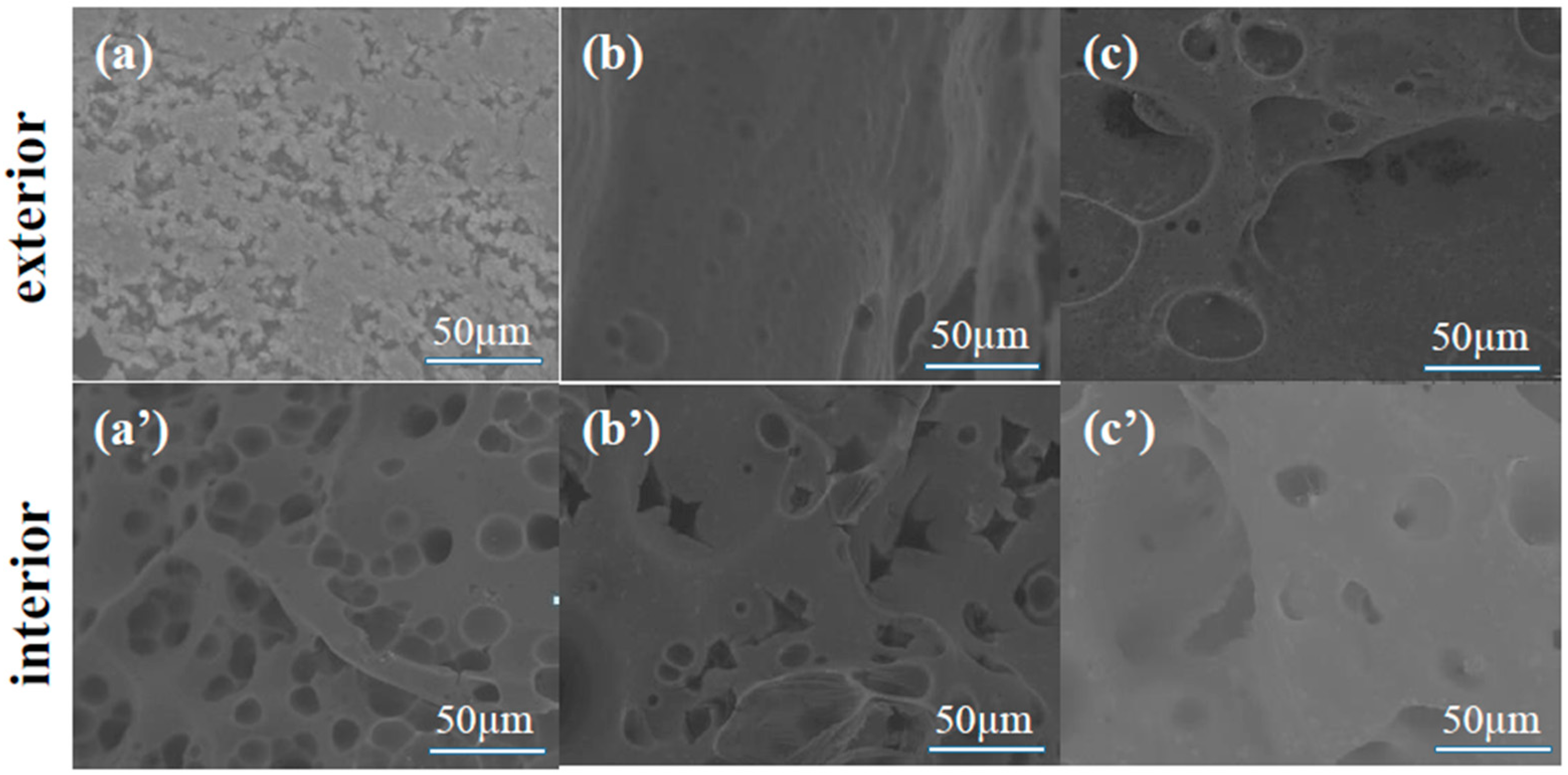
3.2. Morphological Analysis of EP and EP Composites
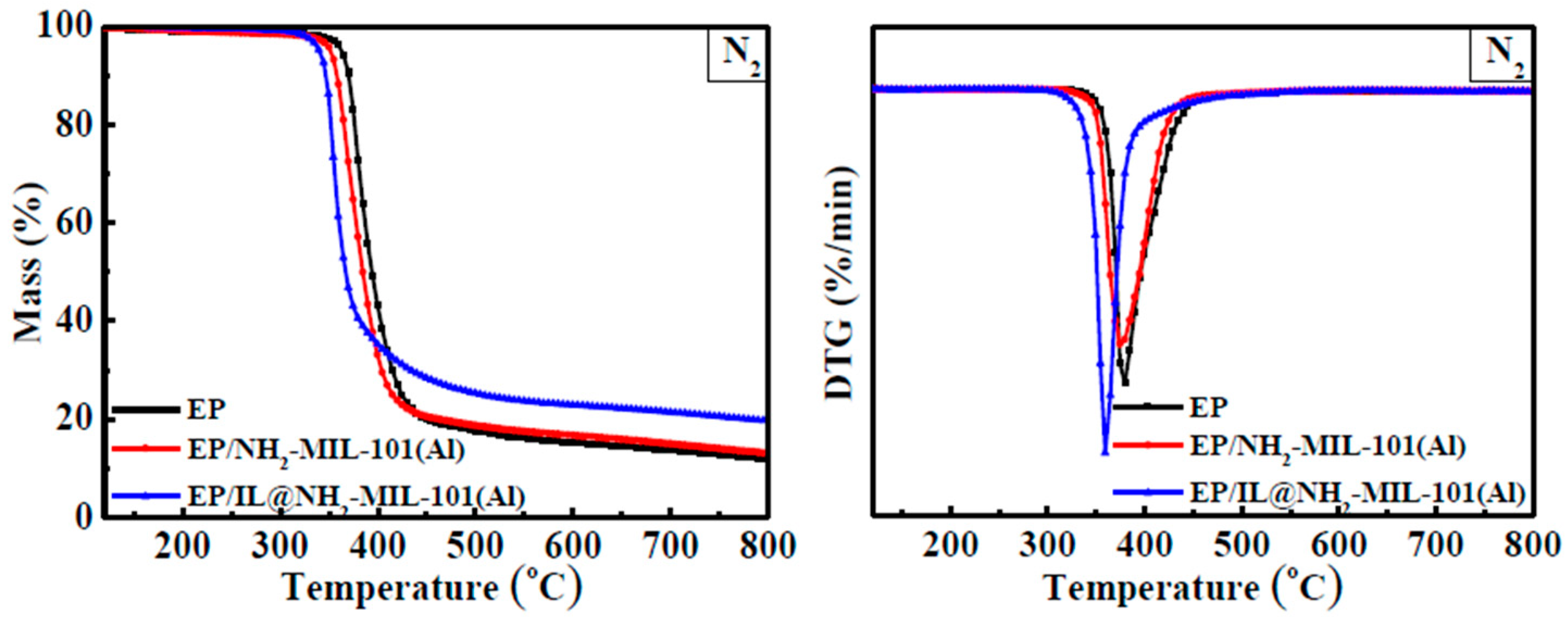
3.3. Thermal Stability of EP and EP Composites
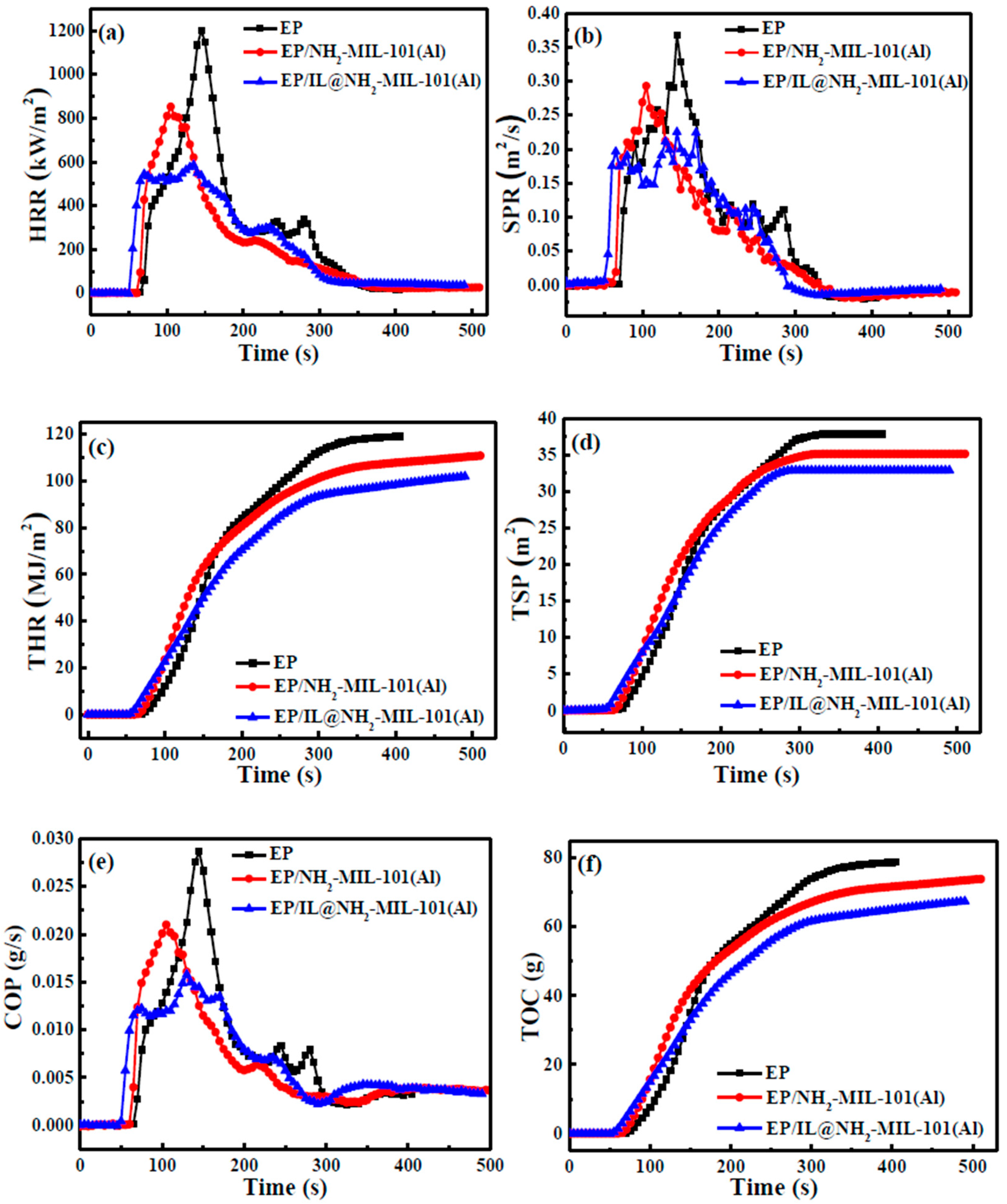
3.4. Flame Retardant Performance of EP Composites
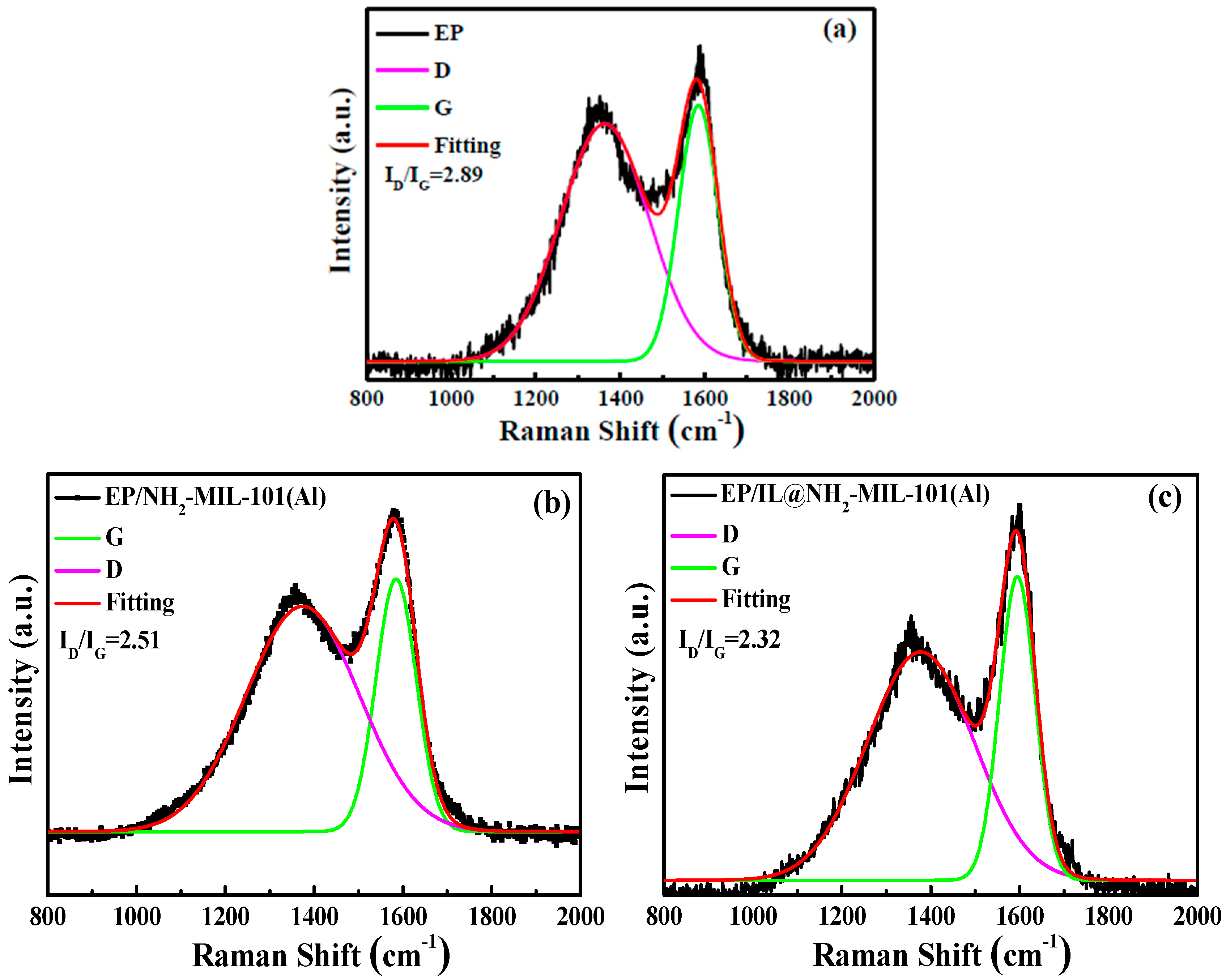
3.5. Analysis of Char Residue
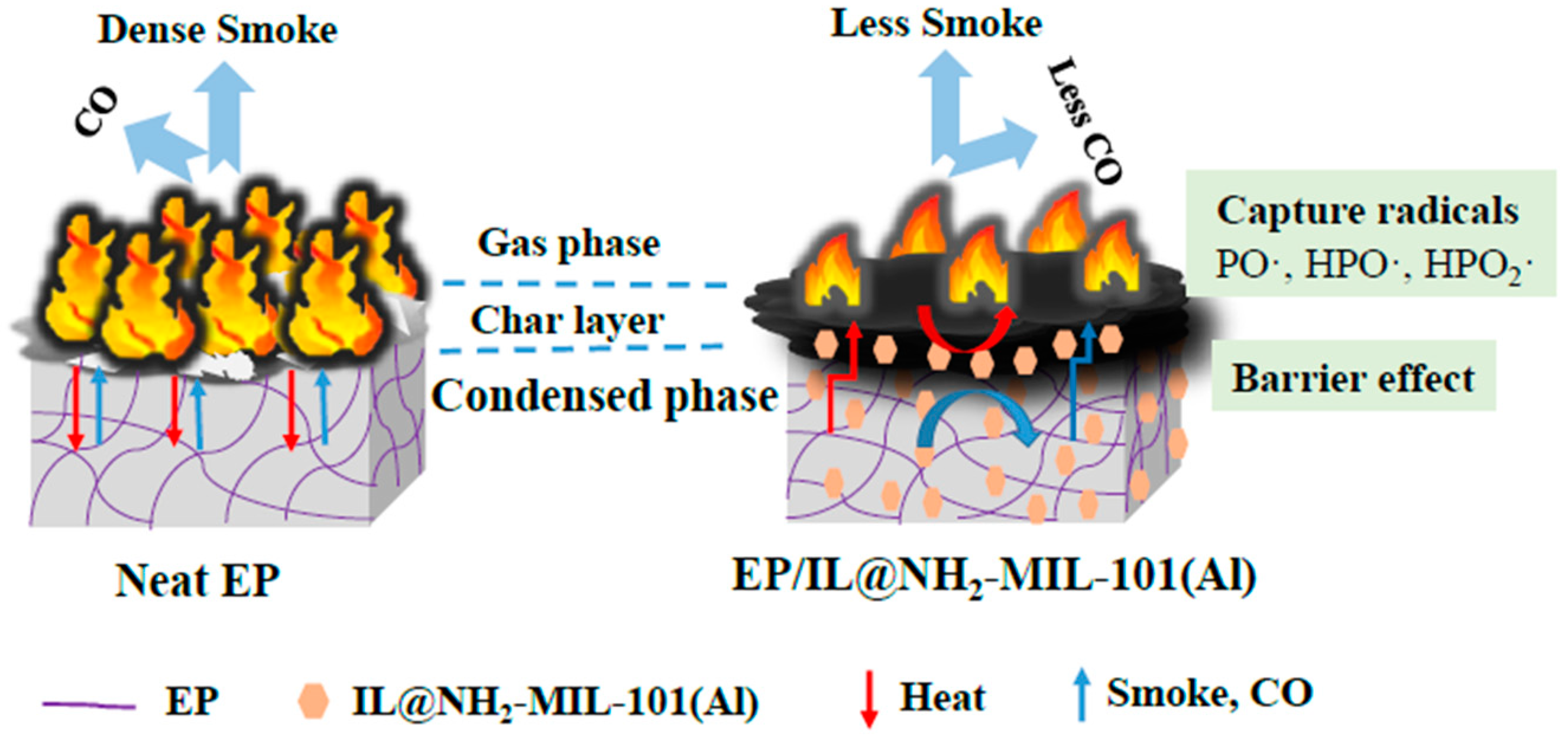
3.6. Proposed Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Martín, C.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Synthesis of novel boron containing epoxy-novolac resins and properties of cured products. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 6332–6344. [Google Scholar] [CrossRef]
- Jin, F.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Wang, N.; Teng, H.; Yang, F.; You, J.; Zhang, J.; Wang, D. Synthesis of k-carrageenan flame-retardant microspheres and its application for waterborne epoxy resin with functionalized graphene. Polymers 2019, 11, 1708. [Google Scholar] [CrossRef]
- Liu, B.; Wang, H.; Guo, X.; Yang, R.; Li, X. Effects of an Organic-Inorganic Hybrid Containing Allyl Benzoxazine and POSS on Thermal Properties and Flame Retardancy of Epoxy Resin. Polymers 2019, 11, 770. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Li, Q.; Wang, Y.; Wang, G. Investigation of the Structure-Property Effect of Phosphorus-Containing Polysulfone on Decomposition and Flame Retardant Epoxy Resin Composites. Polymers 2019, 11, 380. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Wu, W.; Li, M.; Xu, Y.; Chen, G.; Dai, L. A novel POSS-based copolymer functionalized graphene: An effective flame retardant for reducing the flammability of epoxy resin. Polymers 2019, 11, 241. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, B.; Wang, X.; Wang, G.; Ding, D. The flame retardancy and smoke suppression effect of a hybrid containing CuMoO4 modified reduced graphene oxide/layered double hydroxide on epoxy resin. J. Hazard. Mater. 2018, 343, 364–375. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Feng, Y.; Yang, Z.; Yin, B. Synthesis of a multifunctional bisphosphate and its flame retardant application in epoxy resin. Polym. Degrad. Stabil. 2019, 165, 92–100. [Google Scholar] [CrossRef]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of metal-organic frameworks with rod secondary building units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, T.; Chen, Y.; Zou, L.; Feng, D.; Wang, K.; Zhang, Q.; Yuan, S.; Zhong, C.; Zhou, H. A reversible crystallinity-preserving phase transition in metal-organic frameworks: Discovery, mechanistic studies, and potential applications. J. Am. Chem. Soc. 2015, 137, 7740–7746. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Guo, X.; Ying, Y.; Liu, D.; Zhong, C. Composite ultrafiltration membrane tailored by MOF@GO with highly improved water purification performance. Chem. Eng. J. 2017, 313, 890–898. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, L.; Qiu, S.; Zhou, X.; Gui, Z.; Hu, Y. DOPO-modified two-dimensional Co-based metal-organic framework: Preparation and application for enhancing fire safety of poly(lactic acid). ACS Appl. Mater. Interfaces 2018, 10, 8274–8286. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, G.; Xu, J.; Liu, Y.; Chen, R.; Yan, H. Modification of diatomite with melamine coated zeolitic imidazolate framework-8 as an effective flame retardant to enhance flame retardancy and smoke suppression of rigid polyurethane foam. J. Hazard. Mater. 2019, 379, 120819. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Hu, W.; Gui, Z.; Hu, Y. Preparation of metal-organic frameworks and their application as flame retardants for polystyrene. Ind. Eng. Chem. Res. 2017, 56, 2036–2045. [Google Scholar] [CrossRef]
- Qi, X.; Zhou, D.; Zhang, J.; Hu, S.; Haranczyk, M.; Wang, D. Simultaneous improvement of mechanical and fire-safety properties of polymer composites with phosphonate-loaded MOF additives. ACS Appl. Mater. Interfaces 2019, 11, 20325–20332. [Google Scholar] [CrossRef]
- Hou, Y.; Hu, W.; Zhou, X.; Gui, Z.; Hu, Y. Vertically aligned nickel 2-methylimidazole metal-organic framework fabricated from graphene oxides for enhancing fire safety of polystyrene. Ind. Eng. Chem. Res. 2017, 56, 8778–8786. [Google Scholar] [CrossRef]
- Xu, B.; Xu, W.; Wang, G.; Liu, L.; Xu, J. Zeolitic imidazolate frameworks-8 modified graphene as a green flame retardant for reducing the fire risk of epoxy resin. Polym. Adv. Technol. 2018, 29, 1733–1743. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, S.; Pan, Y.; Zhang, S.; Nie, S.; Wei, P.; Zhang, X.; Wang, R.; Wang, D. Nickel metal-organic framework derived hierarchically mesoporous nickel phosphate toward smoke suppression and mechanical enhancement of intumescent flame retardant wood Fiber/poly(lactic acid) composites. ACS Sustain. Chem. Eng. 2019, 7, 9272–9280. [Google Scholar] [CrossRef]
- Ma, J.; Ying, Y.; Guo, X.; Huang, H.; Liu, D.; Zhong, C. Fabrication of mixed-matrix membrane containing metal-organic framework composite with task-specific ionic liquid for efficient CO2 separation. J. Mater. Chem. A 2016, 4, 7281–7288. [Google Scholar] [CrossRef]
- Tome, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef] [PubMed]
- Giernoth, R. Task-specific ionic liquids. Angew. Chem. Int. Ed. 2010, 49, 2834–2839. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ge, N.; Hu, L.; Gui, H.; Wang, Z.; Ding, Y. Synthesis of a novel ionic liquid containing phosphorus and its application in intumescent flame retardant polypropylene system. Polym. Adv. Technol. 2013, 24, 568–575. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Zhu, Y.; Guo, Z.; Su, S. Increasing the efficiency of intumescent flame retardant polypropylene catalyzed by polyoxometalate based ionic liquid. J. Mater. Chem. A 2013, 1, 15242–15246. [Google Scholar] [CrossRef]
- Shi, Y.; Fu, T.; Xu, Y.; Li, D.; Wang, X.; Wang, Y. Novel phosphorus-containing halogen-free ionic liquid toward fire safety epoxy resin with well-balanced comprehensive performance. Chem. Eng. J. 2018, 354, 208–219. [Google Scholar] [CrossRef]
- Gui, H.; Xu, P.; Hu, Y.; Wang, J.; Yang, X.; Bahader, A.; Ding, Y. Synergistic effect of graphene and an ionic liquid containing phosphonium on the thermal stability and flame retardancy of polylactide. RSC Adv. 2015, 5, 27814–27822. [Google Scholar] [CrossRef]
- Chen, S.; Wang, C.; Li, J. Effect of alkyl groups in organic part of polyoxo-metalates based ionic liquids on properties of flame retardant polypropylene. Thermochim. Acta 2016, 631, 51–58. [Google Scholar] [CrossRef]
- Sonnier, R.; Dumazert, L.; Livi, S.; Nguyen, T.; Duchet-Rumeau, H.; Vahabi, P. Laheurte, Flame retardancy of phosphorus-containing ionic liquid based epoxy networks. Polym. Degrad. Stab. 2007, 92, 956–961. [Google Scholar]
- Chen, S.; Li, J.; Zhu, Y.; Su, S. Roles of anion of polyoxometalate-based ionic liquids in properties of intumescent flame retardant polypropylene. RSC Adv. 2014, 4, 32902–32913. [Google Scholar] [CrossRef]
- Xing, W.; Yang, W.; Yang, W.; Hu, Q.; Si, J.; Lu, H.; Yang, B.; Song, L.; Hu, Y.; Yuen, R.K.K. Functionalized carbon nanotubes with phosphorus-and nitrogen-containing agents: Effective reinforcer for thermal, mechanical, and flame-retardant properties of polystyrene nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 26266–26274. [Google Scholar] [CrossRef]
- Serra-Crespo, P.; Ramos-Fernandez, E.V.; Gascon, J.; Kapteijn, F. Synthesis and characterization of an amino functionalized MIL-101(Al): Separation and catalytic properties. Chem. Mater. 2011, 23, 2565–2572. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Ren, X.; Zhang, W.; Zhang, T.; Liu, X.; Du, T.; Li, T.; Wang, J. Internally extended growth of core-shell NH2-MIL-101(Al)@ZIF-8 nanoflowers for the simultaneous detection and removal of Cu(II). J. Mater. Chem. A 2018, 6, 21029–21038. [Google Scholar] [CrossRef]
- Tan, Y.; Shao, Z.; Chen, X.; Long, J.; Chen, L.; Wang, Y. Novel multifunctional organic-inorganic hybrid curing agent with high flame-retardant efficiency for epoxy resin. ACS Appl. Mater. Interfaces 2015, 32, 17919–17928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, Y.; Liu, X.; Huo, T.; Qin, Y. Preparation of durable flame retardant PAN fabrics based on amidoximation and phosphorylation. Appl. Surf. Sci. 2018, 428, 395–403. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Z.; Shi, Y.; Chang, H.; Zhang, Y.; Luo, J.; Lu, C. A preliminary study on the thermal degradation behavior and flame retardancy of high impact polystyrene/magnesium hydroxide/microencapsulated red phosphorus composite with a gradient structure. Polym. Degrad. Stab. 2014, 105, 21–30. [Google Scholar] [CrossRef]
- Wang, P.; Liao, D.; Hu, X.; Pan, N.; Li, W.; Wang, D.; Yao, Y. Facile fabrication of biobased PeNeC-containing nano-layered hybrid: Preparation, growth mechanism and its efficient fire retardancy in epoxy. Polym. Degrad. Stab. 2019, 159, 153–162. [Google Scholar] [CrossRef]
- Xiao, F.; Wu, K.; Luo, F.; Yao, S.; Lv, M.; Zou, H.; Lu, M. Influence of Ionic Liquid-based Metal-organic Hybrid on Thermal Degradation, Flame retardancy, and Smoke Suppression Properties of Epoxy Resin Composites. J. Mater. Sci. 2018, 53, 10135–10146. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, L.; Rao, W.; Qi, W.; Guo, D.; Liao, W.; Wang, Y. Latent curing epoxy system with excellent thermal stability, flame retardance and dielectric property. Chem. Eng. J. 2018, 347, 223–232. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.; Luo, X.; Guo, D.; Zhong, H.; Chen, L.; Wang, Y. A novel phosphorus-containing semi-aromatic polyester toward flame retardancy and enhanced mechanical properties of epoxy resin. Chem. Eng. J. 2020, 380, 122471–122480. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, Z.; Chen, R.; Wang, J.; Zhou, H. Synthesis of DOPO-base pyrazine derivative and its effect on flame retardancy and thermal stability of epoxy resin. Polym. Adv. Technol. 2019, 30, 2331–2339. [Google Scholar] [CrossRef]
- Yang, G.; Wu, W.; Wang, Y.; Jiao, Y.; Lu, L.; Qu, H.; Qin, X. Synthesis of a novel phosphazene-based flame retardant with active amine groups and its application in reducing the fire hazard of Epoxy Resin. J. Hazard. Mater. 2019, 366, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qiu, Y.; Qian, L.; Chen, Y.; Xu, B. Strengthen flame retardancy of epoxy thermoset by montmorillonite particles adhering phosphorus-containing fragments. J. Appl. Polym. Sci. 2020, 47500, 269–276. [Google Scholar] [CrossRef]






| Samples | Element | |||||
|---|---|---|---|---|---|---|
| EP | C | N | O | |||
| EP/NH2-MIL-101(Al) | C | N | O | Al | ||
| EP/IL@NH2-MIL-101(Al) | C | N | O | Al | P | Mo |
| Samples | Nitrogen | ||
|---|---|---|---|
| T5% (°C) | Tmax (°C) | R (wt. %) | |
| EP | 363.2 | 377.8 | 11.2 |
| EP/NH2-MIL-101(Al) | 351.6 | 371.5 | 13.2 |
| EP/IL@NH2-MIL-101(Al) | 340.2 | 354.0 | 19.7 |
| Samples | TTI (s) | PHRR (Kw/m2) | THR (MJ/m2) | SPR (m2/s) | TSP (m2) | COP (g/s) | TOC (g) |
|---|---|---|---|---|---|---|---|
| EP | 63 | 1201.31 | 119.05 | 0.37 | 37.86 | 0.029 | 78.77 |
| EP/NH2-MIL-101(Al) | 65 | 851.64 | 110.79 | 0.29 | 35.15 | 0.021 | 73.86 |
| EP/IL@NH2-MIL-101(Al) | 53 | 585.72 | 101.92 | 0.23 | 32.91 | 0.016 | 67.44 |
| Flame Retardant | Re-PHRR (%) | Re-TSP (%) | Re-COP (%) | Content (wt. %) | Ref. |
|---|---|---|---|---|---|
| PMAIL | 31.00 | 15.41 | 22.50 | 6 | [37] |
| IDOP | 5.02 | 13.90 | 26.98 | 5 | [38] |
| PBHDDP | 19.13 | 0.40 | ----- | 12 | [39] |
| DHBAP | 26.30 | 60.90 | −70.30 | 8 | [40] |
| PBFA | 26.92 | 48.05 | ----- | 9 | [41] |
| TGD | 22.04 | 2.10 | −0.15 | 3 | [42] |
| IL@NH2-MIL-101(Al) | 51.24 | 13.07 | 44.83 | 3 | Our work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Guo, X.; Ma, S.; Xie, J.; Xu, J.; Ma, J. Novel Phosphorus-Nitrogen-Containing Ionic Liquid Modified Metal-Organic Framework as an Effective Flame Retardant for Epoxy Resin. Polymers 2020, 12, 108. https://doi.org/10.3390/polym12010108
Huang R, Guo X, Ma S, Xie J, Xu J, Ma J. Novel Phosphorus-Nitrogen-Containing Ionic Liquid Modified Metal-Organic Framework as an Effective Flame Retardant for Epoxy Resin. Polymers. 2020; 12(1):108. https://doi.org/10.3390/polym12010108
Chicago/Turabian StyleHuang, Rong, Xiuyan Guo, Shiyue Ma, Jixing Xie, Jianzhong Xu, and Jing Ma. 2020. "Novel Phosphorus-Nitrogen-Containing Ionic Liquid Modified Metal-Organic Framework as an Effective Flame Retardant for Epoxy Resin" Polymers 12, no. 1: 108. https://doi.org/10.3390/polym12010108
APA StyleHuang, R., Guo, X., Ma, S., Xie, J., Xu, J., & Ma, J. (2020). Novel Phosphorus-Nitrogen-Containing Ionic Liquid Modified Metal-Organic Framework as an Effective Flame Retardant for Epoxy Resin. Polymers, 12(1), 108. https://doi.org/10.3390/polym12010108





