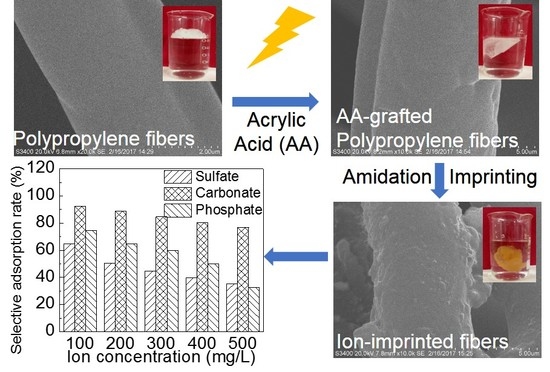
Ion-Imprinted Polypropylene Fibers Fabricated by the Plasma-Mediated Grafting Strategy for Efficient and Selective Adsorption of Cr(VI)
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Instruments
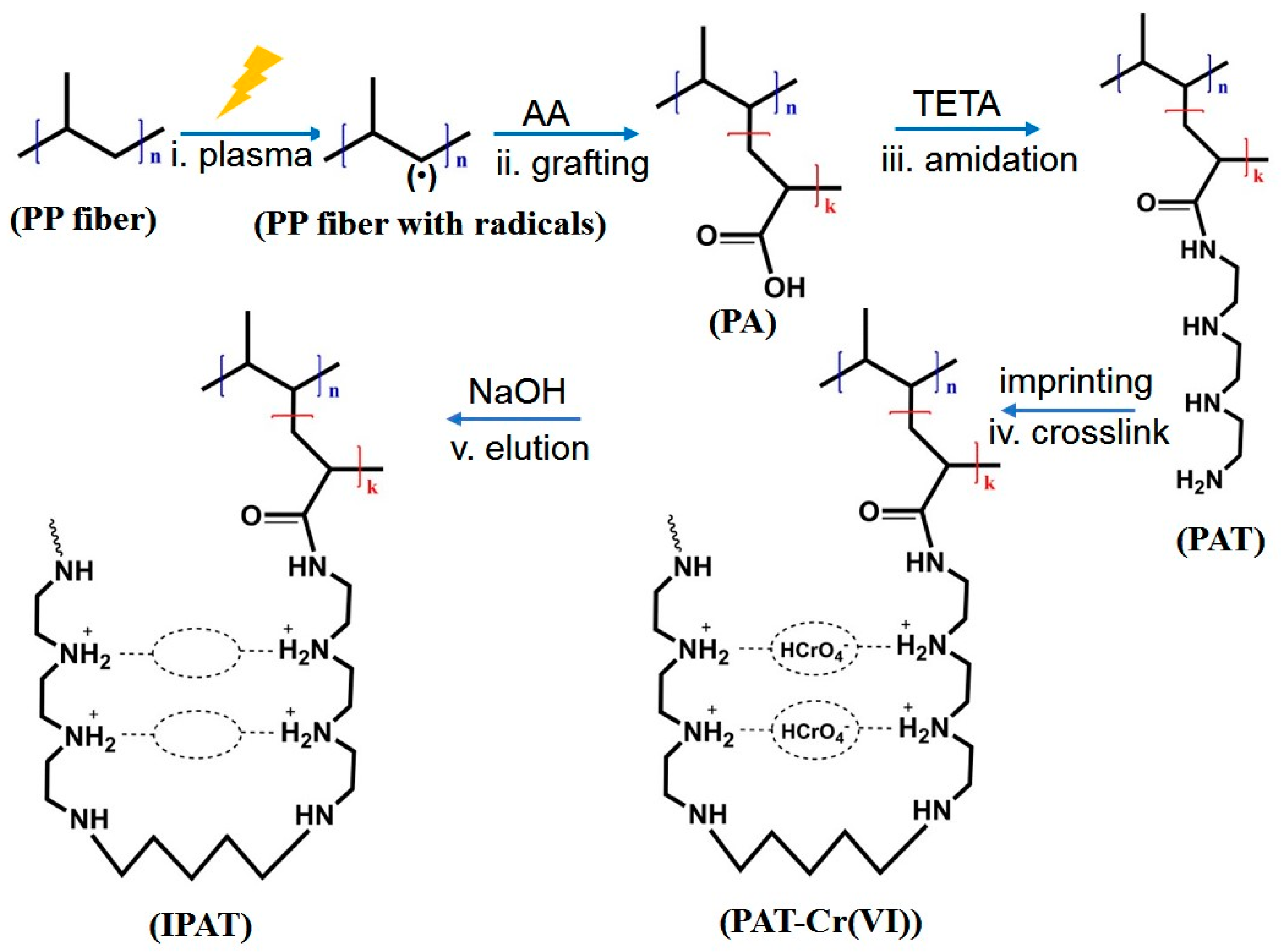
2.2. Preparation of Ion-Imprinted Fibers
2.3. Analytic Methods
2.3.1. Grafting Degree
2.3.2. Amino Content
2.3.3. Crosslink Degree of IPAT
2.4. Characterizations
2.5. Adsorption and Regeneration
3. Results and Discussion
3.1. Optimization of Preparation Conditions
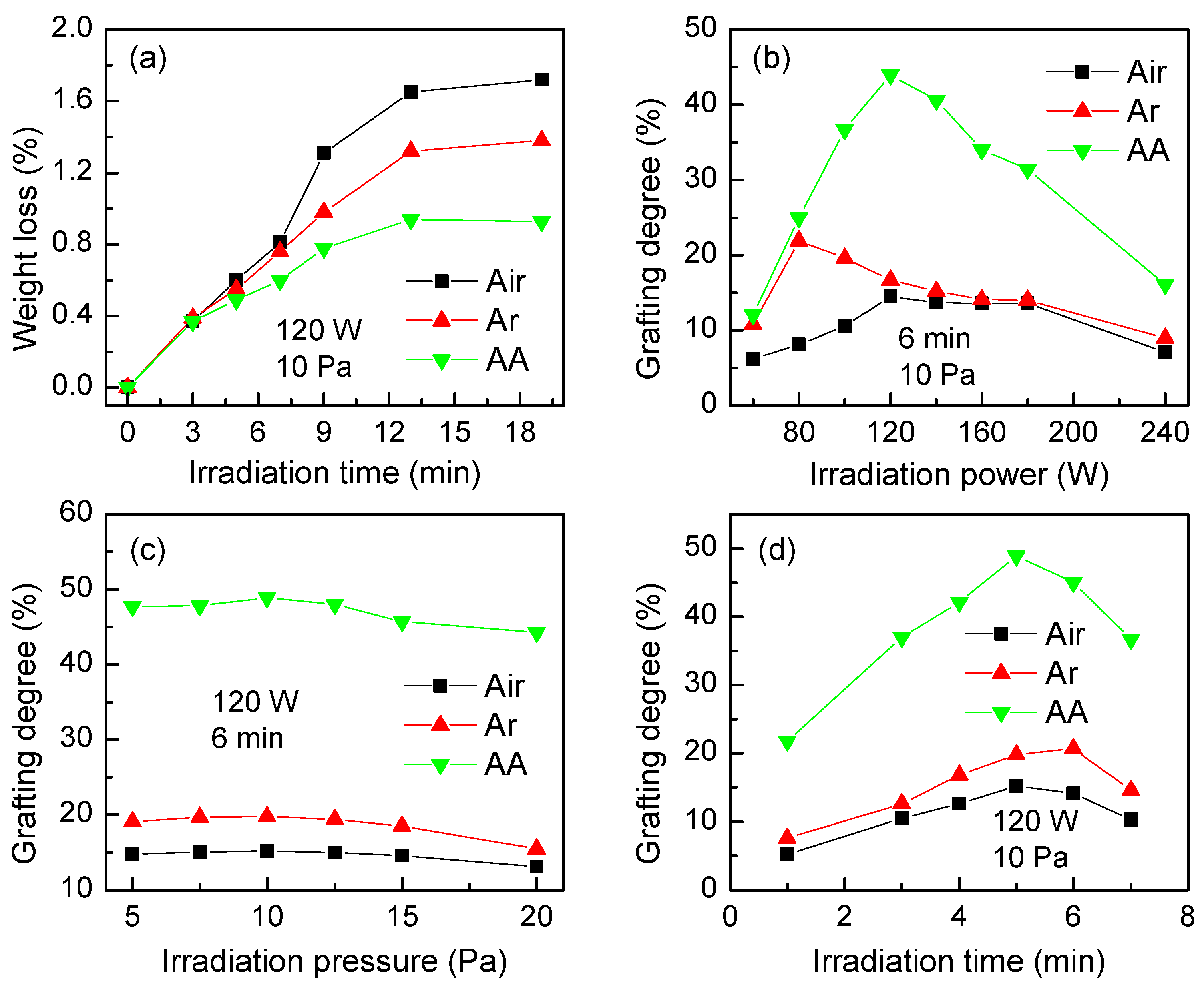
3.1.1. Plasma Irradiation
3.1.2. Grafting Conditions
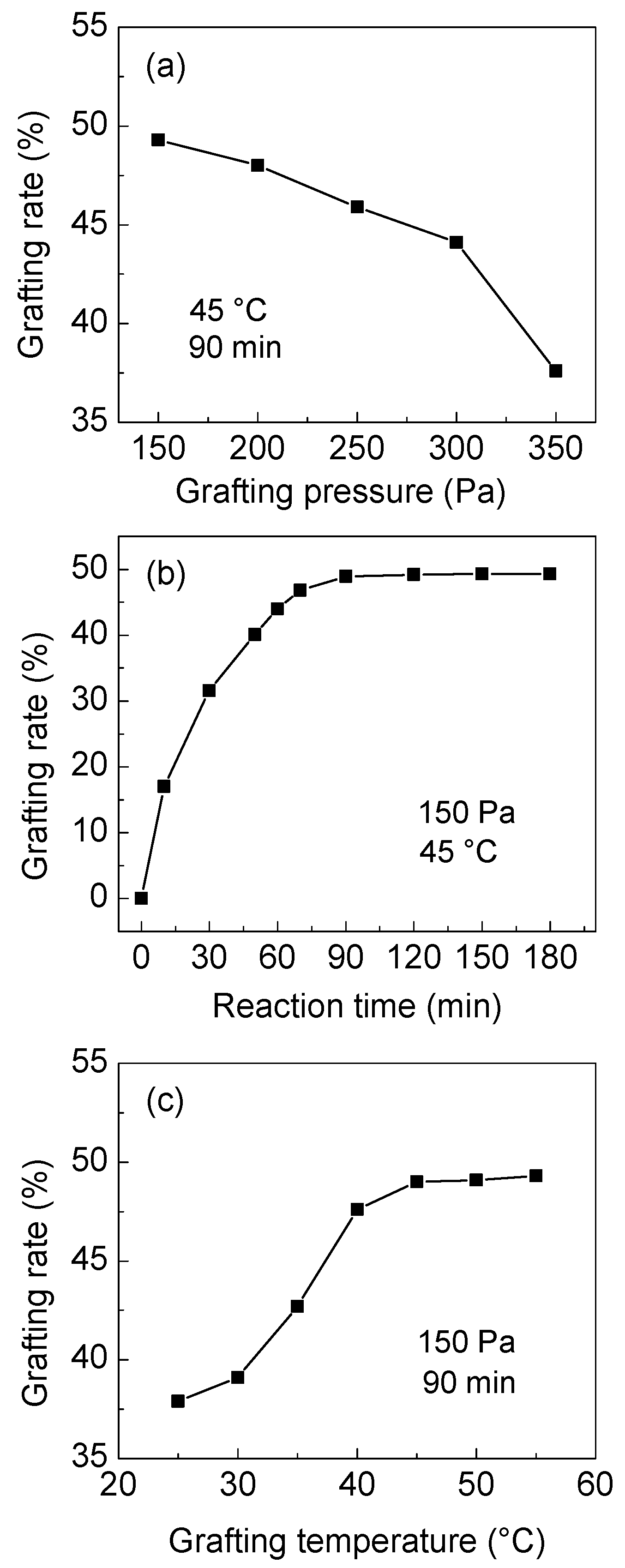
3.1.3. Amidation Efficiency of PAT and Grafting Degree of PA
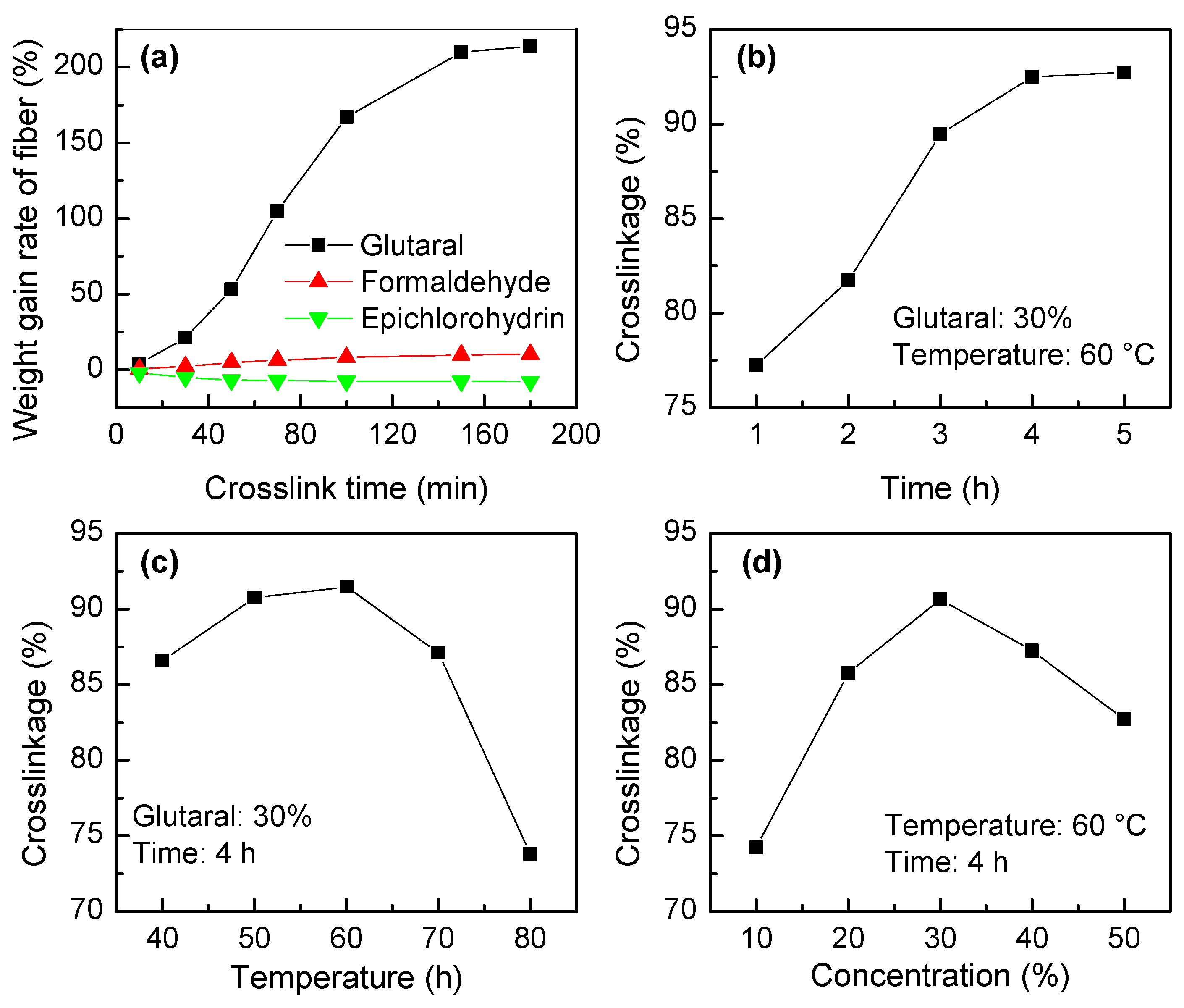
3.1.4. Crosslinking Reaction
3.2. Characterization
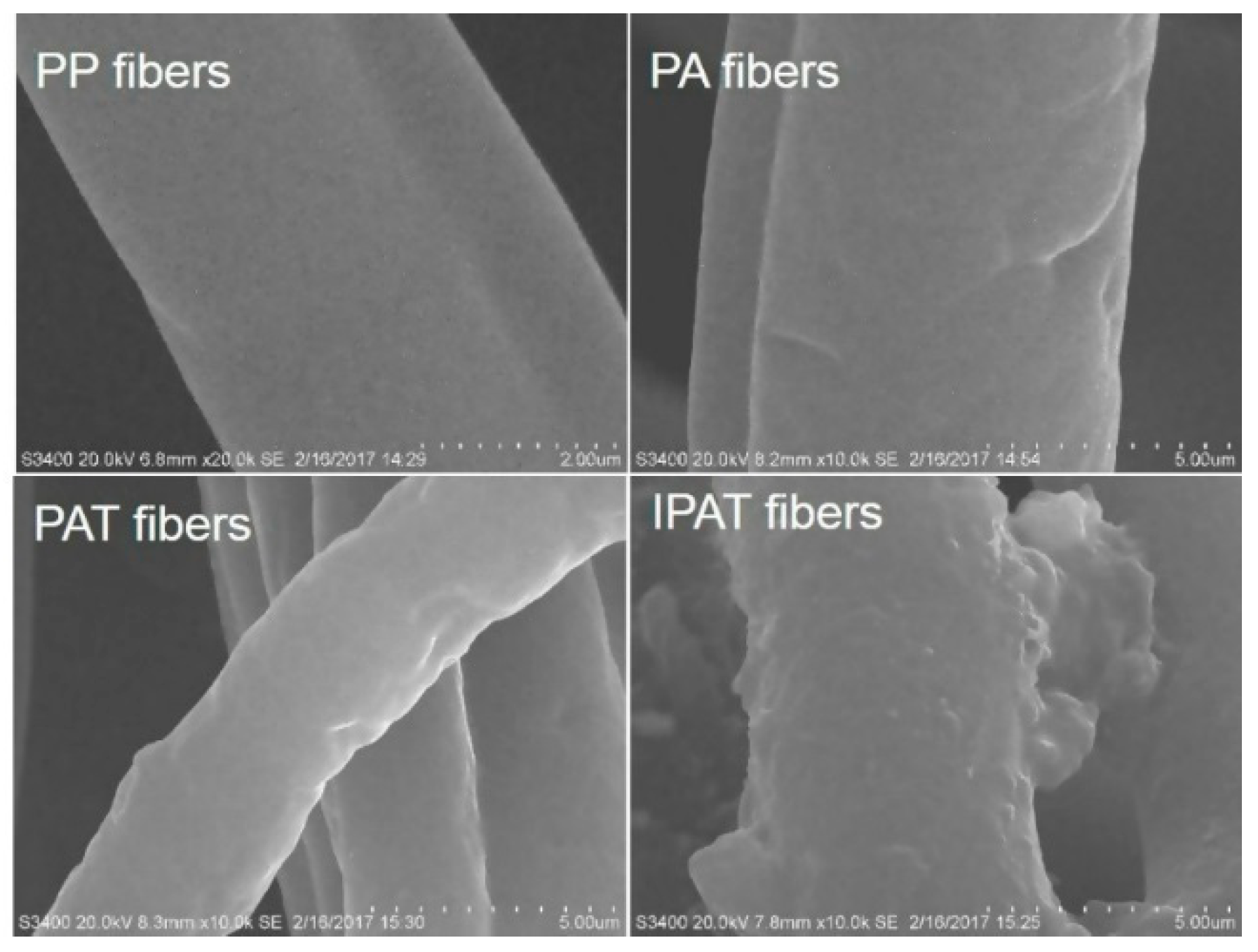
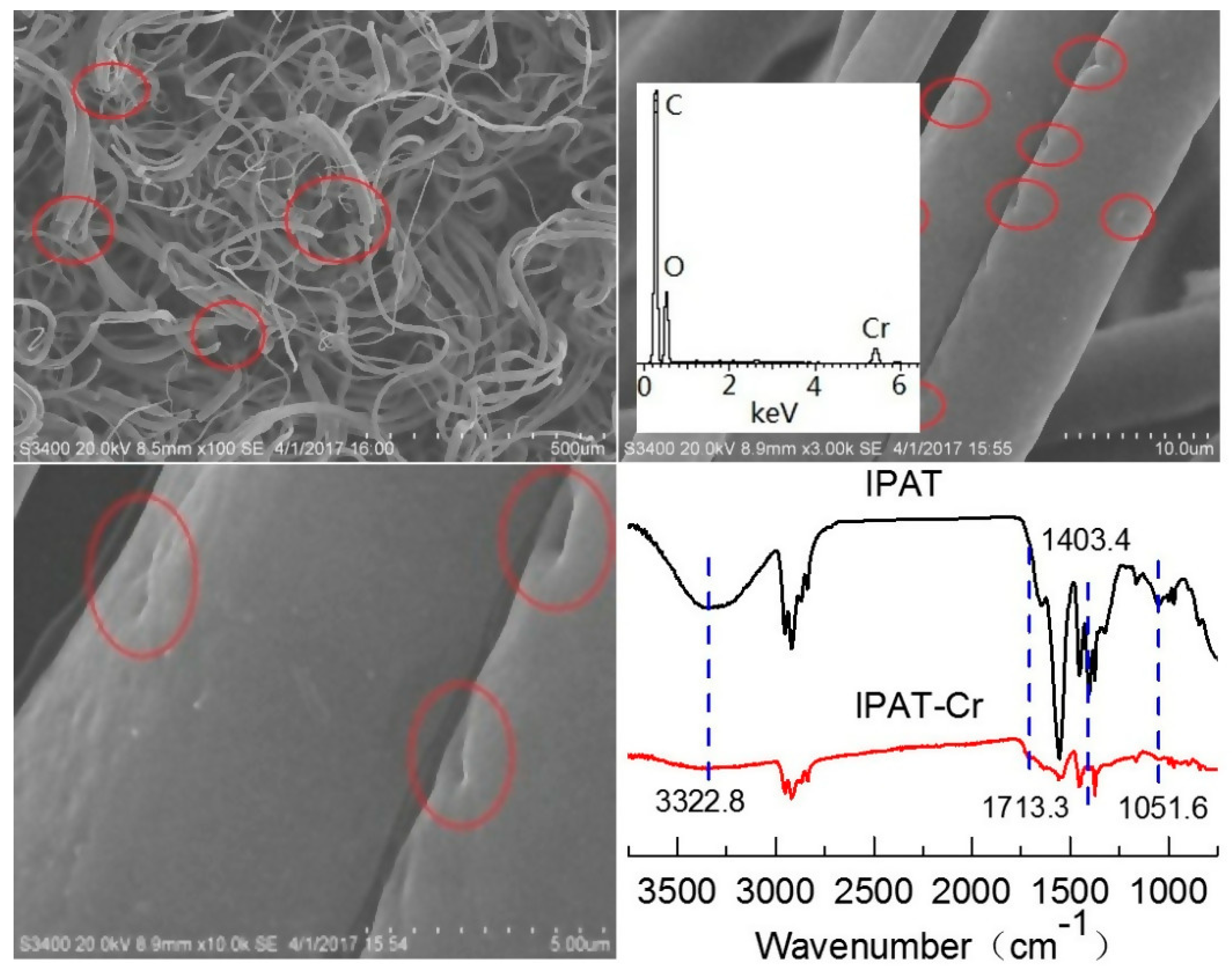
3.2.1. SEM
3.2.2. FTIR
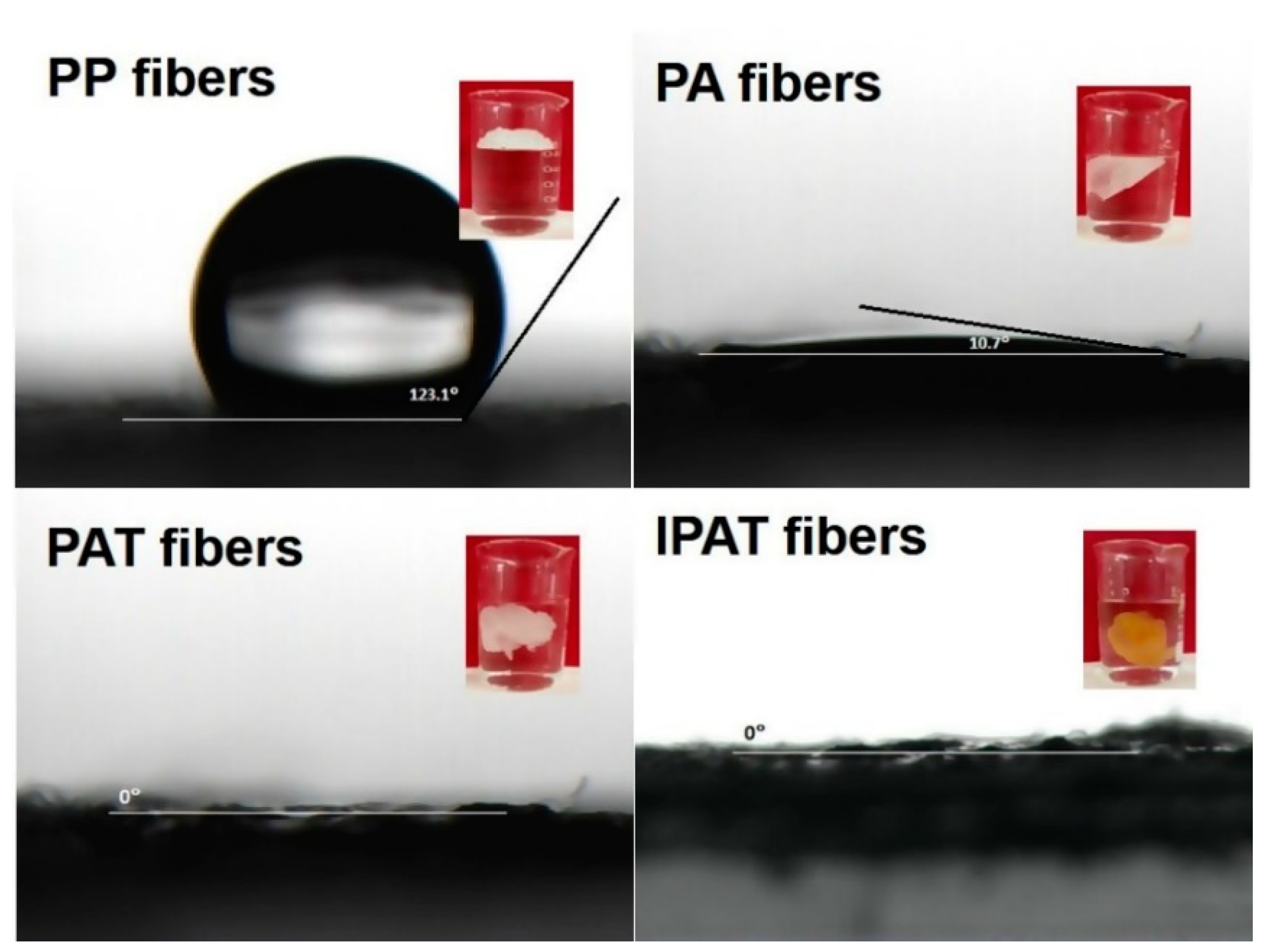
3.2.3. Water Contact Angle
3.3. Adsorption of Cr(VI)
3.3.1. Effect of pH on the Adsorption Capacity of IPAT
3.3.2. Adsorption Kinetics
3.3.3. Adsorption Isotherm
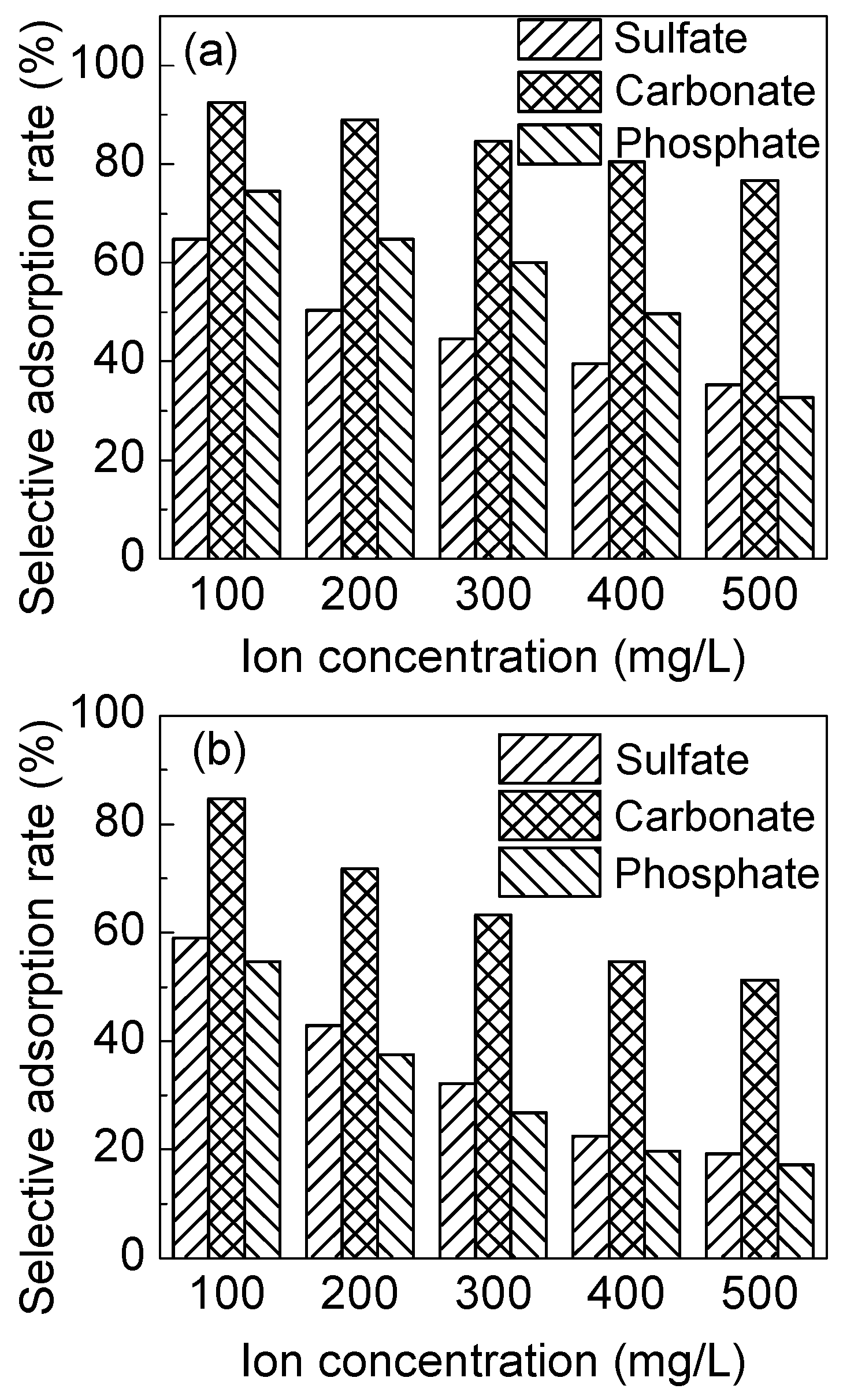
3.3.4. Adsorption Selectivity
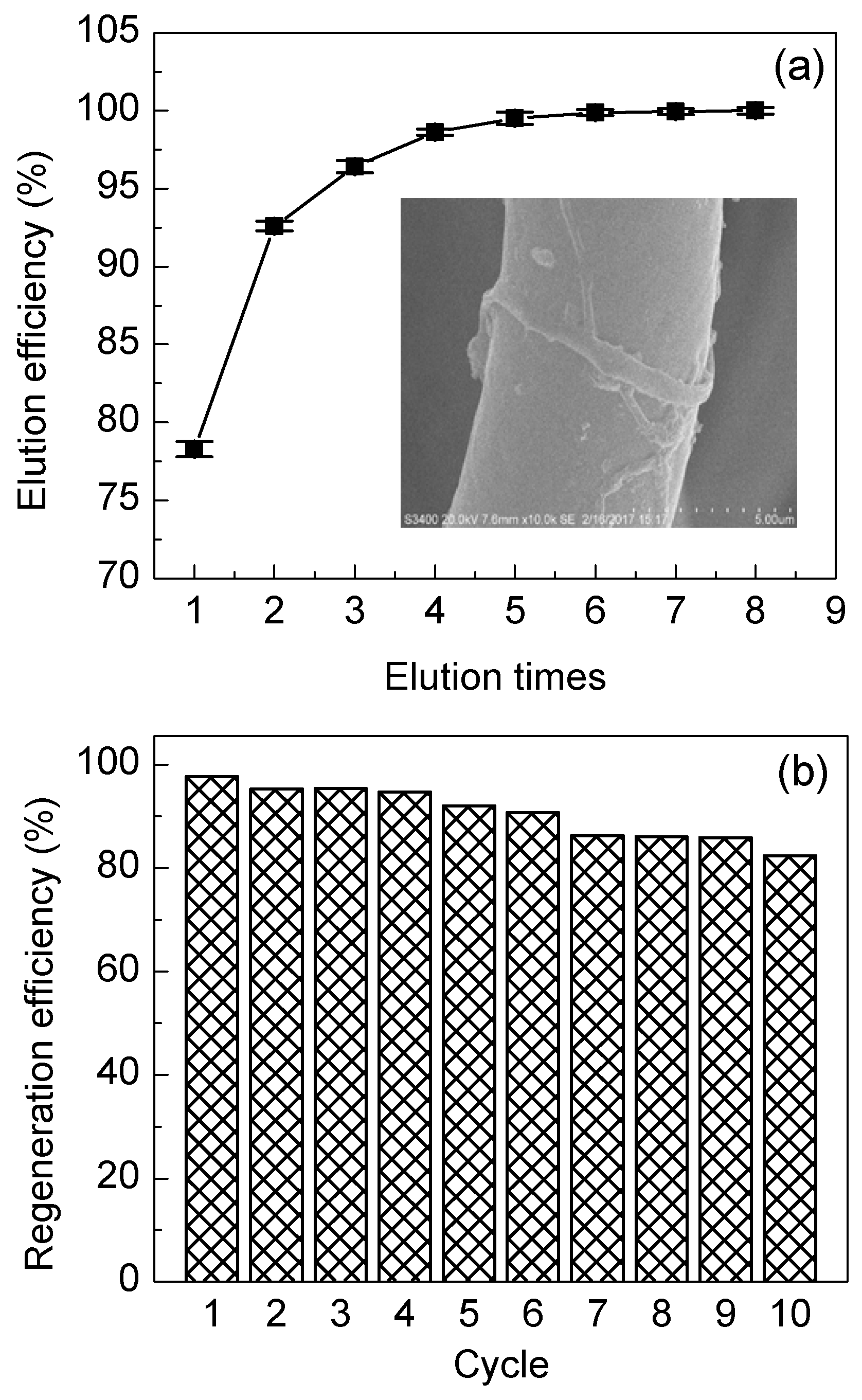
3.3.5. Desorption and Regeneration
3.3.6. The Effect of the Oxidation of Cr(VI) on the IPAT Structural Integrity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kimbrough, D.E.; Cohen, Y. A critical assessment of chromium in the environment. Crit. Rev. Environ. Control 1999, 29, 1–46. [Google Scholar] [CrossRef]
- Dubey, S.P.; Gopal, K. Adsorption of chromium(VI) on low cost adsorbents derived from agricultural waste material: A comparative study. J. Hazard. Mater. 2007, 145, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Erdem, M.; Tumen, F. Chromium removal from aqueous solution by the ferrite process. J. Hazard. Mater. 2004, 109, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.S.W.; Poddar, T.K. New membrane technology for removal and recovery of chromium from waste waters. Environ. Prog. Sustain. 2000, 20, 44–52. [Google Scholar] [CrossRef]
- Luo, S.; Qin, F. Fabrication uniform hollow Bi2S3 nanospheres via Kirkendall effect for photocatalytic reduction of Cr(VI) in electroplating industry wastewater. J. Hazard. Mater. 2017, 340, S508503713X. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, K.; Jha, M. Biosorption of Cr(VI) from aqueous solutions by Eichhornia crassipes. Chem. Eng. J. 2006, 117, 71–77. [Google Scholar] [CrossRef]
- Wang, W.; Li, M. Column adsorption of chromium(VI) by strong alkaline anion-exchange fiber. J. Appl. Polym. Sci. 2012, 126, 1733–1738. [Google Scholar] [CrossRef]
- Dognani, G.; Hadi, P. Effective chromium removal from water by polyaniline-coated electrospun adsorbent membrane. Chem. Eng. J. 2019, 372, 341–351. [Google Scholar] [CrossRef]
- Hayashi, N.; Chen, J. Nitrogen-containing fabric adsorbents prepared by radiation grafting for removal of chromium from wastewater. Polymers 2018, 10, 744. [Google Scholar] [CrossRef]
- Huang, R.; Ma, X. A novel ion-imprinted polymer based on graphene oxide-mesoporous silica nanosheet for fast and efficient removal of chromium (VI) from aqueous solution. J. Colloid Interface Sci. 2018, 514, 544–553. [Google Scholar] [CrossRef]
- Wei, Q.F.; Mather, R.R. Functional nanostructures generated by plasma-enhanced modification of polypropylene fibre surfaces. J. Mater. Sci. 2005, 40, 5387–5392. [Google Scholar] [CrossRef]
- Gul Dincmen, M.; Hauser, P.J. Plasma induced graft polymerization of three hydrophilic monomers on nylon 6,6 Fabrics for enhancing antistatic property. Plasma Chem. Plasma Proc. 2016, 36, 1377–1391. [Google Scholar] [CrossRef]
- Basarir, F.; Choi, E.Y. Electrochemical properties of PP membranes with plasma polymer coatings of acrylic acid. J. Membrane Sci. 2005, 260, 66–74. [Google Scholar] [CrossRef]
- Chen, H.; Guo, M. Green and efficient synthesis of an adsorbent fiber by plasma-induced grafting of glycidyl methacrylate and its Cd(II) adsorption performance. Fibers Polym. 2018, 19, 722–733. [Google Scholar] [CrossRef]
- Tseng, C.; Wang, C. Polypropylene fibers modified by plasma treatment for preparation of Ag nanoparticles. J. Phys. Chem. B 2006, 110, 4020–4029. [Google Scholar] [CrossRef]
- Haji, A.; Shoushtari, A.M. Grafting of poly(propylene imine) dendrimer on polypropylene nonwoven: Preparation optimization, characterization, and application. Fibers Polym. 2019, 20, 913–921. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, H. Surface grafting of styrene on polypropylene by argon plasma and its adsorption and regeneration of BTX. J. Appl. Polym. Sci. 2018, 135, 46171. [Google Scholar] [CrossRef]
- Noor, S.; Xiakeer, S. Controlled levofloxacin release and antibacterial properties of β-cyclodextrins-grafted polypropylene mesh devices for hernia repair. Polymers 2018, 10, 493. [Google Scholar]
- Khlyustova, A.; Galmiz, O. Underwater discharge plasma-induced coating of poly(acrylic acid) on polypropylene fiber. J. Mater. Sci. 2015, 50, 3504–3509. [Google Scholar] [CrossRef]
- Buček, A.; Popelka, A. Acrylic acid plasma treatment of polypropylene nonwoven fabric. Fibres Text. East. Eur. 2016, 24, 161–164. [Google Scholar] [CrossRef][Green Version]
- Sciarratta, V.; Vohrer, U. Plasma functionalization of polypropylene with acrylic acid. Surf. Coat. Technol. 2003, 174–175, 805–810. [Google Scholar] [CrossRef]
- Nishide, H.; Tsuchida, E. Selective adsorption of metal ions on poly(4-vinylpyridine) resins in which the ligand chain is immobilized by crosslinking. Macromol. Chem. Phys. 1976, 177, 2295–2310. [Google Scholar] [CrossRef]
- Li, T.; Chen, S. Preparation of an ion-imprinted fiber for the selective removal of Cu2+. Langmuir 2011, 27, 6753–6758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, S. Surface ion-imprinted polypropylene nonwoven fabric for potential uranium seawater extraction with high selectivity over vanadium. Ind. Eng. Chem. Res. 2017, 56, 1860–1867. [Google Scholar] [CrossRef]
- Yang, S.; Ji, G. Polypropylene nonwoven fabric modified with oxime and guanidine for antibiofouling and highly selective uranium recovery from seawater. J. Radioanal. Nucl. Chem. 2019, 321, 323–332. [Google Scholar] [CrossRef]
- Guo, M.; Liang, H. Study on melt-blown processing, web structure of polypropylene nonwovens and its BTX adsorption. Fibers Polym. 2016, 17, 257–265. [Google Scholar] [CrossRef]
- Černáková, L.; Kováčik, D. Surface modification of polypropylene non-woven fabrics by atmospheric-pressure plasma activation followed by acrylic acid grafting. Plasma Chem. Plasma Proc. 2005, 25, 427–437. [Google Scholar] [CrossRef]
- Altay, L.; Bozaci, E. The effect of atmospheric plasma treatment of recycled carbon fiber at different plasma powers on recycled carbon fiber and its polypropylene composites. J. Appl. Polym. Sci. 2019, 136, 47131. [Google Scholar] [CrossRef]
- Hassanpour, S.; Taghizadeh, M. Magnetic Cr(VI) ion imprinted polymer for the fast selective adsorption of Cr(VI) from aqueous solution. J. Polym. Environ. 2018, 26, 101–115. [Google Scholar] [CrossRef]
- Wu, S.; Dai, X. Highly sensitive and selective ion-imprinted polymers based on one-step electrodeposition of chitosan-graphene nanocomposites for the determination of Cr(VI). Carbohydr. Polym. 2018, 195, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Huang, Z. Bagasse cellulose grafted with an amino-terminated hyperbranched polymer for the removal of Cr(VI) from aqueous solution. Polymers 2018, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Pakade, V.; Cukrowska, E. Selective removal of chromium (VI) from sulphates and other metal anions using an ion-imprinted polymer. Water Sa 2011, 37, 529–538. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Arica, M.Y. Synthesis of Cr(VI)-imprinted poly(4-vinyl pyridine-co-hydroxyethyl methacrylate) particles: Its adsorption propensity to Cr(VI). J. Hazard. Mater. 2011, 187, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Tavengwa, N.T.; Cukrowska, E. Synthesis, adsorption and selectivity studies of N-propyl quaternized magnetic poly(4-vinylpyridine) for hexavalent chromium. Talanta 2013, 116, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Zhang, F. Fast removal of Cr(VI) from aqueous solution using Cr(VI)-imprinted polymer particles. Ind. Eng. Chem. Res. 2014, 53, 4434–4441. [Google Scholar] [CrossRef]
- Velempini, T.; Pillay, K. Epichlorohydrin crosslinked carboxymethyl cellulose-ethylenediamine imprinted polymer for the selective uptake of Cr(VI). Int. J. Biol. Macromol. 2017, 101, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, M.; Samadi, S. Selective adsorption of Cr(VI) ions from aqueous solutions using Cr6+-imprinted Pebax/chitosan/GO/APTES nanofibrous adsorbent. Int. J. Biol. Macromol. 2017, 95, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Xu, X. FTIR, Raman, and XPS analysis during phosphate, nitrate and Cr(VI) removal by amine cross-linking biosorbent. J. Colloid Interf. Sci. 2016, 468, 313–323. [Google Scholar] [CrossRef] [PubMed]






| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| Qm (mg/g) | KL (L/mg) | R2 | n | KF (mg/g) | R2 |
| 238.1 | 0.024 | 0.993 | 4.76 | 62.8 | 0.996 |
| Adsorbents | Morphology | qmax (mg/g) | References |
|---|---|---|---|
| styrene/4-VP/EDGMA | Particles 53–90 μm | 37.58 | [32] |
| 4-VP/HEMA | Particles 75–150 μm | 172.12 | [33] |
| Magnetic poly(4-VP) | - | 6.20 | [34] |
| 4-VP/EDGMA | Particles 50–100 μm | 286.56 | [35] |
| SCC/Epichlorohydrin | - | 177.62 | [36] |
| Magnetic | Particles ~70 nm | 39.3 | [29] |
| Pebax/chitosan/GO/APTES | Nanofibers ~90 nm | 204 5 | [37] |
| IPAT | Fiberous ~7 μm | 167 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Xu, J.; Zhu, D.; Wang, D.; Xu, J.; Jiang, H.; Geng, W.; Wei, W.; Lian, Z. Ion-Imprinted Polypropylene Fibers Fabricated by the Plasma-Mediated Grafting Strategy for Efficient and Selective Adsorption of Cr(VI). Polymers 2019, 11, 1508. https://doi.org/10.3390/polym11091508
Luo Z, Xu J, Zhu D, Wang D, Xu J, Jiang H, Geng W, Wei W, Lian Z. Ion-Imprinted Polypropylene Fibers Fabricated by the Plasma-Mediated Grafting Strategy for Efficient and Selective Adsorption of Cr(VI). Polymers. 2019; 11(9):1508. https://doi.org/10.3390/polym11091508
Chicago/Turabian StyleLuo, Zhengwei, Jiahuan Xu, Dongmei Zhu, Dan Wang, Jianjian Xu, Hui Jiang, Wenhua Geng, Wuji Wei, and Zhouyang Lian. 2019. "Ion-Imprinted Polypropylene Fibers Fabricated by the Plasma-Mediated Grafting Strategy for Efficient and Selective Adsorption of Cr(VI)" Polymers 11, no. 9: 1508. https://doi.org/10.3390/polym11091508
APA StyleLuo, Z., Xu, J., Zhu, D., Wang, D., Xu, J., Jiang, H., Geng, W., Wei, W., & Lian, Z. (2019). Ion-Imprinted Polypropylene Fibers Fabricated by the Plasma-Mediated Grafting Strategy for Efficient and Selective Adsorption of Cr(VI). Polymers, 11(9), 1508. https://doi.org/10.3390/polym11091508