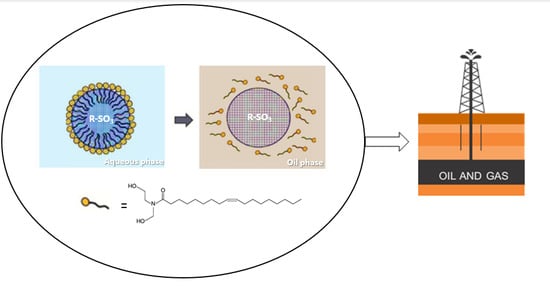
Sulfonated Polystyrene Nanoparticles as Oleic Acid Diethanolamide Surfactant Nanocarriers for Enhanced Oil Recovery Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
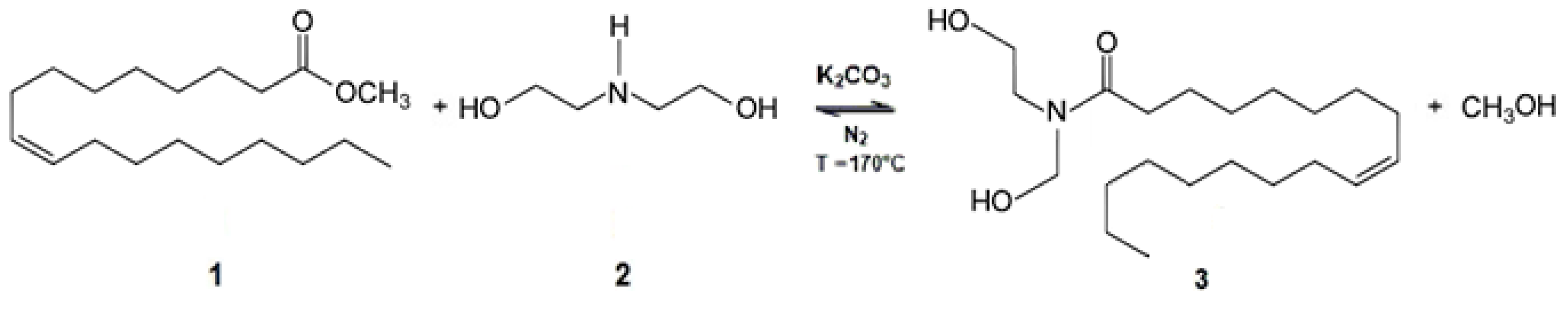
2.2.1. Oleic Acid Diethanolamide (OADA) Synthesis and Characterization
2.2.2. Synthesis of Crosslinked Polystyrene Nanoparticles (NPPS)
2.2.3. Synthesis of Crosslinked Sulfonated Polystyrene Nanoparticles (SPSNP)
2.2.4. Nanoparticles Characterization
2.2.5. Superficial and Interfacial Tension Measurements
2.2.6. Quantification of Surfactant Retention by NPPS
2.2.7. Sand Pre-Treatment and Characterization
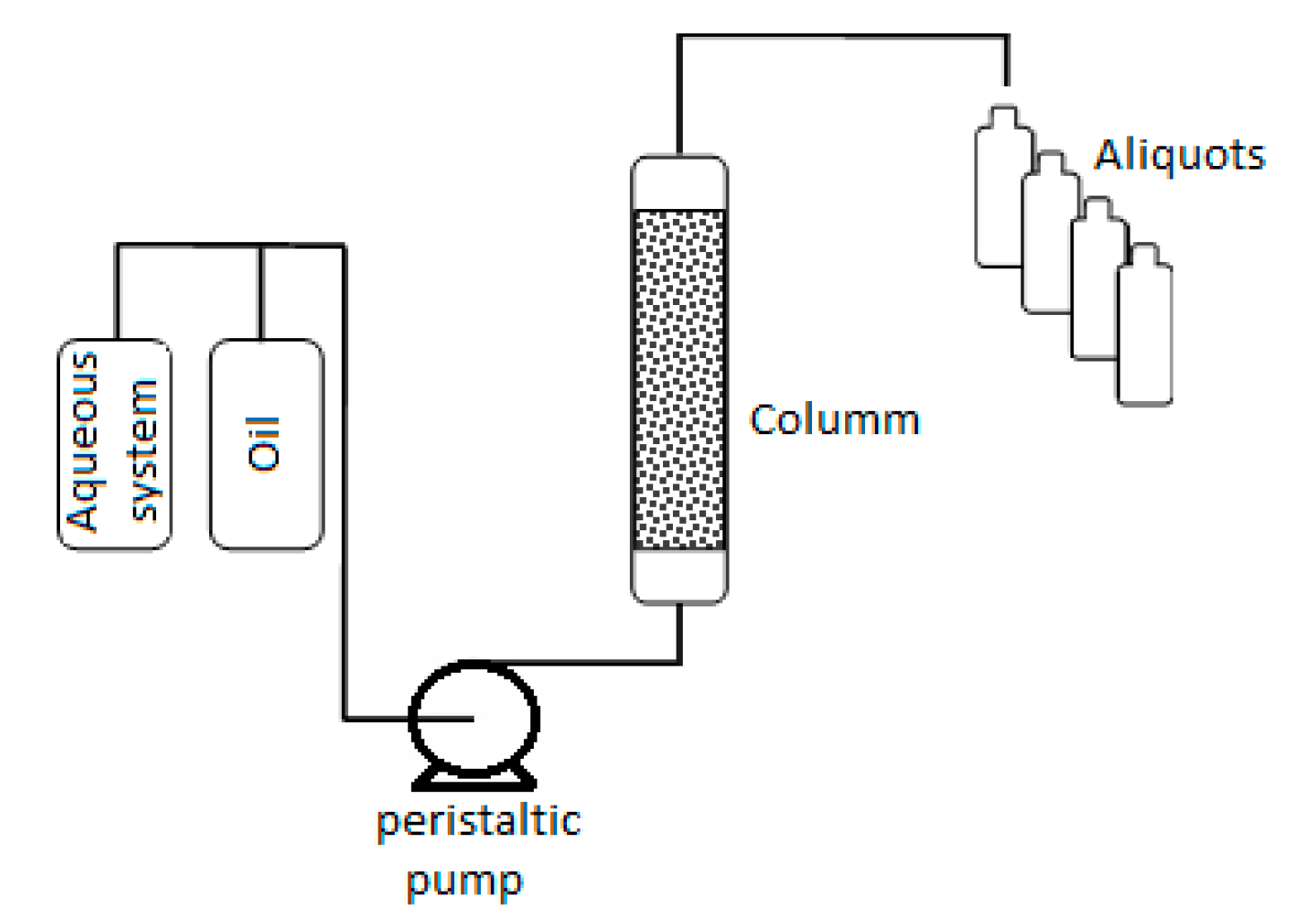
2.2.8. Transport Test Evaluation
2.2.9. Oil Recovery Tests
3. Results and Discussion
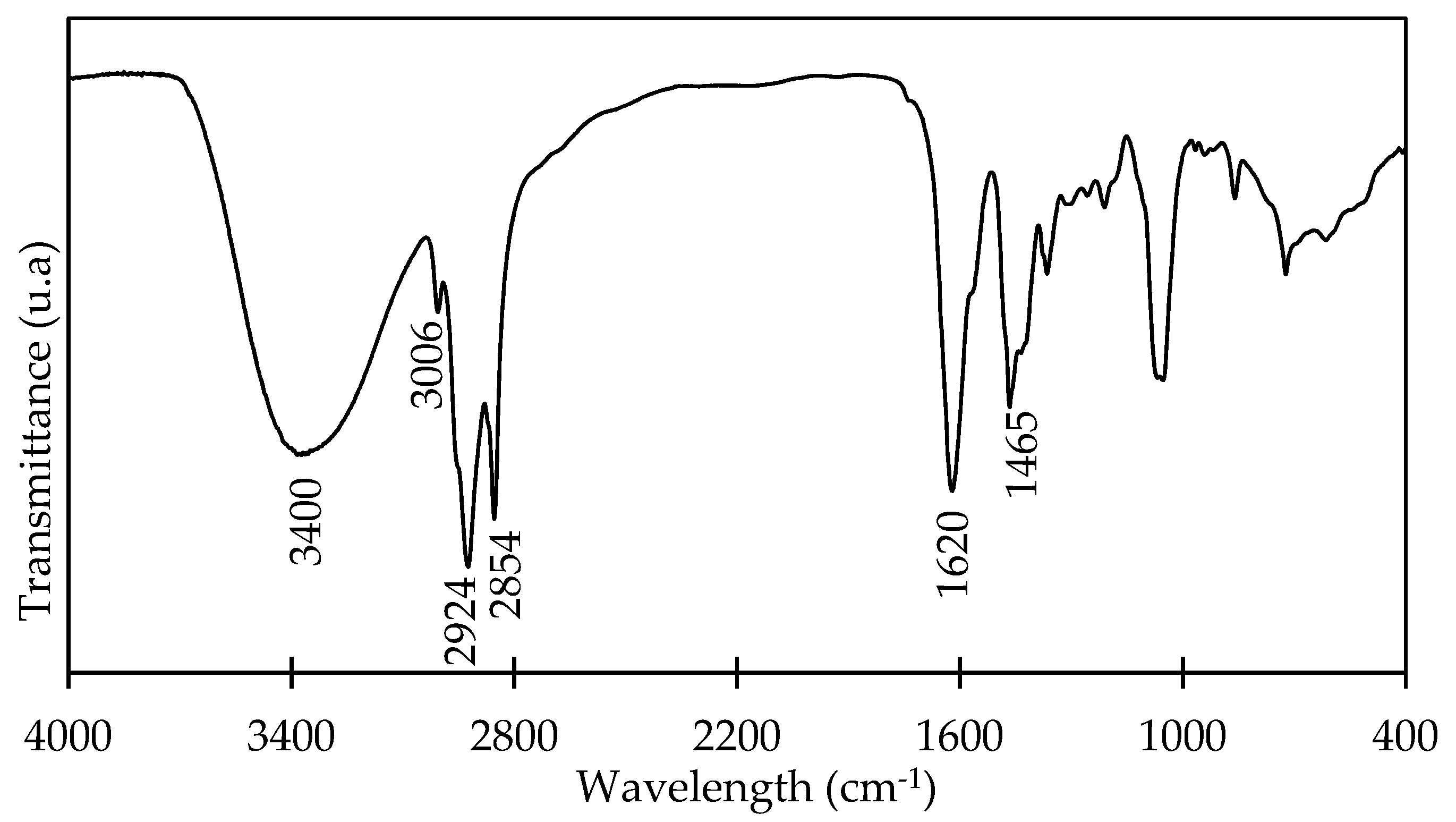
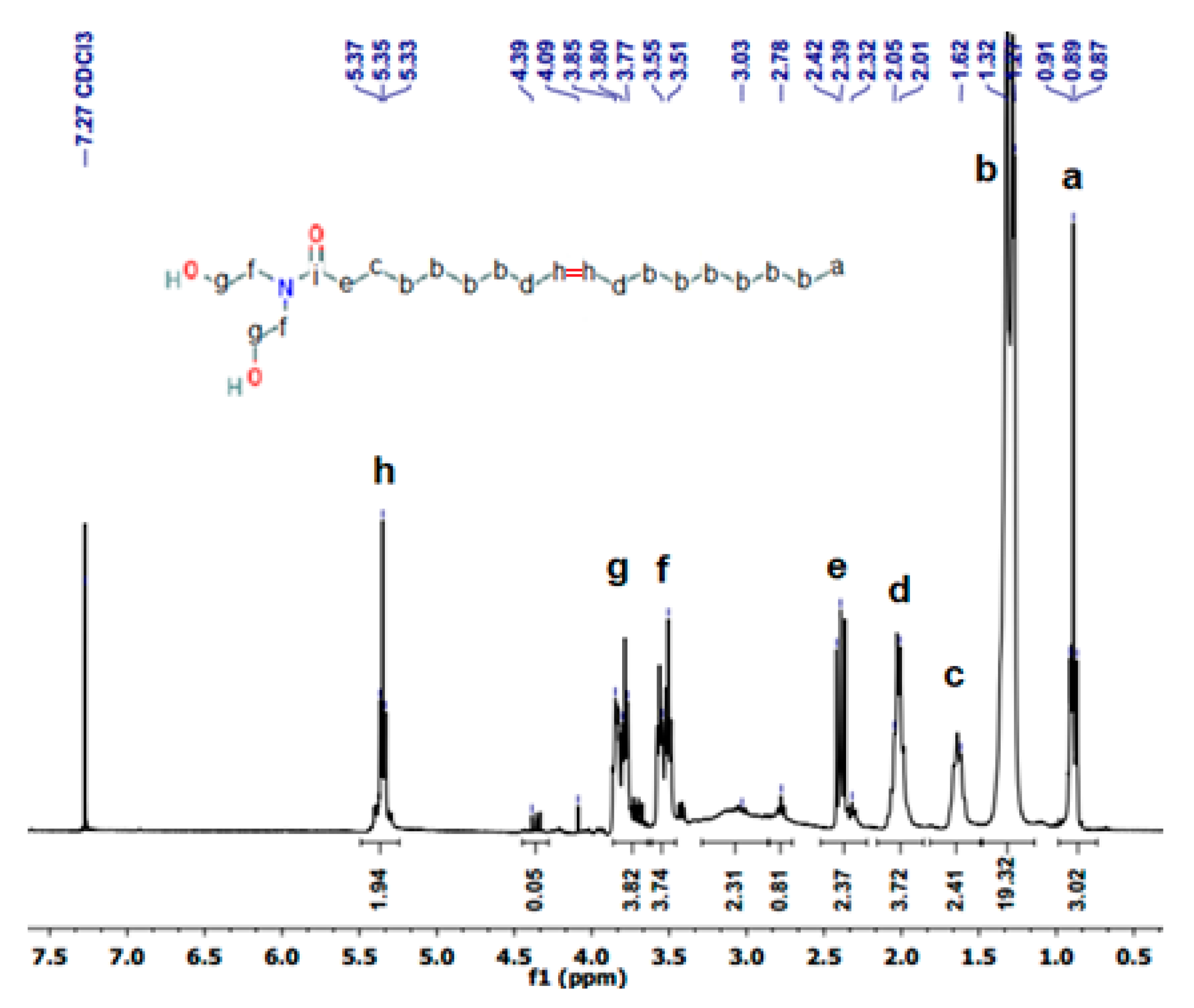
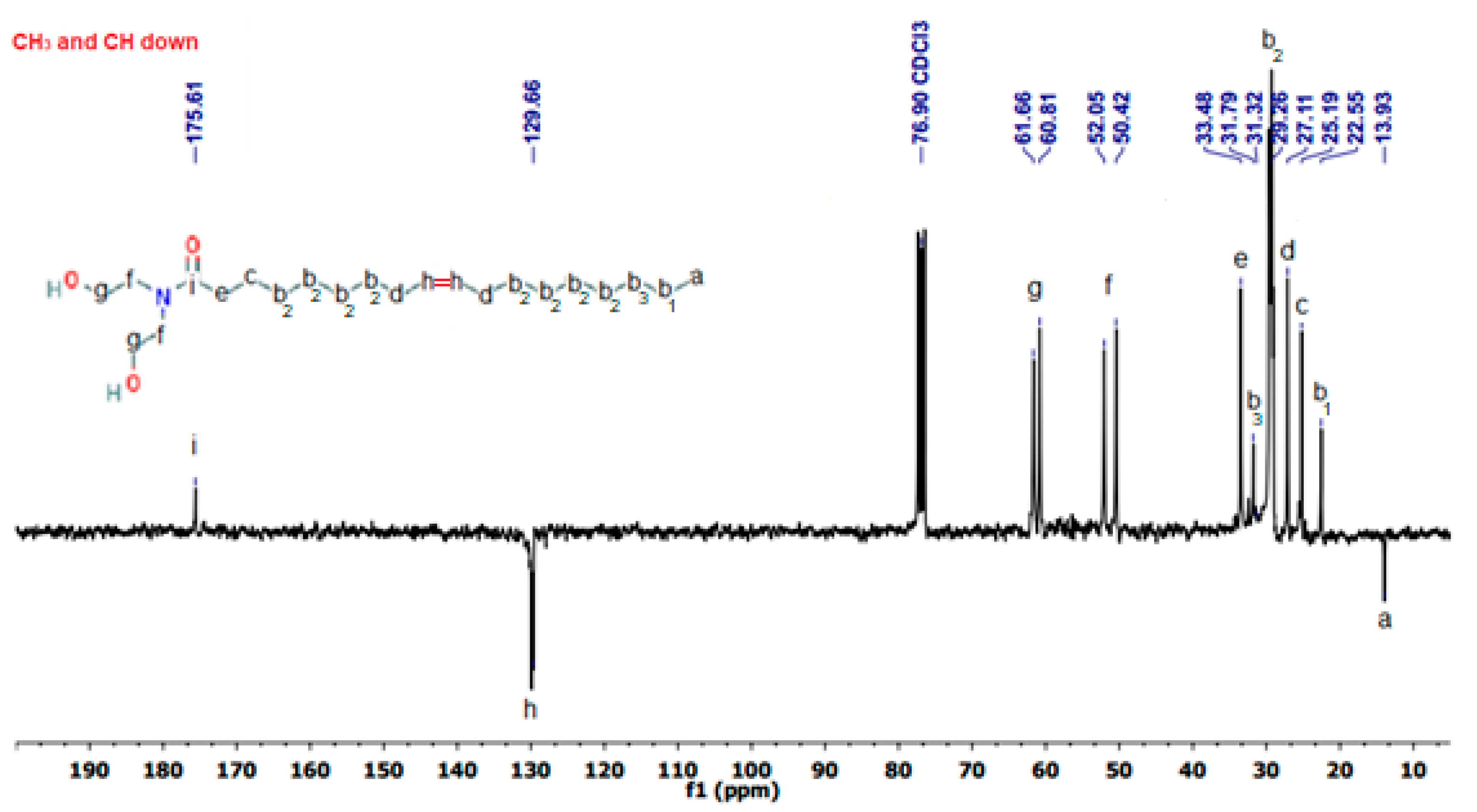
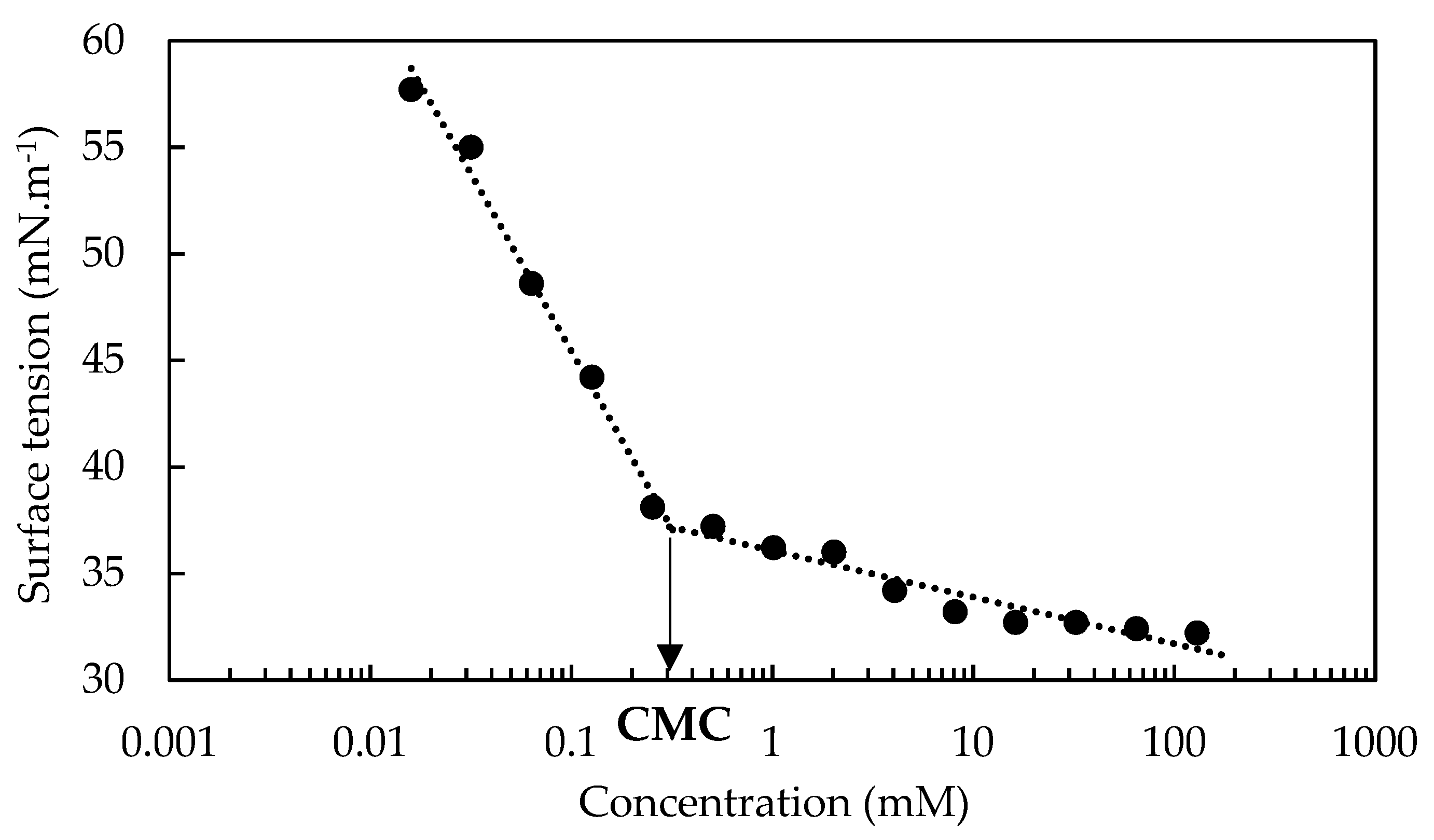
3.1. OADA Characterization
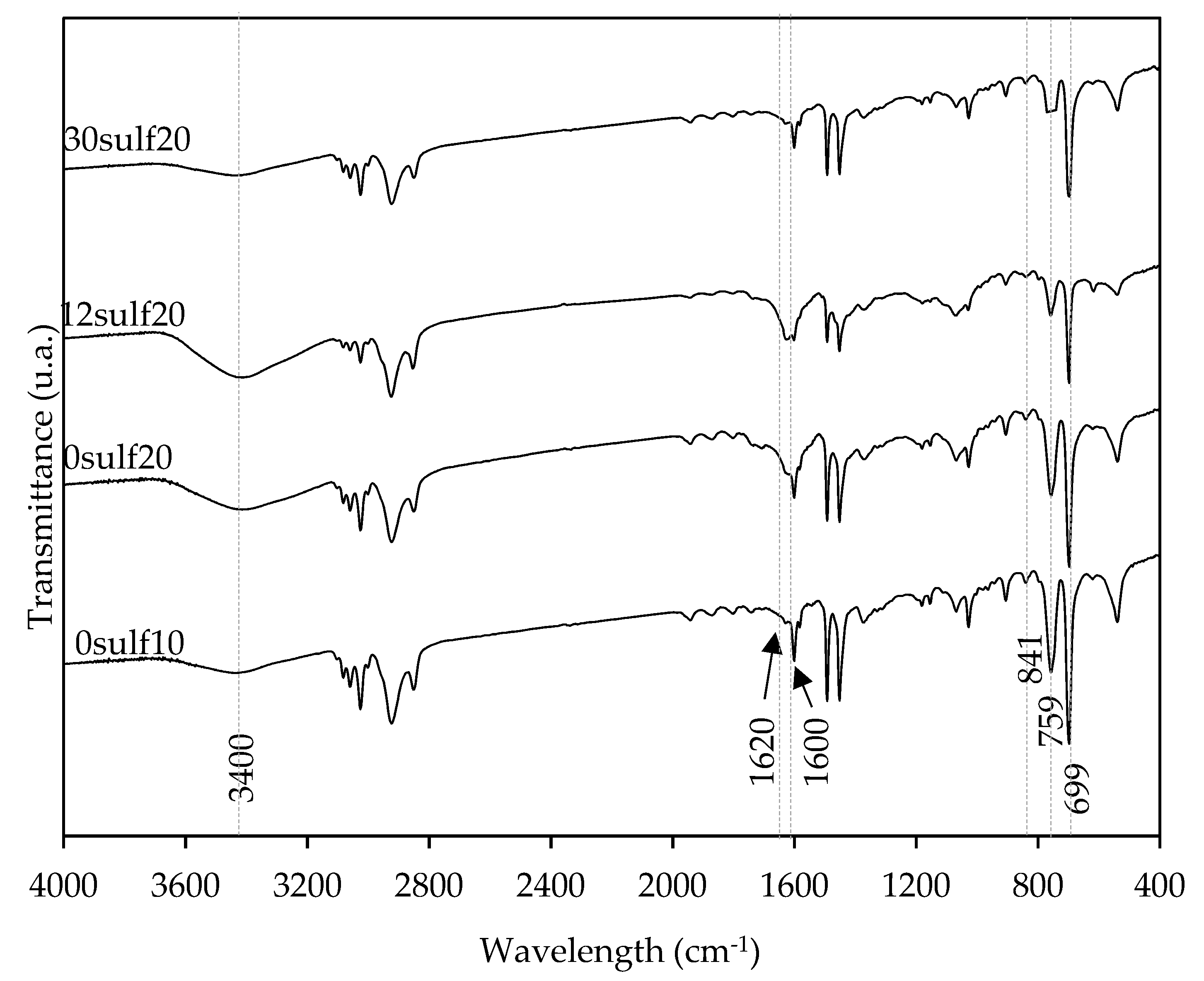
3.2. Nanoparticles Characterization
3.3. Effect of Crosslinking Agent and Surfactantconcentration on the Non-Sulfonated Polysturene Nanoparticles (NPPS) Size and on the Oil/Water Interfacial Tension
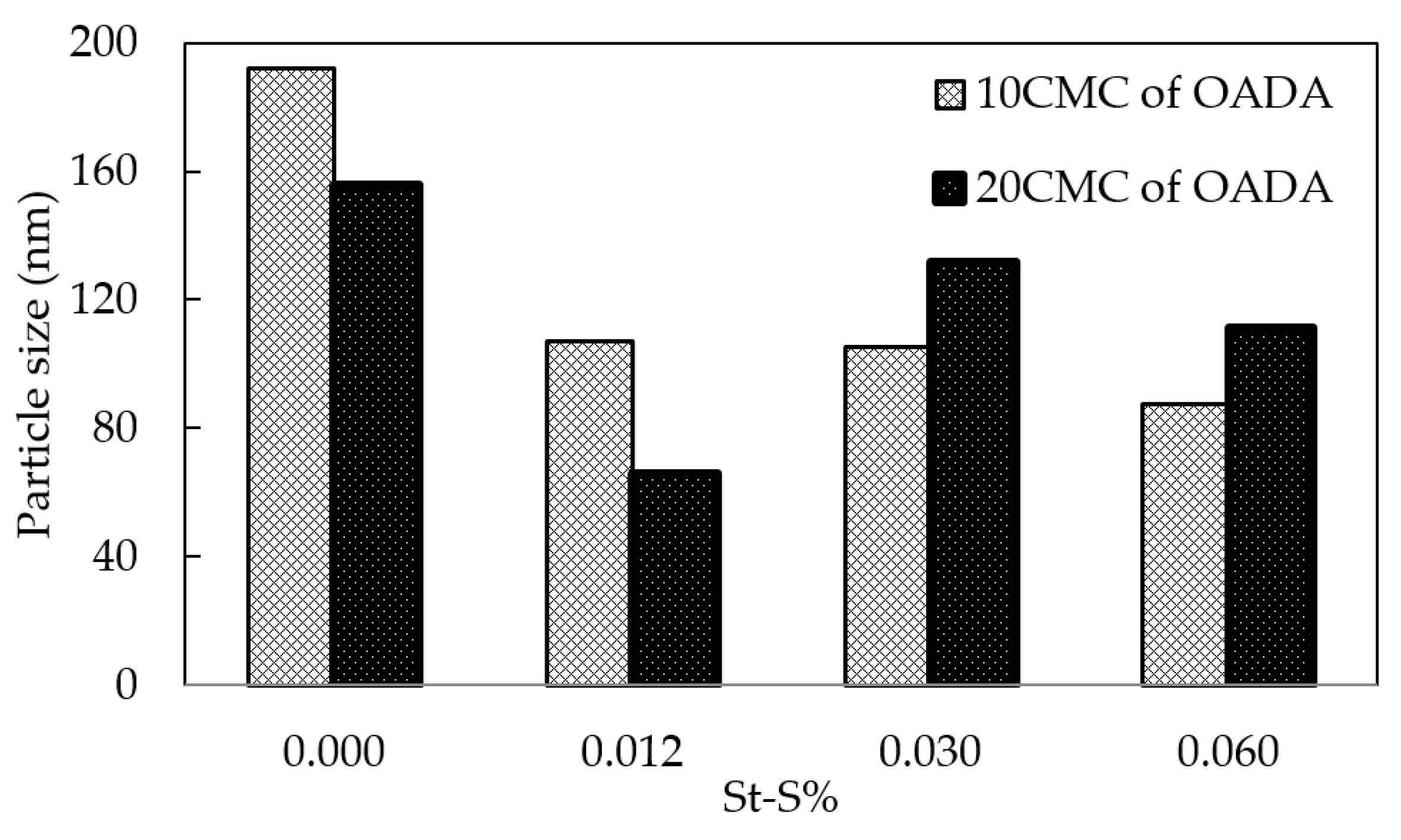
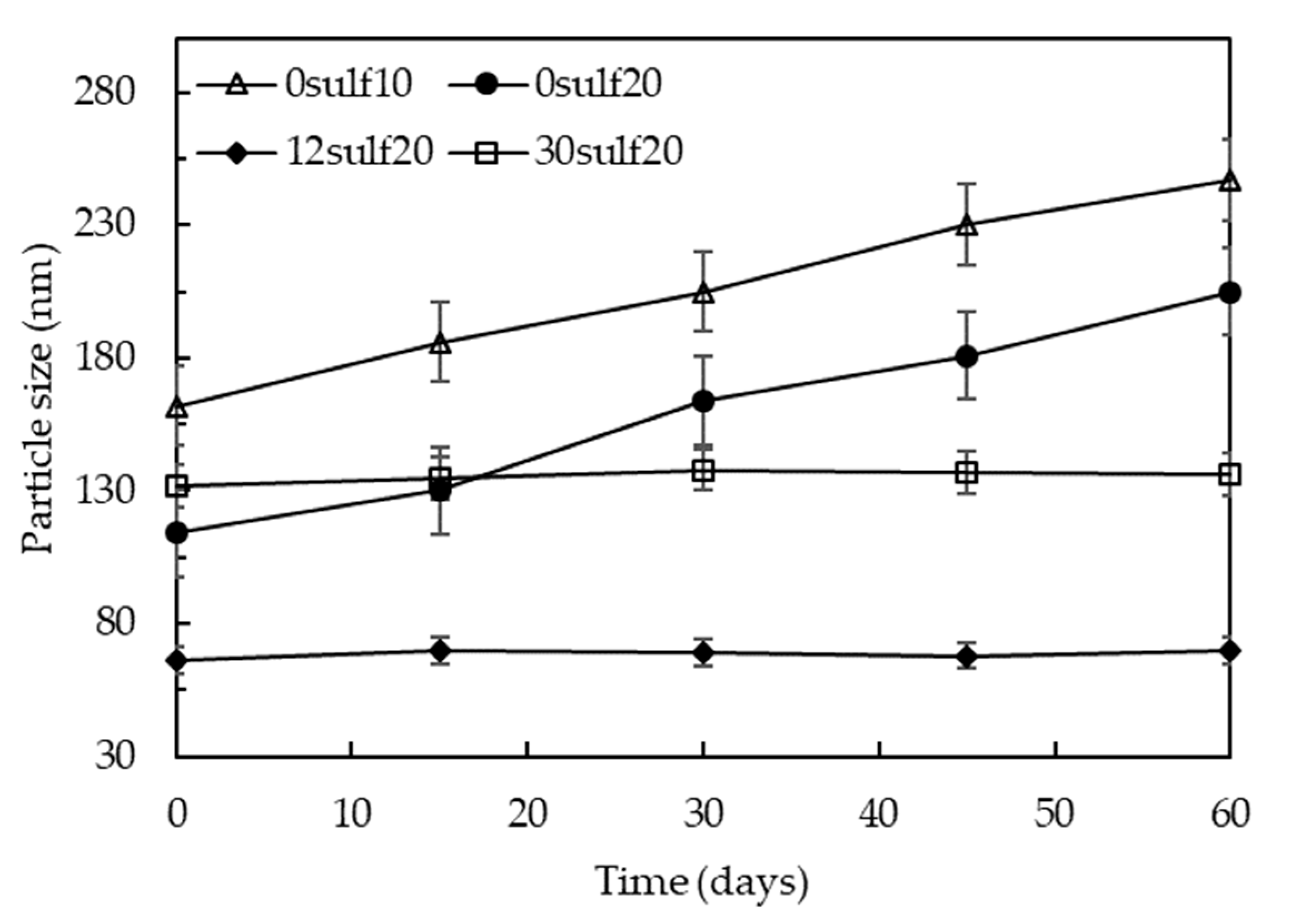
3.4. Effect of the Sulfonate Group Content on SPSNP Size
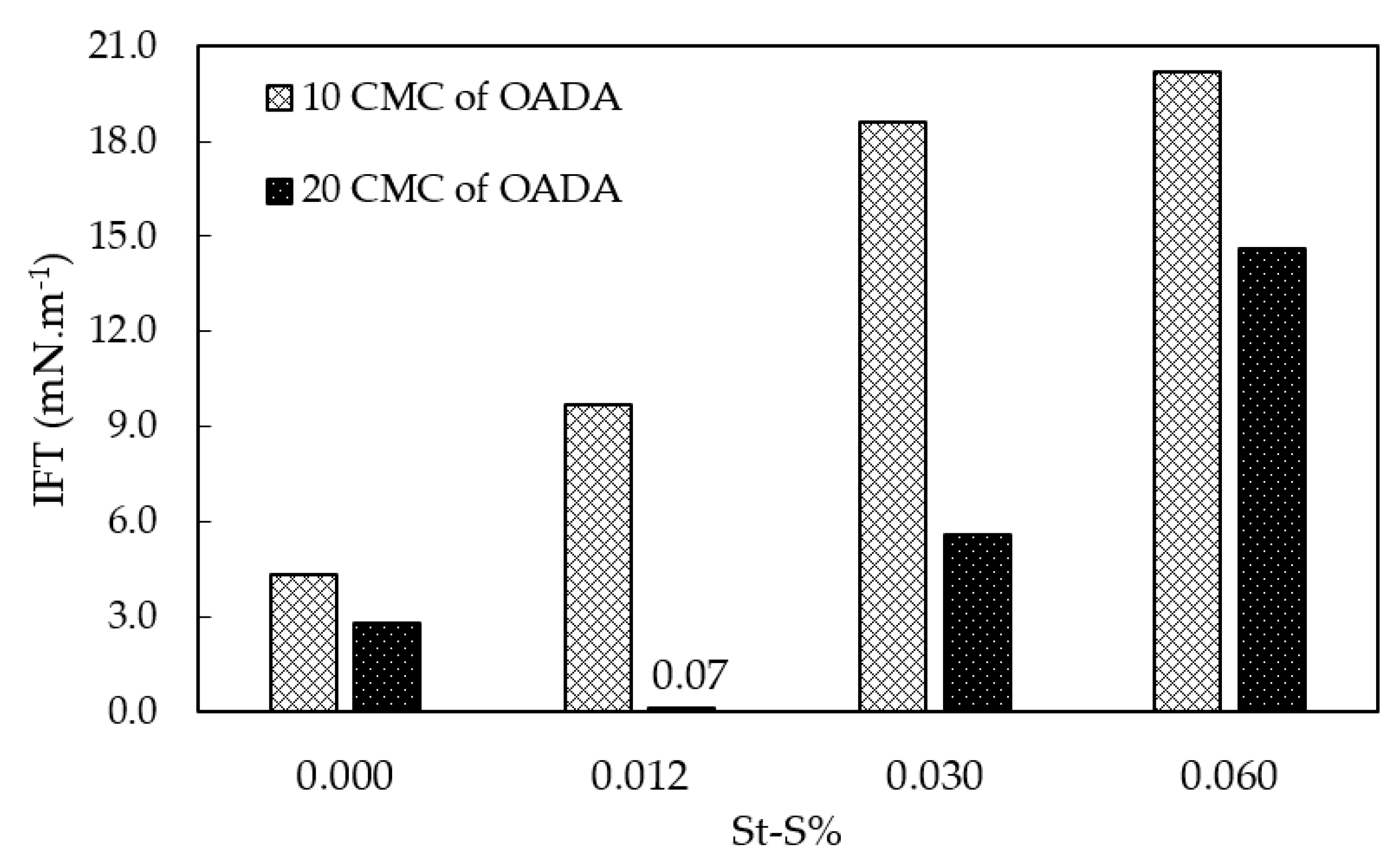
3.5. Effect of the Sulfonate Group Content on Water/Oil IFT Values for the Systems Containing SPSNP
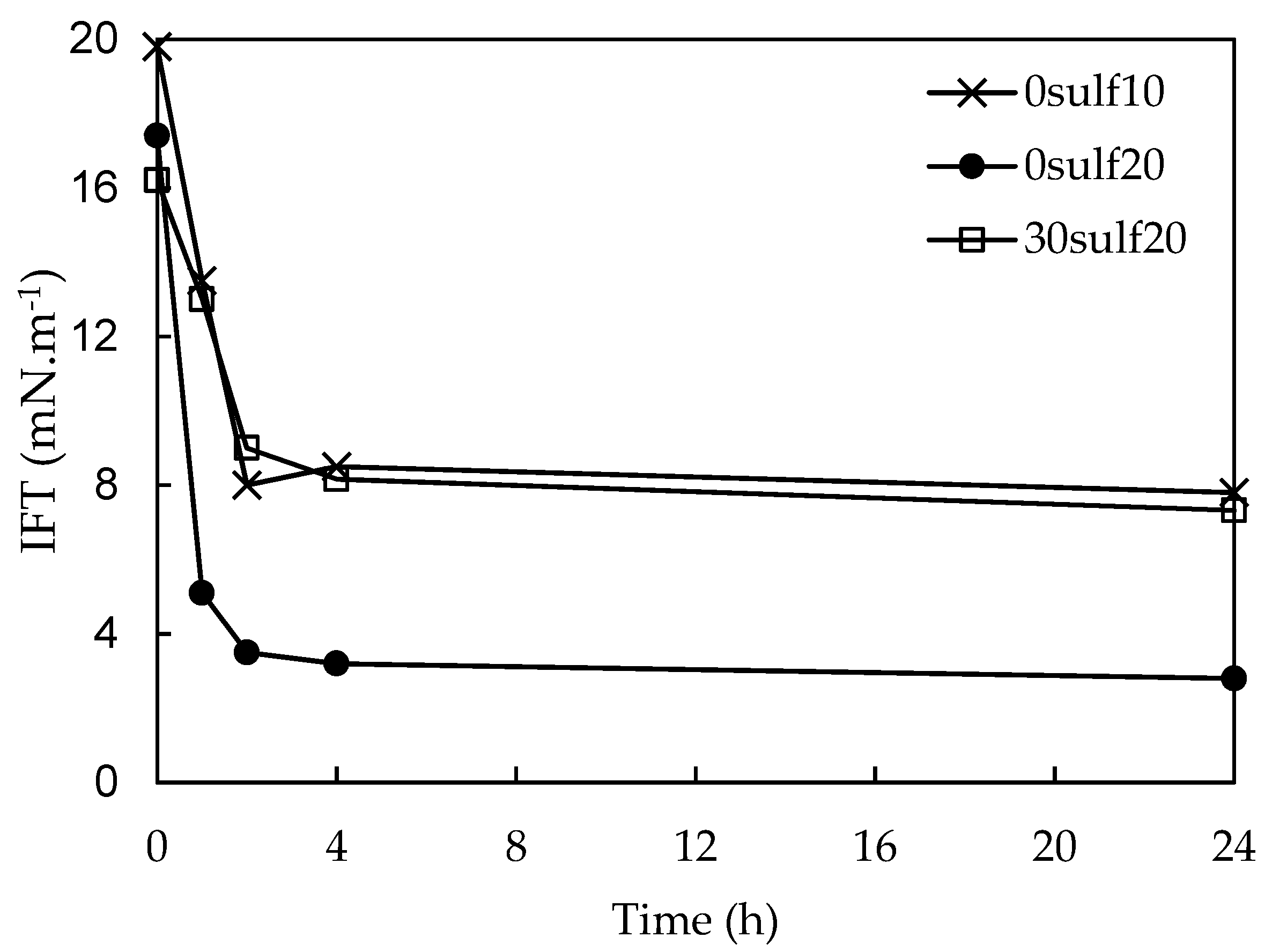
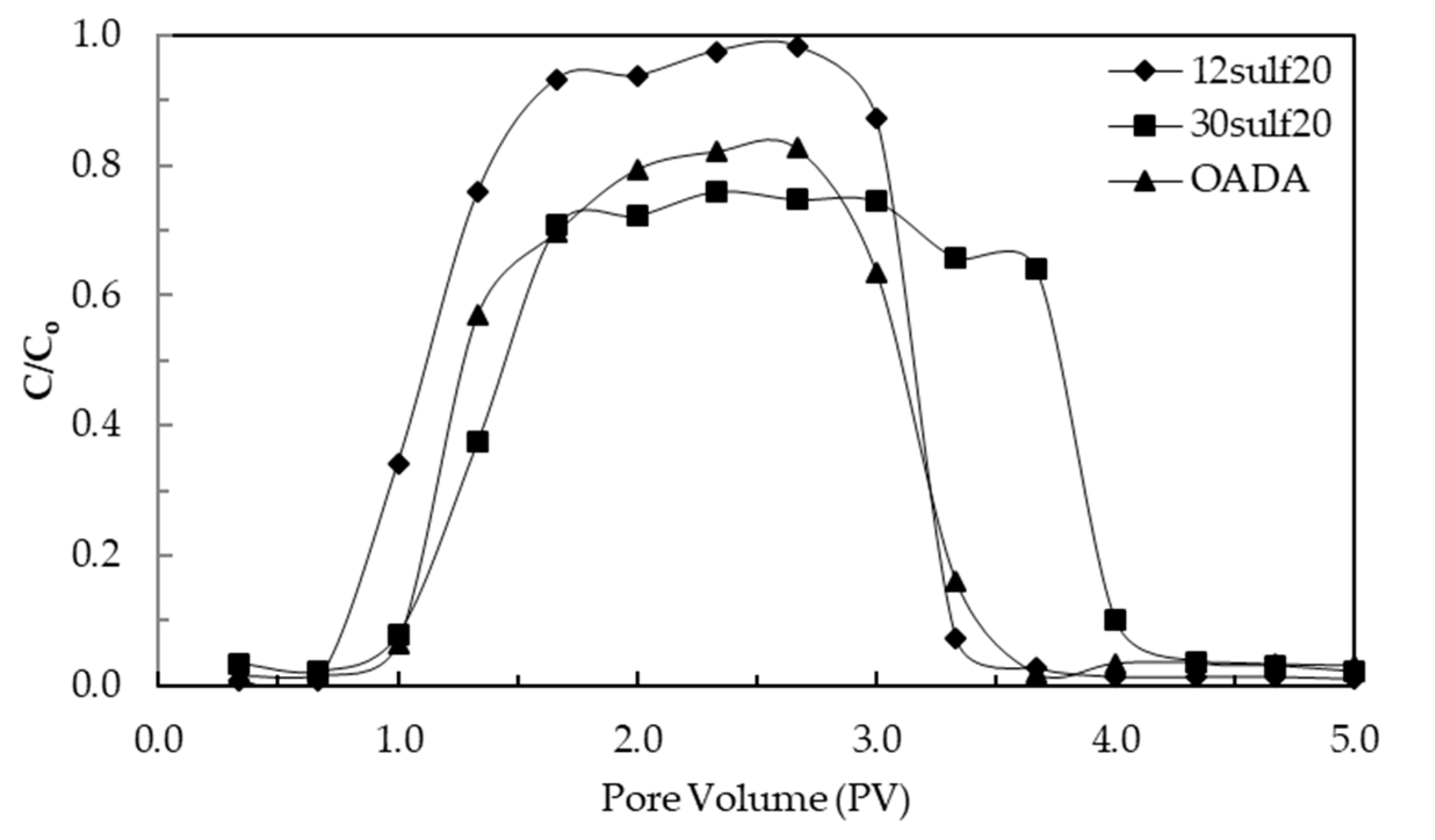
3.6. Surfactant Controlled Release Study
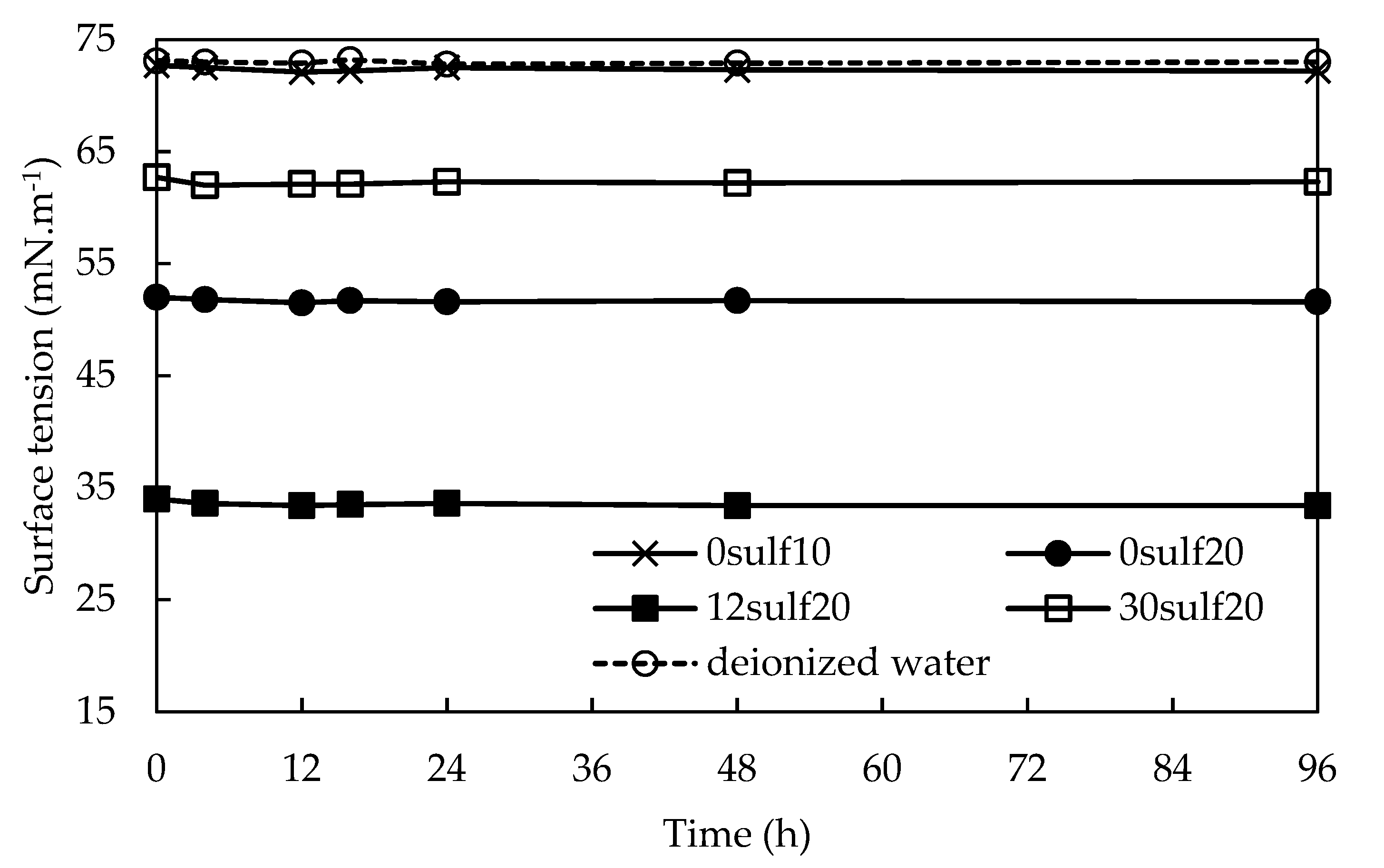
3.7. Nanoparticles Storage Ability
3.8. Quantification of Surfactant Retention by Nanoparticles
3.9. Sand Characterization
3.10. Transport Test Evaluation
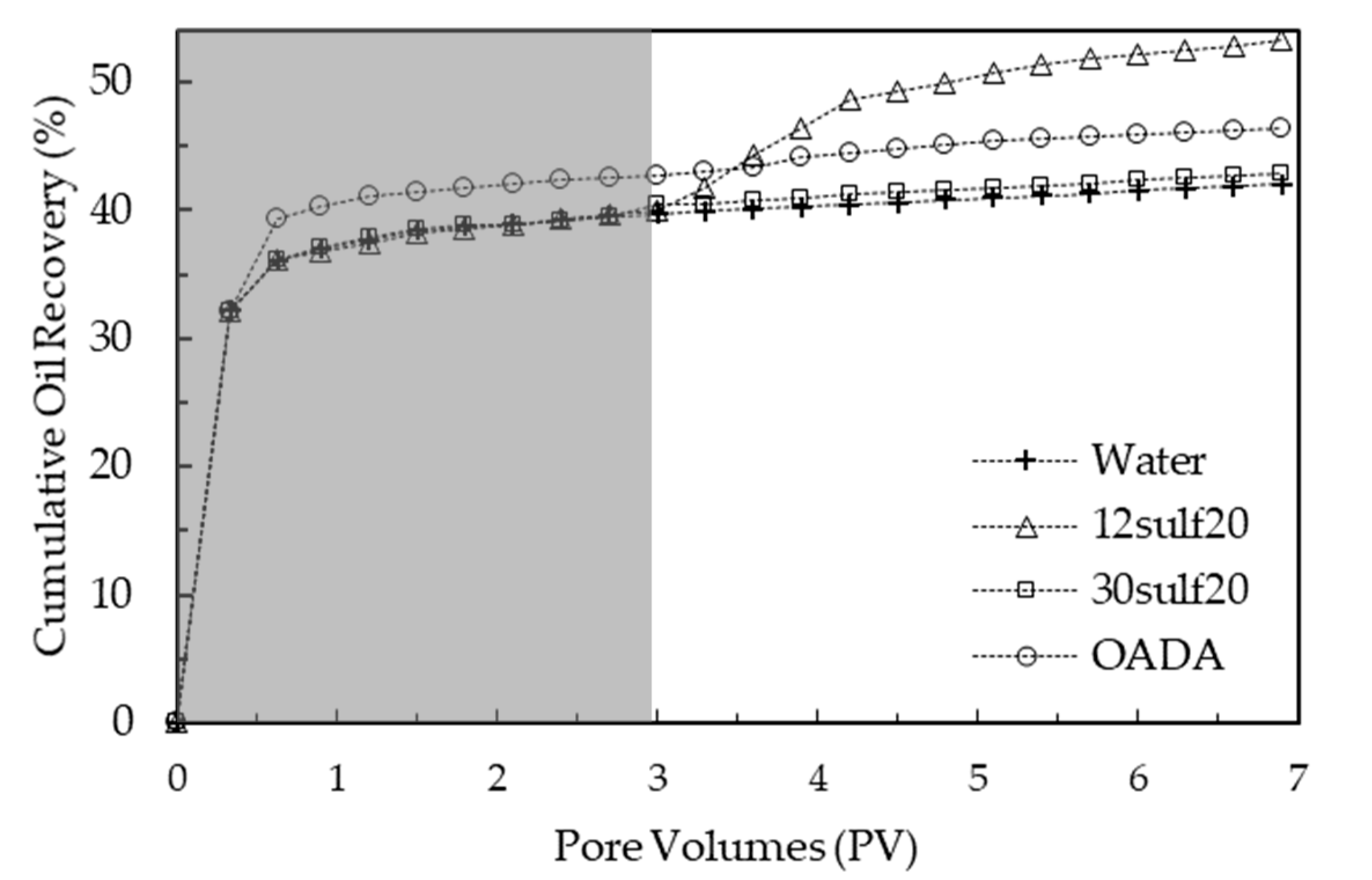
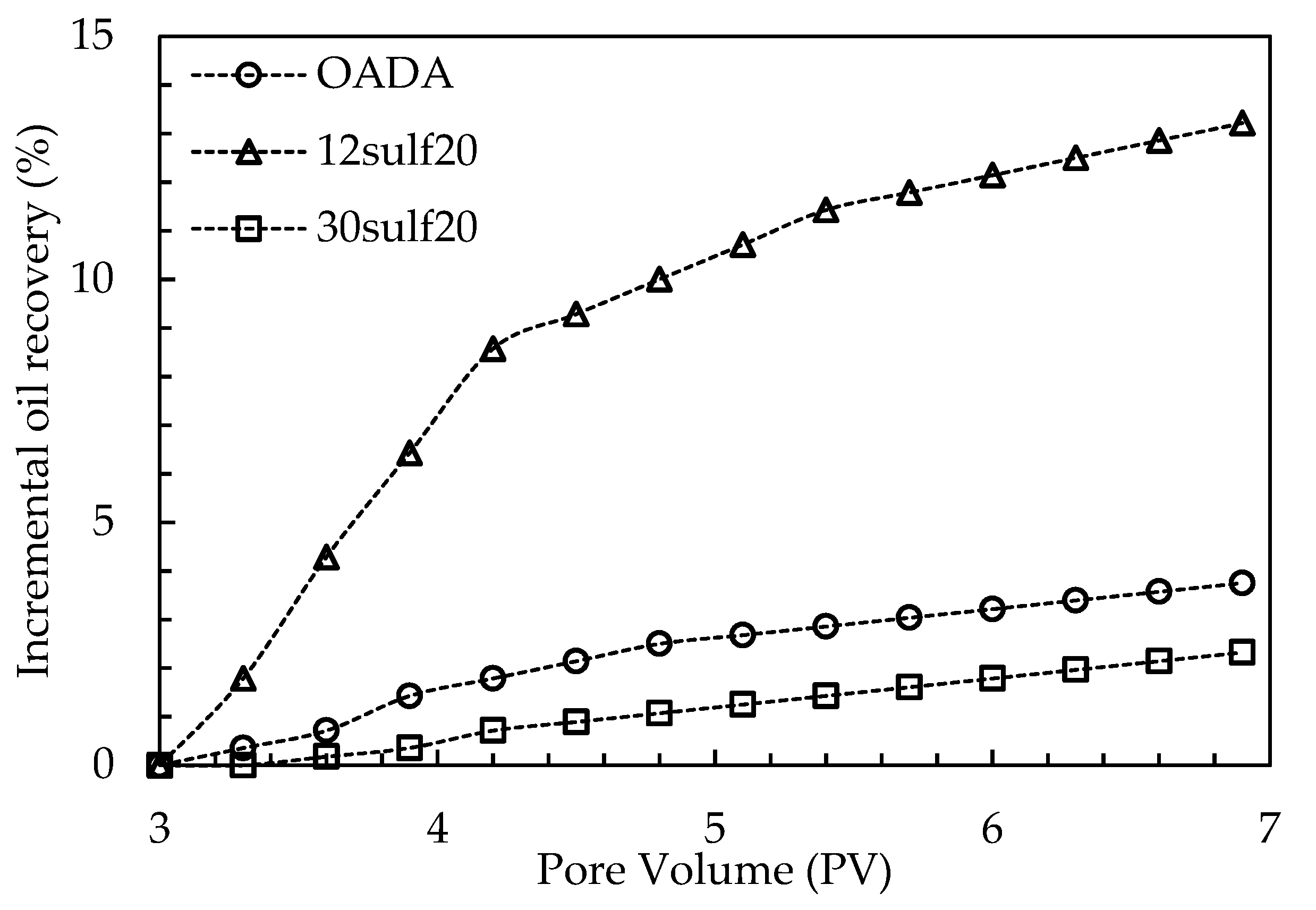
3.11. Oil Displacement Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, D.W.; Willhite, G.P. Enhanced Oil Recovery; Henry, L., Ed.; Doherty Memorial Fund of AIME, Society of Petroleum Engineers: Richardson, TX, USA, 1998; Volume 6, Chapter 1; p. 2. [Google Scholar]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery: Theory and Practice, 1st ed.; Elsevier: Burlington, VT, USA, 2011; pp. 1–9. [Google Scholar]
- Hirasaki, G.; Miller, C.A.; Puerto, M. Recent advances in surfactant EOR. SPE J. 2011, 16, 889–907. [Google Scholar] [CrossRef]
- Iglauer, S.; Wu, Y.; Shuler, P.; Tang, Y.; Goddard, W.A., III. New surfactant classes for enhanced oil recovery and their tertiary oil recovery potential. J. Pet. Sci. Eng. 2010, 71, 23–29. [Google Scholar] [CrossRef]
- Alhassawi, H.; Romero-Zerón, L. New surfactant delivery system for controlling surfactant adsorption onto solid surfaces. Part I: static adsorption tests. Can. J. Chem. Eng. 2015, 93, 1188–1193. [Google Scholar] [CrossRef]
- Avila, J.N.L.; Araujo, L.L.G.C.; Drexler, S.; Rodrigues, J.A.; Nascimento, R.S.V. Polystyrene nanoparticles as surfactant carriers for enhanced oil recovery. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Pei, H.; Zhang, G.; Ge, J.; Zhang, J.; Zhang, Q. Investigation of synergy between nanoparticle and surfactant in stabilizing oil-in-water emulsions for improved heavy oil recovery. Colloids Surf. A 2015, 484, 478–484. [Google Scholar] [CrossRef]
- Zargartalebi, M.; Kharrat, R.; Barati, N. Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 2015, 143, 21–27. [Google Scholar] [CrossRef]
- Esmaeilzadeh, P.; Hosseinpour, N.; Bahramian, A.; Fakhroueian, Z.; Arya, S. Effect of ZrO2 nanoparticles on the interfacial behavior of surfactant solutions at air-water and n-heptane-water interfaces. Fluid Phase Equilib. 2014, 361, 289–295. [Google Scholar] [CrossRef]
- Zargartalebi, M.; Barati, N.; Kharrat, R. Influences of hydrophilic and hydrophobic silica nanoparticles on anionic surfactant properties: interfacial and adsorption behaviors. J. Pet. Sci. Eng. 2014, 119, 36–43. [Google Scholar] [CrossRef]
- Chen, C.; Wang, S.; Kadhum, M.J.; Harwell, J.H.; Shiau, B.J. Using carbonaceous nanoparticles as surfactant carrier in enhanced oil recovery: A laboratory study. Fuel 2018, 222, 561–568. [Google Scholar] [CrossRef]
- Nourafkan, E.; Hu, Z.; Wen, D. Nanoparticle-enabled delivery of surfactants in porous media. J. Colloid Interface Sci. 2018, 519, 44–57. [Google Scholar] [CrossRef]
- Nourafkan, E.; Hu, Z.; Wen, D. Controlled delivery and release of surfactant for enhanced oil recovery by nanodroplets. Fuel 2018, 218, 396–405. [Google Scholar] [CrossRef]
- Rosestolato, J.C.S.; Pérez-Gramatges, A.; Lachter, E.R.; Nascimento, R.S.V. Lipid nanostructures as surfactant carriers for enhanced oil recovery. Fuel 2019, 239, 403–412. [Google Scholar] [CrossRef]
- Maurya, N.K.; Mandal, A. Investigation of synergistic effect of nanoparticle and surfactant in macro emulsion based EOR application in oil reservoirs. Chem. Eng. Res. Des. 2018, 132, 370–384. [Google Scholar] [CrossRef]
- Saien, J.; Bahrami, M. Understanding the effect of different size silica nanoparticles and SDS surfactant mixtures on interfacial tension of n-hexane-water. J. Mol. Liq. 2016, 224, 158–164. [Google Scholar] [CrossRef]
- Freitas, F.A.; Keils, D.; Lachter, E.R.; Maia, C.E.B.; Silva, M.I.P.; Nascimento, R.S.V. Synthesis and evaluation of the potential of nonionic surfactants/mesoporous silica systems as nanocarriers for surfactant controlled release in enhanced oil recovery. Fuel 2019, 241, 1184–1194. [Google Scholar] [CrossRef]
- Holley, D.W.; Ruppel, M.; Mays, J.W.; Urban, V.S.; Baskaran, D. Polystyrene nanoparticles with tunable interfaces and softness. Polymer 2014, 55, 58–65. [Google Scholar] [CrossRef]
- Venier-Julienne, M.C.; Vouldoukis, J.; Monjour, L.; Benoit, J.P. In Vitro Study of the Anti-Leishmanial Activity of Biodegradable Nanoparticles. J. Drug Target. 1995, 3, 23–29. [Google Scholar] [CrossRef]
- Arunbabu, D.; Hazarika, M.; Naik, S.; Jana, T. Synthesis of crosslinked poly(styrene-co-divinylbenzene-co-sulfopropyl methacrylate) nanoparticles by emulsion polymerization: Tuning the particle size and surface charge density. Bull. Mater. Sci. 2009, 32, 633–641. [Google Scholar] [CrossRef][Green Version]
- Chonde, Y.; Liu, L.J.; Krieger, I.M. Preparation and surface modification of poly(vinylbenzyl chloride) latices. J. Appl. Polym. Sci. 1980, 25, 2407–2416. [Google Scholar] [CrossRef]
- Boguslavsky, L.; Baruch, S.; Margel, S. Synthesis and characterization of polyacrylonitrile nanoparticles by dispersion/emulsion polymerizationn process. J. Colloid Interface Sci. 2005, 289, 71–85. [Google Scholar] [CrossRef]
- Chen, Y.W.; Kang, E.T.; Neoh, K.G.; Greiner, A. Preparation of Hollow Silica Nanospheres by Surface-Initiated Atom Transfer Radical Polymerization on Polymer Latex Templates. Adv. Funct. Mater. 2005, 15, 113–117. [Google Scholar] [CrossRef]
- Cen, L.; Neoh, K.G.; Cai, Q.; Kang, E.T. Au-Pt bimetallic nanoparticles formation via viologen-mediated reduction on polymeric nanospheres. J. Colloid Interface Sci. 2006, 300, 190–199. [Google Scholar] [CrossRef]
- Galperin, A.; Margel, D.; Baniel, J.; Dank, G.; Biton, H.; Margel, S. Radiopaque iodinated polymeric nanoparticles for X-ray imaging applications. Biomaterials 2007, 28, 4461–4468. [Google Scholar] [CrossRef]
- Baruch-Sharon, S.; Margel, S. Synthesis and characterization of polychloromethylstyrene nanoparticles of narrow size distribution by emulsion and miniemulsion polymerization processes. Colloid Polym. Sci. 2010, 288, 869–877. [Google Scholar] [CrossRef]
- Wutzer, H.; Samhaber, W.H. Exploring the limits of emulsion polymerization for the synthesis of polymer nanoparticles. Monatsh. Chem. 2007, 138, 357. [Google Scholar] [CrossRef]
- Rajput, S.D.; Mahulikar, P.P.; Gite, V.V. Biobased dimer fatty acid containing two pack polyurethane for wood finished coatings. Prog. Org. Coat. 2014, 77, 38–46. [Google Scholar] [CrossRef]
- Former, B.M.; Holmberger, K.; Klingskog, E.G.; Bergström, K. Fatty amide ethoxylates and self-assembly. J. Surf. Det. 2001, 4, 175–183. [Google Scholar] [CrossRef]
- Madhumanchi, S.; Chakrabarti, P.P.; Rao Bhamidipati, V.S.K. Preparation and surface active properties of coconut and sunflower protein-based diethanolamides. Biomass Conv. Bioref. 2016, 6, 377–383. [Google Scholar] [CrossRef]
- Brijmohan, S.B.; Swier, S.; Weiss, R.A.; Montgomery, T.S. Synthesis and characterization of cross-linked sulfonated polystyrene nanoparticles. Ind. Eng. Chem. Res. 2005, 44, 8039–8045. [Google Scholar] [CrossRef]
- Alhassawi, H.; Romero-Zerón, L. Novel Surfactant Delivery System for Controlling SurfactantAdsorption onto Solid Surfaces. Part III: Oil Displacement Tests. Can. J. Chem. Eng. 2015, 93, 1539–1546. [Google Scholar] [CrossRef]
- Romero-Zerón, L.B.; Kittisrisawai, S. Evaluation of a surfactant carrier for the effective propagation and target release of surfactants within porous during enhanced oil recovery. Part I: Dynamic adsorption study. Fuel 2015, 148, 238–245. [Google Scholar] [CrossRef]
- Kittisrisawai, S.; Romero-Zerón, L.B. Complexation of Surfactant/β-Cyclodextrin to inhibit surfactant adsorption onto sand, kaolin, and shale for applications in enhanced oil recovery processes. Part III: Displacement evaluation. J. Surf. Det. 2015, 18, 797–809. [Google Scholar] [CrossRef]
- Tulloch, A.P.; Mazurek, M. 13C nuclear magnetic resonance spectroscopy of saturated, unsaturated, and oxygenated fatty acid methyl esters. Lipids 1976, 11, 228–234. [Google Scholar] [CrossRef]
- Schramm, L.L.; Stasiuk, E.N.; Marangoni, D.G. Surfactants and their applications. Annu. Rep. Prog. Chem. Sec. C Phys. Chem. 2003, 99, 3–48. [Google Scholar] [CrossRef]
- Al-Sahhaf, T.; Suttar, A.; Elkamel, A. Producing ultralow interfacial tension at the oil/water interface January 2002. Pet. Sci. Technol. 2002, 20, 773–788. [Google Scholar] [CrossRef]
- Yang, J.C.C.; Jablonsky, M.J.; Mays, J.W. NMR and FT-IR studies of sulfonated styrene-based homopolymers and copolymers. Polymer 2002, 43, 5125–5132. [Google Scholar] [CrossRef]
- Su, Z.; Hsu, S.L.; Li, X. Spectroscopic and thermal studies of sulfonated syndiotactic polystyrene. Macromolecules 1994, 27, 287–291. [Google Scholar] [CrossRef]
- Kai, J.; Sheng-Li, C.; Peng, D.; Renxiao, L. Synthesis of monodisperse crosslinked polystyrene microspheres. Pet. Sci. 2008, 5, 375–378. [Google Scholar] [CrossRef][Green Version]
- Turner, S.R.; Weiss, R.A.; Lundberg, R.D. The emulsion copolymerization of styrene and sodium styrene sulfonate. J. Polym. Sci. 1985, 23, 535–548. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, C.L.; Shen, S.L.; Zhang, K.; Meng, H.; Guo, K.; Chen, J.F. Effects of silica sources on the properties of magnetic hollow silica. Colloids Surf. A 2009, 334, 131–136. [Google Scholar] [CrossRef]
- Rotureau, E.; Leonard, M.; Marie, E.; Dellacherie, E.; Camesano, T.A.; Durand, A. From polymeric surfactants to colloidal systems (1): Amphiphilic dextrans for emulsion preparation. Colloids Surf. A 2006, 288, 131–137. [Google Scholar] [CrossRef]
- Rotureau, E.; Leonard, M.; Marie, E.; Dellacherie, E.; Camesano, T.A.; Durand, A. From polymeric surfactants to colloidal systems (2): Preparation of colloidal dispersions. Colloids Surf. A 2006, 288, 62–70. [Google Scholar] [CrossRef]
- Zhang, F.; Cao, L.; Yang, W. Preparation of monodisperse and anion-charged polystyrene microspheres stabilized with polymerizable sodium styrene sulfonate by dispersion polymerization. Macromol. Chem. Phys. 2010, 211, 744–751. [Google Scholar] [CrossRef]
- Arunbabu, D.; Sanga, Z.; Seenimeera, K.M.; Jana, T. Emulsion copolymerization of styrene and sodium styrene sulfonate: kinetics, monomer reactivity ratios and copolymer properties. Polym. Internat. 2008, 58, 88–96. [Google Scholar] [CrossRef]
- Bon, S.A.; Colver, P.J. Pickering miniemulsion polymerization using laponite clay as a stabilizer. Langmuir 2007, 23, 8316–8322. [Google Scholar] [CrossRef]
- Mirzataheri, M.; Mahdavian, A.R.; Atai, M. Nanocomposite particles with core-shell morphology IV: An efficient approach to the encapsulation of Cloisite 30B by poly (styrene-co-butyl acrylate) and preparation of its nanocomposite latex via miniemulsion polymerization. Colloid Polym. Sci. 2009, 287, 725–732. [Google Scholar] [CrossRef]
- Singh, V.S.; Pandey, B.R. Role of Adsorption in Improved Oil Recovery by Surfactant Flooding. J. Scient. Indus. Res. 1982, 41, 54–57. [Google Scholar]
- Leja, J. Surface Chemistry of Froth Flotation; Plenum Press: New York, NY, USA, 1982; pp. 485–498. [Google Scholar]
- Harkot, J.; Jan’czuk, B. The role of adsorption of dodecylethyldimethylammonium bromide and benzyldimethyldodecylammonium bromide surfactants in wetting of polytetrafluoroethylene and poly(methyl methacrylate) surfaces. Appl. Surf. Sci. 2009, 255, 3623–3628. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Liu, J.; Sun, D.; Yin, B.; Wang, X. Adsorption kinetic of 3-alkoxy-2-hydroxypropyl trimethylammonium chloride at oil-water interface. Appl. Surf. Sci. 2012, 261, 237–241. [Google Scholar] [CrossRef]
- Somasundaran, P.; Huang, U.L. Adsorption/aggregation of surfactants and their mixtures at solid liquid interfaces. Adv. Colloid Interface Sci. 2000, 88, 179–208. [Google Scholar] [CrossRef]
- Prado-Herrero, P.; Garcia-Guinea, J.; Crespo-Feo, E.; Correcher, V. Temperature-induced transformation of metavariscite to berlinite. Phase Transit. 2010, 83, 440–449. [Google Scholar] [CrossRef]
- Gregora, I.; Magneron, N.; Simon, P.; Luspin, Y.; Raimboux, N.; Philippot, E. Raman study of AlPO4 (berlinite) at the α-β transition. J. Phys. Condens. Matter 2003, 15, 4487. [Google Scholar] [CrossRef]
- Chen, C.; Feng, B.; Hu, S.; Zhang, Y.; Li, S.; Gao, L.; Zhang, X.; Yu, K. Control of aluminum phosphate coating on mullite fibers by surface modification with polyethylenimine. Ceram. Int. 2018, 44, 216–224. [Google Scholar] [CrossRef]
- Olajire, A.A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Tabatabal, A.; Gonzalez, M.V.; Harwell, J.H.; Scamehorn, J.F. Reducing Surfactant Adsorption in Carbonate Reservoirs. SPE Reserv. Eng. 1993, 8, 117–122. [Google Scholar]
- Zhang, R.; Somasundaran, P. Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Adv. Colloid Interface Sci. 2006, 123, 213–229. [Google Scholar] [CrossRef]
- Bera, A.; Kumar, T.; Ojha, K.; Mandal, A. Adsorption of surfactants on sand surface in enhanced oil recovery: Isotherms, kinetics and thermodynamic studies. Appl. Surf. Sci. 2013, 284, 87–99. [Google Scholar] [CrossRef]
- Markowitz, M.A.; Klaehn, J.; Hendel, R.A.; Qadriq, S.B.; Golledge, S.L.; Castner, D.G.; Gaber, B.P. Direct synthesis of metal-chelating mesoporous silica: Effects of added organosilanes on silicate formation and adsorption properties. J. Phys. Chem. B 2000, 104, 10820–10826. [Google Scholar] [CrossRef]
- Somasundaran, P.; Krishnakumar, S. Adsorption of surfactants and polymers at the solid-liquid interface. Colloids Surf. A 1997, 123, 491–513. [Google Scholar] [CrossRef]
- Brinck, J.; Jönsson, B.; Tiberg, F. Kinetics of nonionic surfactant adsorption and desorption at the silica-water interface: One component. Langmuir 1998, 14, 1058–1071. [Google Scholar] [CrossRef]
- Rodriguez Pin, E.; Roberts, M.; Yu, H.; Huh, C.; Bryant, S.L. Enhanced migration of surface-treated nanoparticles in sedimentary rocks. In SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009; Society of Petroleum Engineers: Richardson, TX, USA, 2009. [Google Scholar] [CrossRef]


| Sample | OADA 1 (g) | St 2 (mL) | St-S 3 (g) | St-S 3 (mol % of Total Monomer) |
|---|---|---|---|---|
| 0sulf10 | 0.2888 (10CMC 4) | 10.00 | - | 0 |
| 0sulf20 | 0.5779 (20CMC 4) | 10.00 | - | 0 |
| 12sulf20 | 0.5779 (20CMC 4) | 10.00 | 0.022 | 0.012 |
| 30sulf20 | 0.5779 (20CMC 4) | 9.99 | 0.054 | 0.030 |
| Sample | OADA | DVB (v/v) % | Particle Size (nm) | PdI | IFT (mN·m−1) |
|---|---|---|---|---|---|
| 01 | 10CMC | 0.5 | 192 | 0.150 | 35.1 |
| 02 | 10CMC | 1.5 | 176 | 0.282 | 17.9 |
| 03 | 10CMC | 3.0 | 162 | 0.274 | 4.3 |
| 04 | 10CMC | 4.5 | 128 | 0.226 | 12.1 |
| 05 | 10CMC | 6.0 | 127 | 0.180 | 26.5 |
| 06 | 10CMC | 9.0 | 138 | 0.136 | 29.7 |
| 07 | 20CMC | 3.0 | 114 | 0.204 | 2.8 |
| System | Particle Size (nm) | Retention% | Qe (mg/g) |
|---|---|---|---|
| 0sulf10 | 162 | 56.2 | 17.2 |
| 0sulf20 | 114 | 35.0 | 21.6 |
| 12sulf20 | 66 | 34.4 | 21.2 |
| 30sulf20 | 132 | 19.8 | 12.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
P. C. Caplan, S.; B. G. Silva, T.; D. S. Franscisco, A.; R. Lachter, E.; S. V. Nascimento, R. Sulfonated Polystyrene Nanoparticles as Oleic Acid Diethanolamide Surfactant Nanocarriers for Enhanced Oil Recovery Processes. Polymers 2019, 11, 1513. https://doi.org/10.3390/polym11091513
P. C. Caplan S, B. G. Silva T, D. S. Franscisco A, R. Lachter E, S. V. Nascimento R. Sulfonated Polystyrene Nanoparticles as Oleic Acid Diethanolamide Surfactant Nanocarriers for Enhanced Oil Recovery Processes. Polymers. 2019; 11(9):1513. https://doi.org/10.3390/polym11091513
Chicago/Turabian StyleP. C. Caplan, Shalimar, Thaís B. G. Silva, Agatha D. S. Franscisco, Elizabeth R. Lachter, and Regina S. V. Nascimento. 2019. "Sulfonated Polystyrene Nanoparticles as Oleic Acid Diethanolamide Surfactant Nanocarriers for Enhanced Oil Recovery Processes" Polymers 11, no. 9: 1513. https://doi.org/10.3390/polym11091513
APA StyleP. C. Caplan, S., B. G. Silva, T., D. S. Franscisco, A., R. Lachter, E., & S. V. Nascimento, R. (2019). Sulfonated Polystyrene Nanoparticles as Oleic Acid Diethanolamide Surfactant Nanocarriers for Enhanced Oil Recovery Processes. Polymers, 11(9), 1513. https://doi.org/10.3390/polym11091513