1. Introduction
The urinary bladder epithelium, called the urothelium, is considered to be one of the most impermeable epithelia in the body and it is a very effective blood–urine barrier [
1,
2]. The urothelium consists of three cell layers: superficial, intermediate, and basal cells. Under physiological conditions, the urothelium has a very low turnover rate. However, after an injury, undifferentiated basal and partially differentiated intermediate cells rapidly proliferate and differentiate into terminally differentiated superficial cells, also called umbrella cells [
3,
4,
5].
Due to its mucoadhesion, permeability enhancement, and antimicrobial activity, chitosan is widely used in medical research [
6,
7]. Many drug delivery systems with chitosan intended for intravesical application have been developed, aiming to increase the residence time of the drug in the bladder, ensure better delivery of the drug to the target cells, and avoid systemic side effects [
8,
9,
10,
11]. Our previous studies showed that chitosan induces desquamation of the urothelial cells, followed by rapid regeneration of the urothelium in ex vivo and in vivo conditions [
4,
5]. The influence of chitosan on the urothelial structure and function is dependent on time and concentration, with more pronounced damage at longer exposure and higher concentrations of the polymer [
12]. Desquamation of the urothelium induced by chitosan could be beneficial in the case of recurrent urinary tract infections. During urinary tract infection, uropathogenic
Escherichia coli can persist within the urothelial cells, and these latent intracellular reservoirs are not affected by antibiotic treatments [
13]. The repeated use of chitosan together with quinolone antibiotic ciprofloxacin eradicated uropathogenic
Escherichia coli, which could prevent relapsing bacteriuria [
14].
The polymer charge plays an important role in the ability of the polymer to interact with the urothelial surface. In ex vivo experiments, positively charged polymers such as poly-L-arginine and chitosan had the most pronounced influence on the permeability of the urothelium [
15]. In a study performed on the intestinal Caco-2 cell line, all polycations investigated (chitosan, polyethylenimine, and poly-L-lysine) induced an increase in tight junction permeability associated with changes in the F-actin cytoskeleton and in the localization of tight junctional proteins [
16].
In spite of the fact that chitosan proved to be very effective as a co-treatment with antibiotics in fighting bacterial cystitis [
14], the low pH value around 4.5 required to disperse chitosan was found to cause discomfort in human patients and required analgesic treatment (unpublished results). Thus, the aim of our study was to establish whether the cationic poly (amino acid) poly-L-lysine (PLL), which can be dispersed at physiological pH value, could be used as a desquamation inducer of the urothelium as an alternative to chitosan. We aimed to establish how the urothelial barrier function and structure are influenced by the polymer’s concentration, molecular weight, and treatment time. To be used as a desquamation inducer, poly-L-lysine should mainly exfoliate the superficial urothelial cells, and rapid restoration of urothelial function and structure should occur after the treatment. The function of the urothelium was evaluated ex vivo by measuring transepithelial electrical resistance using side-by-side diffusion cells (Ussing chambers), and urothelial structure was analyzed after ex vivo experiments and after in vivo exposure to poly-L-lysine by scanning electron microscopy.
2. Material and Methods
2.1. Materials
Poly-L-lysine hydrobromide (PLL) with molecular weights 30–70 kDa and 70–150 kDa were obtained from Sigma–Aldrich Chemie (Steinheim, Germany). Dispersions of PLL at 0.001% and 0.01% (w/v) were prepared in a phosphate buffer (PB), which consisted of 0.67 g of Na2HPO4, 0.19 g of KH2PO4, and 8.00 g of NaCl in 1 l of deionized water. The pH of the dispersions was adjusted to 6.5.
2.2. Animals for Ex Vivo Experiments
Nine young adult (8 to 9 weeks old) female Wistar rats (Harlan, Italy) were used for ex vivo experiments. The rats were housed at the Medical Experimental Center at the Faculty of Medicine (Ljubljana, Slovenia) in open-bar cages (two to five per cage) with bedding (Lignocel 3/4, Germany) and under controlled conditions of temperature (22 ± 1 °C) and humidity (55 ± 10%), and a 12 h/12 h light–dark cycle (light from 7 am to 7 pm) with unlimited access to food (standard rodent diet; Mucerola, Italy) and tap water. All procedures involving rats were approved by the National Ethical Committee and the Administration of the Republic of Slovenia for Food Safety, Veterinary and Plant Protection (permit number U34401-4/2013/3). Animal care and treatment were in accordance with Slovenian and international legislation and policy (Directive 2010/63/EU) on the protection of animals used for research purposes.
2.3. Ex Vivo Experiments
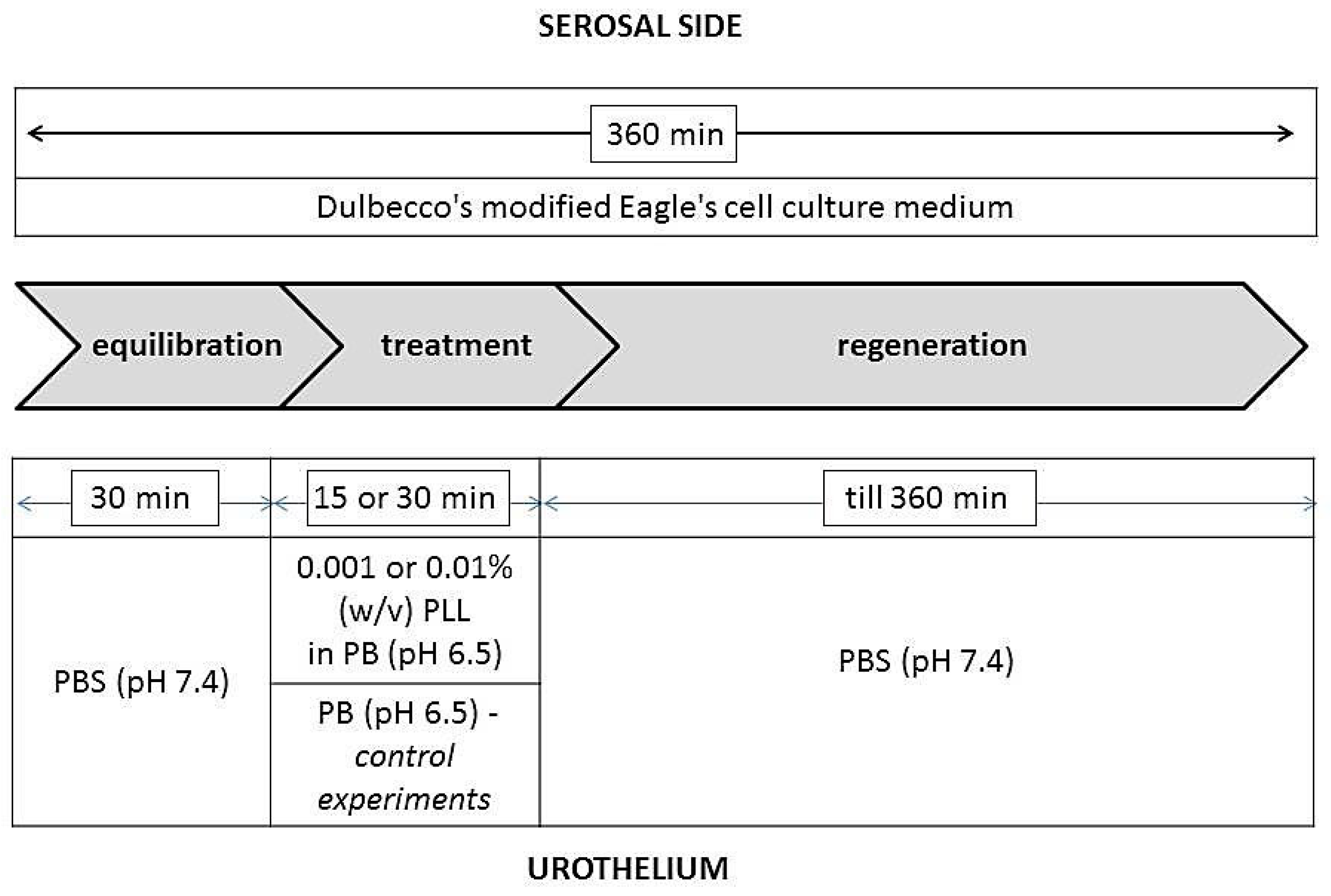
After the rats were sacrificed by CO2 inhalation, their urinary bladders were isolated and immediately placed in Dulbecco’s modified Eagle’s cell culture medium (DMEM; Sigma–Aldrich Chemie, Steinheim, Germany). The corpus of the urinary bladder was halved and the tissue was first placed onto an insert with a circular aperture (3 mm diameter, 0.07 cm² exposure area). Of the two rodent species only rat urinary bladders were used for transepithelial electrical resistance (TEER) measurement, because bladder halves of these animals entirely covered the aperture in the insert of the diffusion chamber and by this enabled the TEER measurements. Mouse bladders were found to be too small and the TEER measurements were not possible due to leakage at the edge of the aperture in the insert. No pharmacological agents were used to relax the bladder smooth muscles. The insert was then placed between EasyMount® half-chambers of an Ussing chamber (Physiologic Instruments, San Diego, CA, USA). In diffusion chambers, the temperature was maintained at 37 °C and the incubation solutions were constantly oxygenated and stirred by gas (95% O2, 5% CO2).
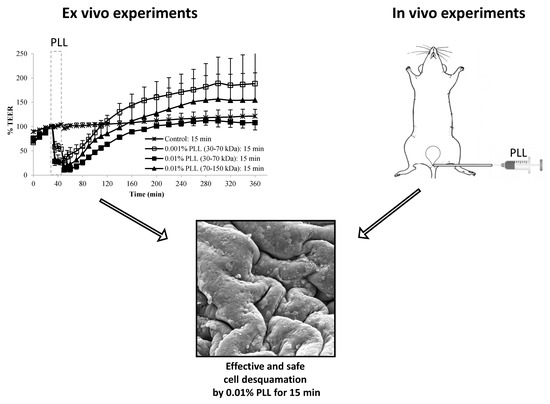
During the entire duration of the experiments, the serosal side of the tissue was exposed to DMEM, and the urothelium was exposed to various media as shown in
Figure 1. During the treatment period, the urothelium was exposed to a 0.001% or 0.01% (
w/
v) dispersion of PLL with pH 6.5 and a molecular weight of 30 to 70 kDa or 70 to 150 kDa. In the control experiments, the urothelium was exposed to PB with pH 6.5 because PLL dispersions were prepared in this buffer. The duration of the treatment period was 15 or 30 min. The experiments were performed in three or four replicates.
In the ex vivo experiments with the most promising results, the tissue structure was also analyzed. The morphological evaluation of urothelial desquamation was performed after the treatment period and at the end of the regeneration period. At these particular time points, the tissue was removed from the insert of the diffusion chamber and prepared for observation by scanning electron microscopy.
Measuring of Transepithelial Electrical Resistance (TEER)
During the ex vivo experiment, the potential difference (PD) and passing current (I) were measured by a multi-channel voltage-current clamp (model VCC MC6, Physiologic Instruments, San Diego, CA, USA). Two pairs of Ag/AgCl electrodes were connected to the diffusion chambers via 3 M KCl/3.5% agar bridges. The experiments were performed under open circuit conditions with the current set to zero. For each measurement, a PD value was clamped to 20 mV and the necessary current was recorded. Electrical resistance was calculated according to Ohm’s law (R = (20 mV – PD)/I). To obtain TEER, the fluid resistance measured before mounting the tissue in the diffusion chamber was first subtracted and the value obtained was then multiplied by the exposure area (0.07 cm²).
Electrophysiological parameters were recorded during the equilibration period at 10 min intervals, in the treatment period at 5 min intervals, and during the regeneration period initially at 10 min intervals (up to 120 min) and then at 20 min intervals. Because the TEER values determined already varied considerably during the equilibration period, all measurements were presented as a percentage of the TEER value at the end of the equilibration period for each tissue separately. This ensured better comparability and repeatability of data. A two-tailed unpaired Student’s t-test (α = 0.05) was applied to evaluate the differences in TEER values between different experimental conditions at particular time points.
2.4. Animals for In Vivo Experiments
For the in vivo experiments, we used ten adult (10 to 12 weeks old) female C57BL/6JOLaHsd mice (Harlan, Italy), six mice for experiments with 0.01% PLL of both molecular weights and four mice for experiments with 0.001% PLL of both molecular weights. The mice were housed at the Medical Experimental Center at the Faculty of Medicine (Ljubljana, Slovenia) in open-bar cages (two to five per cage) with bedding (Lignocel 3/4, Germany) and enrichment material (paper towel) and under controlled conditions of temperature (22 ± 1 °C) and humidity (55 ± 10%), and a 12 h/12 h light–dark cycle (light from 7 am to 7 pm) with unlimited access to food (standard rodent diet; Mucerola, Italy) and tap water. All procedures involving mice were approved by the National Ethical Committee and the Administration of the Republic of Slovenia for Food Safety, Veterinary and Plant Protection (permit number U34401-4/2016/8). Animal care and treatment were in accordance with Slovenian and international legislation and policy (Directive 2010/63/EU) on the protection of animals used for research purposes.
2.5. In Vivo Experiments
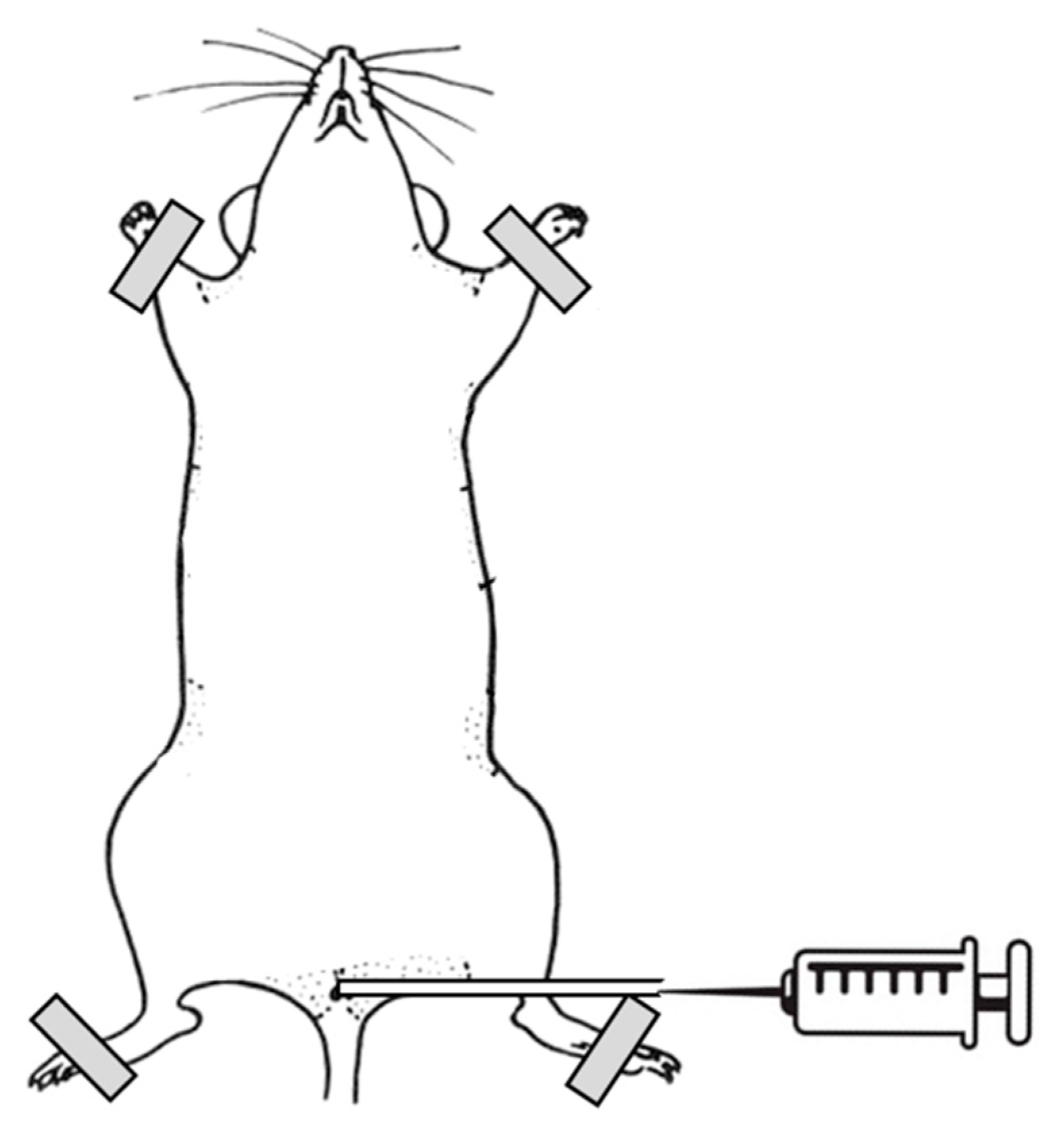
The mice were anesthetized intraperitoneally with ketamine HCl (100 mg/kg) and xylazine (10 mg/kg), and placed in the dorsal position. A polyethylene catheter with a 0.28 mm inner diameter (Intramedic, Becton Dickinson, USA) was inserted into the bladder through the urethra and sheathed over a 29 G needle (Micro-Fine Plus
TM, Becton Dickinson, USA) connected to a 1 mL injection syringe. The bladder of each animal was emptied with mild manual pressure on the abdomen. Then, 80 µl of PLL dispersion (30–70 kDa or 70–150 kDa, 0.001% or 0.01%) were instilled intravesically for 15 min (
Figure 2). All infusions of PLL were performed gradually and at a slow rate to avoid injury or vesicoureteral reflux. After the elapsed time, the catheter and injection syringe were removed from the bladder and the mouse was sacrificed with CO
2. The abdomen was opened, and the fully filled and distended urinary bladder was ligated with surgical thread. The bladder was then excised from the animal, cut longitudinally into halves, and further prepared for scanning electron microscopy.
2.6. Scanning Electron Microscopy
The tissue from the ex vivo and in vivo experiments was fixed in a mixture of 2% paraformaldehyde (Merck KgaA, Germany) and 2% glutaraldehyde (Serva, Germany) in 0.1 M cacodylate buffer (pH 7.4; Serva, Germany) for 3 to 4 h at 4 °C. The tissue samples were then rinsed in 0.1 M cacodylate buffer and post-fixed in 1% osmium tetroxide (Serva, Germany) in the same buffer for 1 h at 4 °C. Specimens were critical-point dried, attached to aluminum holders with silver paste (Silver DAG1415, Plano GmbH, Germany), sputter-coated with gold, and examined in a Tescan Vega3 scanning electron microscope at 30 kV (Brno, Czech Republic).
4. Discussion
Cell desquamation is part of the physiological renewal in some tissues (e.g., the epidermis), but it can be enhanced by various inducers or types of stress conditions [
18,
19]. The urothelium is a very stable epithelium, but it quickly responds to various pathological stimuli with intense cell desquamation [
4,
5,
20]. However, the desquamation should be limited to the upper urothelial cell layers, without provoked bleeding, and has to be reversible. Namely, rapid regeneration of urothelial function and structure is essential to restore the blood–urine barrier. Our previous ex vivo and in vivo studies showed that chitosan induces desquamation of the urothelial cells, with rapid regeneration of the urothelium after the removal of the polymer [
4,
5], and that its effects on urothelial structure and function are time- and concentration-dependent [
12]. The aim of this study was to establish whether another cationic polymer, PLL, affects the urothelium similar to chitosan and could be used as an alternative desquamation inducer. In contrast to chitosan, which has to be dispersed in a buffer with acidic pH value, PLL can be dispersed in a buffer at close to neutral pH value. This could be important to reduce discomfort of human patients during potential intravesical application by clinicians.
A PLL with a molecular weight from 30 kDa to 300 kDa was used in the studies where epithelial permeability was examined [
16]. It has also been used in urinary bladder pretreatment in orthotopic bladder tumor model to enhance the electrostatic interactions between tumor cells and the urothelium to increase the tumor implantation rate [
21,
22]. However, the results of our previous study proved that PLL (molecular weight 70–150 kDa, 0.01% concentration) caused desquamation of urothelial cells and, therefore, enabled tumor cells to attach and implant, which is in accordance with the findings of the present study [
23].
In ex vivo experiments performed with Ussing chambers, we aimed to establish how the concentration and molecular weight of PLL as well as treatment time influence the permeability of the urothelium. During these experiments, TEER was determined, which is a sensitive measure of the ion permeability of the urothelium [
2]. Our previous studies already proved that in ex vivo experiments with Ussing chambers, the urinary bladders isolated from animals maintain the barrier function and cellular ultrastructure for at least 6 h [
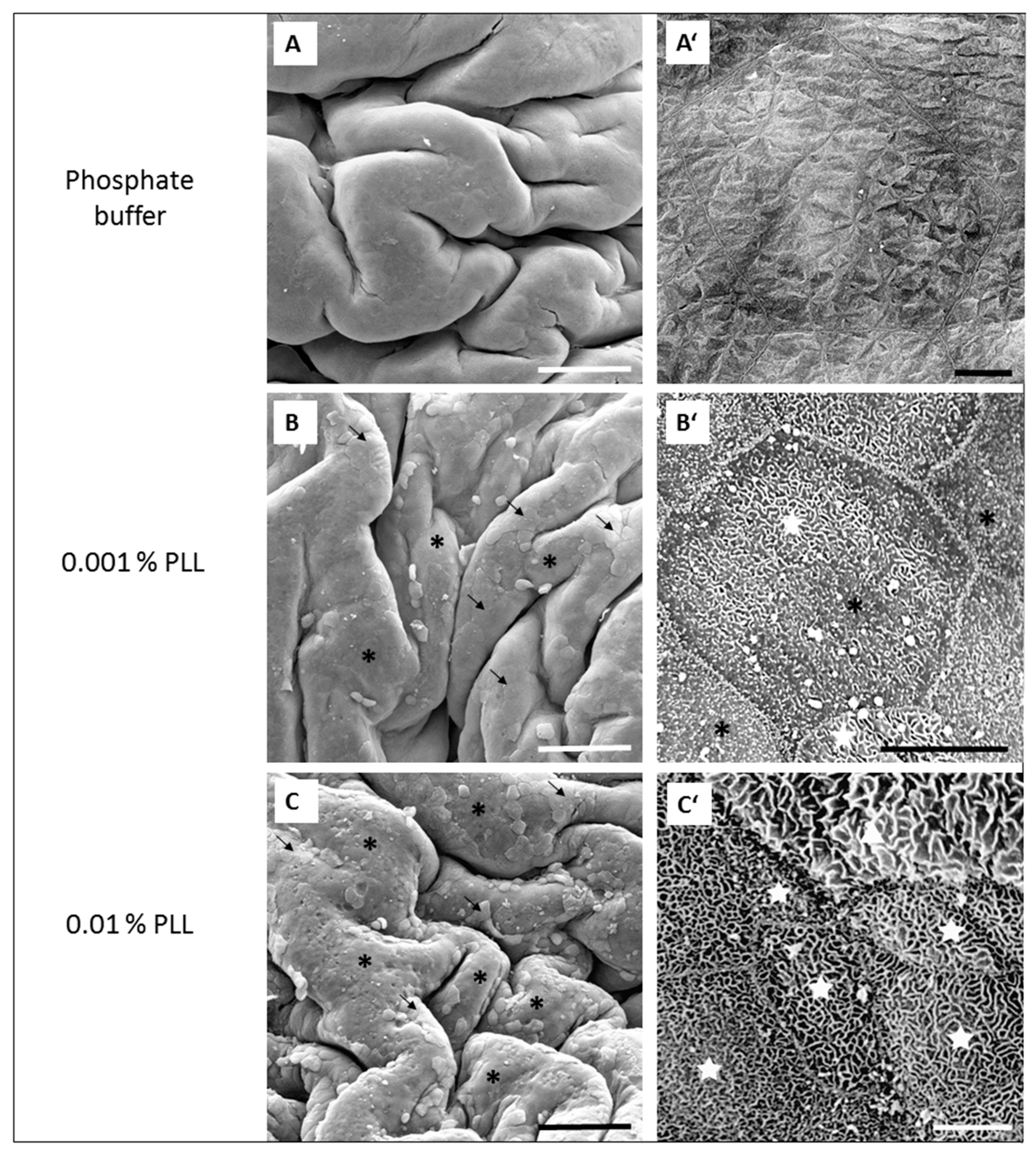
5]. This was also confirmed in this study because at the end of the experiment (at 360 min) the TEER was higher than at the beginning of the measurement, and the luminal surface of the urinary bladders was intact, with no observable damage or cell death (
Figure 5E).
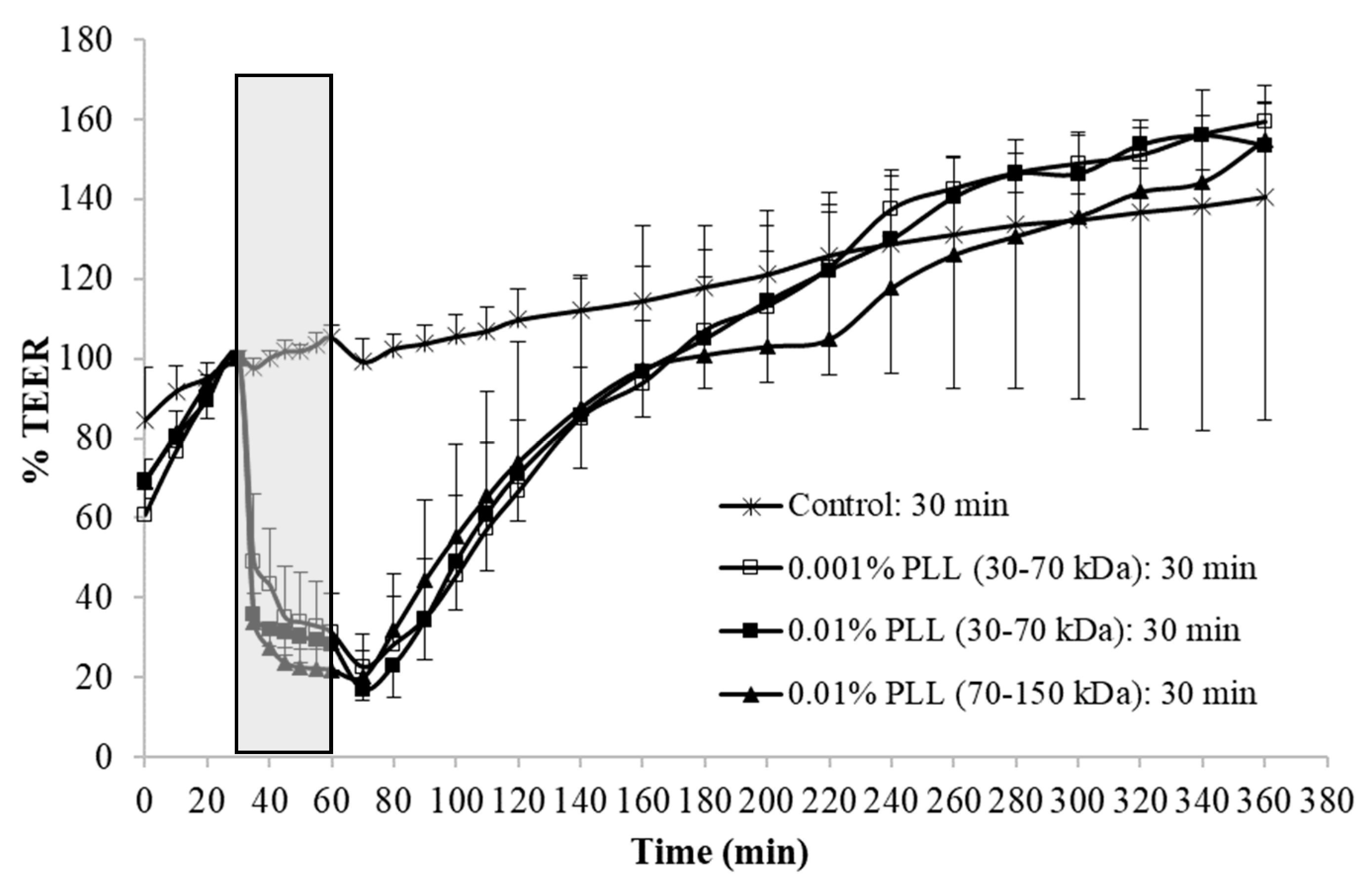
When the urothelium was exposed for 30 min to 0.01% PLL of different molecular weight (30–70 kDa or 70–150 kDa), there was a similar drop in TEER, followed by a comparable rate of TEER restoration (
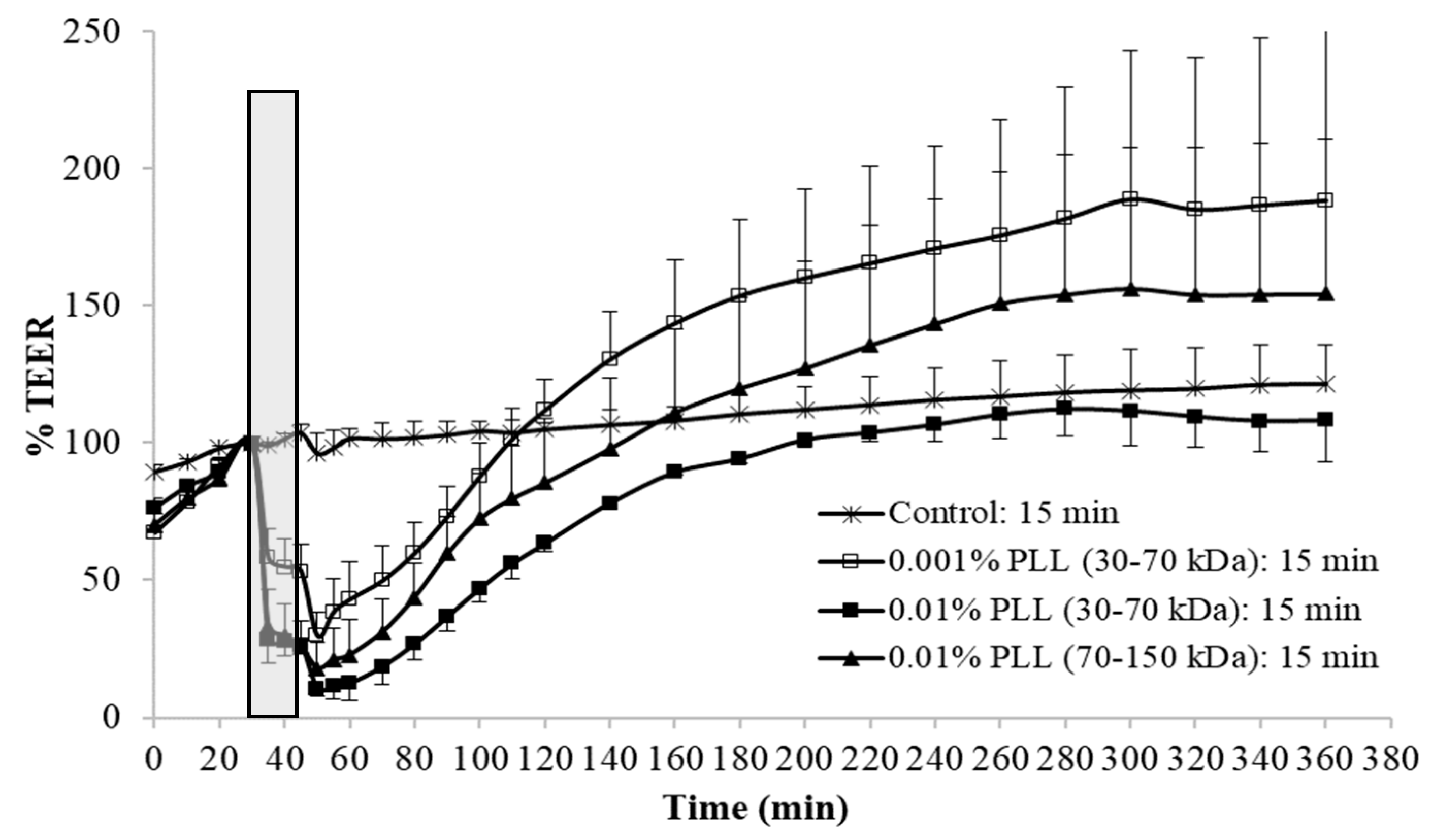
Figure 4). At a shorter exposure time (15 min), there was also no significant difference in drop of TEER during the exposure of the urothelium to 0.01% PLL of a different molecular weight. However, after treatment with PLL of a higher molecular weight, TEER restoration was slightly faster (
Figure 3).
At the end of the treatment period, drop in TEER was significantly smaller when the urothelium was exposed for 15 min to 0.001% PLL compared to either a higher concentration of PLL (0.01%) or longer exposure time (30 min;
Figure 3 and
Figure 4). Only the minority of the superficial cells were exfoliated at the end of 15 min exposure to 0.001% PLL (
Figure 5B), and this treatment with PLL would probably not be enough to remove the reservoirs of
Escherichia coli in the superficial urothelial cells.
Fifteen-minute exposure to 0.01% PLL or 30 min exposure to either concentration of PLL resulted in the same reduction of TEER at the end of the treatment period (approximately 10 to 30% of the baseline value). Morphologically, 15 min exposure of the urothelium to 0.01% PLL resulted in extensive umbrella cell desquamation. However, it is important that the desquamation was limited to the superficial cells only (
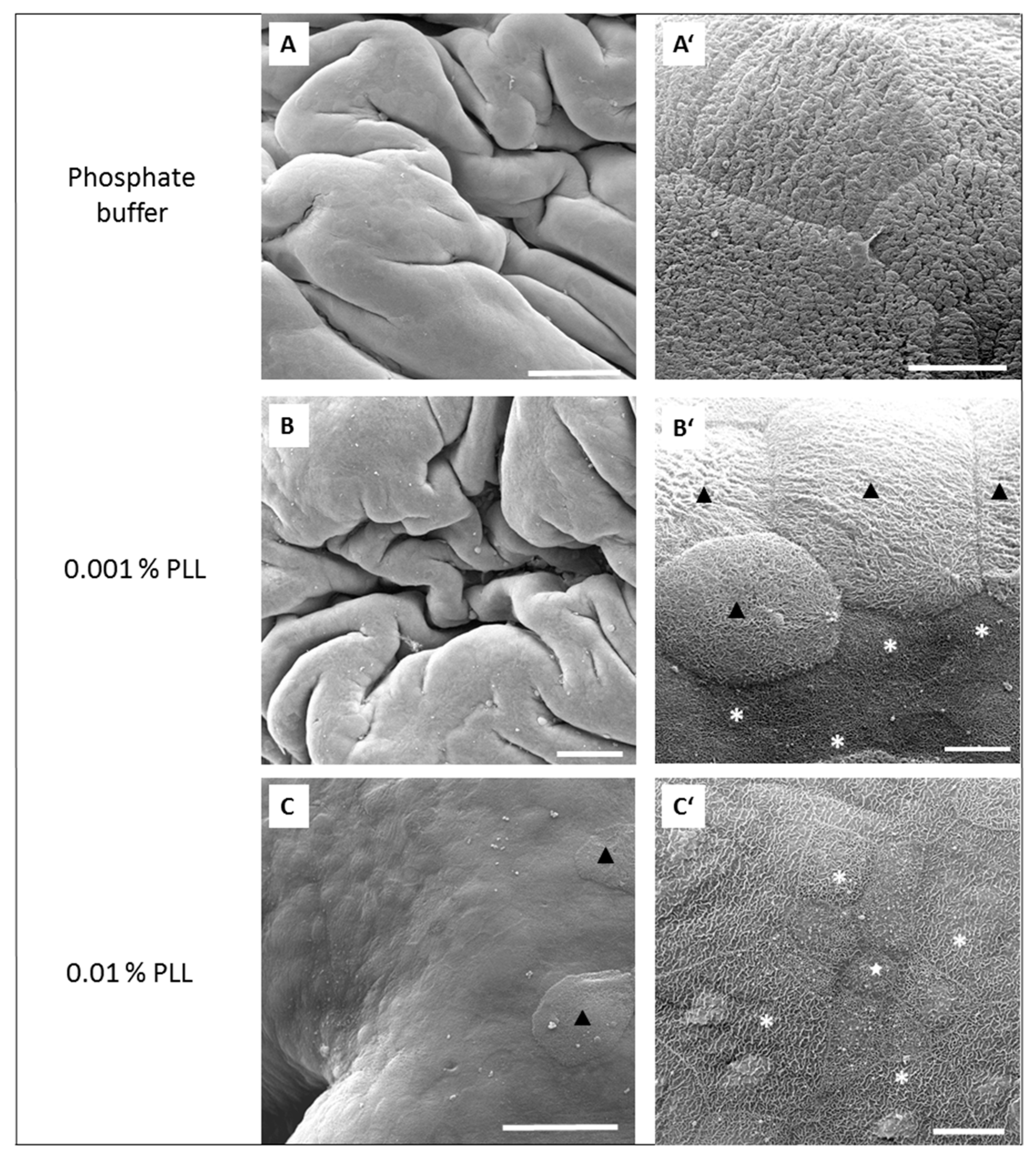
Figure 5C,C’). With more extensive desquamation of urothelial cells, more time was needed during the regeneration period to attain the TEER values of the control tissue. After 15 min treatment with 0.01% PLL, TEER values reached the control values at approximately 2.5 to 3 h after the end of the treatment, and at the end of the regeneration period no cell desquamation was observed. Newly exposed intermediate cells were not finally differentiated, but they were already at a higher stage of cell differentiation compared to the end of the treatment period (
Figure 6C,C’). This is in agreement with the fact that urothelial regeneration after induced injury is much faster than physiological renewal [
4,
5,
24].
In the regeneration period the urothelium recovered from induced desquamation. The rate of recovery was different among tissue pieces, which is expected in biological samples. Moreover, TEER values of the control tissue also gradually increased throughout the experiment, while the relative TEER values were normalized to the TEER value at the end of the equilibration period. Both facts increase the standard deviations of the measured values at the later time points. In some cases, TEER values at the end of ex vivo experiments were even higher compared to the values at the end of the equilibration period. However, as shown in our previous study, this was not a consequence of hyperplasia [
5].
Therefore, from the ex vivo experiments we concluded that 15 min treatment with 0.01% PLL yields promising results for potential use of PLL as a desquamation inducer of the urothelium. The influence of PLL on urothelial structure and function is mostly influenced by PLL concentration, whereas the treatment time and the molecular weight of the polymer had a minor effect. Comparing the influence of chitosan on the permeability of isolated pig urothelium, it was time and concentration dependent [
12].
We evaluated urothelial desquamation and regeneration using TEER measurement as a quantitative method on the one hand, and analyzed the urothelial structure by scanning electron microscopy on the other hand. By these two methods we examined the level of functional and structural regeneration of this tissue after induced desquamation. Scanning electron microscopy has been proven in our previous studies [
5,
17] as the most appropriate method to detect changes in the differentiation stage of urothelial cells. Namely, differentiation levels of these cells are best reflected in the structure of apical plasma membrane. The structuration of the apical plasma membrane into microvilli (cells at low differentiation stage), ropy ridges (cells at high differentiation stage) or specifically scalloped surface (terminally differentiated cells) has been shown as a relevant marker of urothelial cell differentiation [
4,
5,
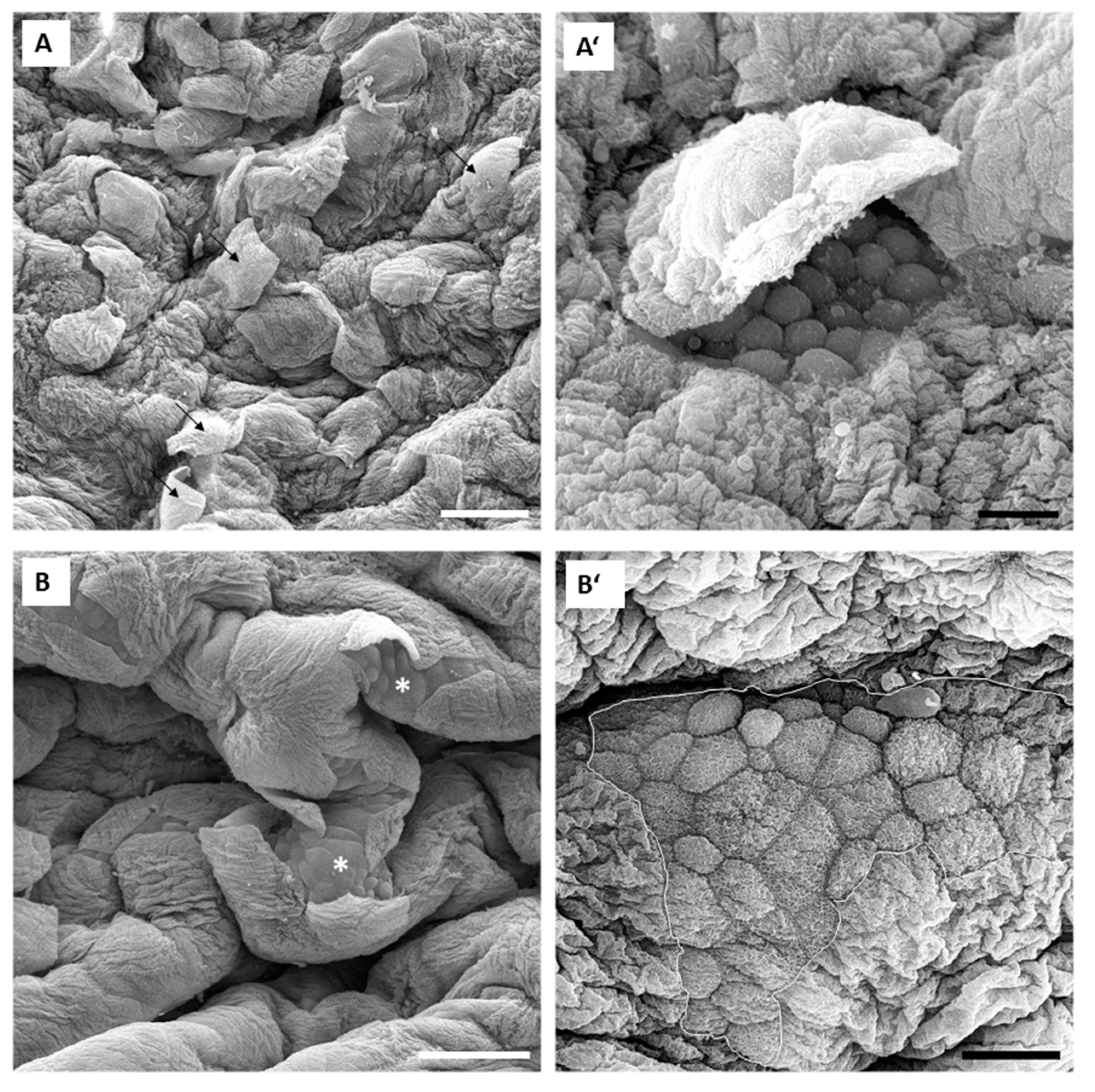
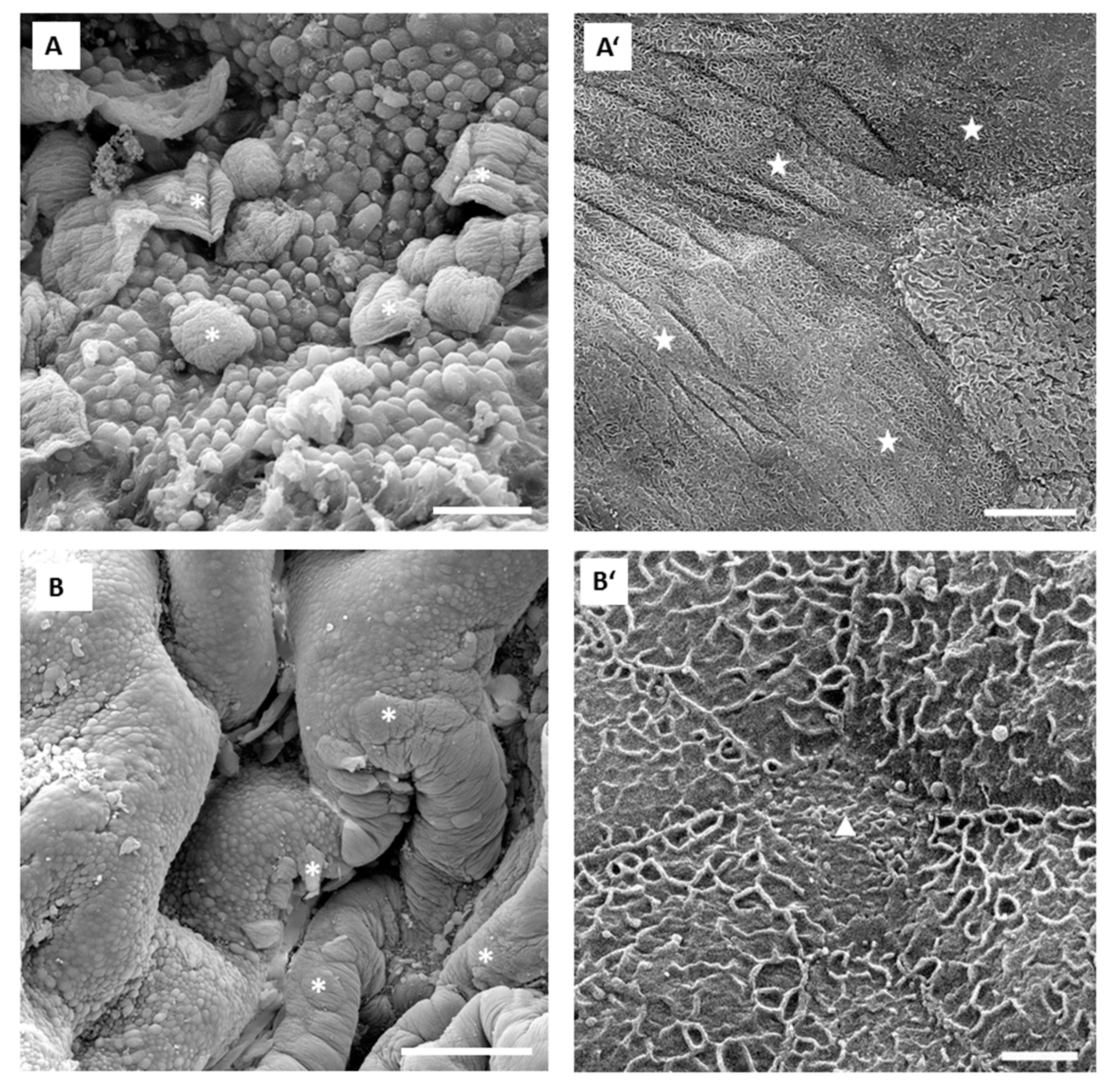
17]. Our results obtained in in vivo experiments are in accordance with the results of ex vivo experiments performed with isolated urinary bladders. In in vivo conditions, 0.001% concentration of PLL was also too low for the desired effect. Namely, only individual umbrella cells were exfoliated when the urothelium was exposed to 0.001% PLL with molecular weights of either 30 to 70 kDa or 70 to 150 kDa (
Figure 7). On the other hand, exposure to 0.01% PLL resulted in intense cell desquamation that was limited to the superficial cell layer (
Figure 8), with no apoptotic or necrotic cells on the urothelial surface.
In the in vivo experiments with 0.01% PLL, the molecular weight of the polymer influenced the desquamation effect because at a higher molecular weight the majority of the urothelium was exfoliated (
Figure 8). The PLL with a higher molecular weight has a larger number of amino groups, which are positively charged at pH 6.5 because the pKa of PLL is approximately 10.5 [
25]. Consequently, we expected better contact between such PLL with higher density of positively charge and negatively charged urothelial surfaces. Namely, the urothelium is covered with the glycosaminoglycan layer, mainly composed of heparin, dermatan, chondroitin sulphate, and hyaluronic acid [
26]. On the other hand, there was no significant difference between 0.01% PLL of 30–70 kDa and 70–150 kDa on urothelial permeability (
Figure 4), while in the experiments performed with the human intestinal Caco-2 cell line, PLL of a higher molecular weight (300 kDa) had a significantly greater effect on TEER and inulin passage compared to PLL with a molecular weight of 30 kDa [
16]. The different effects of PLL on epithelial permeability could be explained by the different structures of both epithelia. The urothelium is a three-layered epithelium with complex architecture, whereas the Caco-2 cell line grows as a monolayer. The insignificant influence of PLL molecular weight on urothelium permeability might be also due to the relatively small difference between the molecular weights tested in our study. However, in the in vivo experiments with 0.01% PLL more intense cell desquamation was present with a molecular weight of 70–150 kDa compared to molecular weight of 30–70 kDa (
Figure 7). Consequently, it would not be rational to use a PLL with an even higher molecular weight.
Therefore, 15 min exposure of the urothelium to a 0.01% concentration of PLL results in uniform desquamation of the superficial cell layer, followed by rapid regeneration of the urothelium. In comparison to chitosan, the main advantage of PLL is that the desquamation is limited to only a superficial cell layer. Namely, in comparable ex vivo experiments, 15 min exposure of the urothelium to 0.005% chitosan resulted in intense desquamation of superficial cells, but intermediate cells were also removed to some extent [
5]. In addition, in the experiments performed with Caco-2 cells, TEER recovery was much faster after PLL treatment compared to chitosan when the same concentration of polymers was used [
16].
The mechanism by which PLL induces desquamation of urothelial cells is not known. No studies have yet been published evaluating the effect of this polymer on urothelial permeability and structure. In Caco-2 cells, PLL influenced tight junctions through morphological changes of the F-actin cytoskeleton and redistribution of the tight junction proteins ZO-1 and occludin [
16].
It is also worth mentioning that the results of ex vivo experiments are in accordance with the results obtained by in vivo experiments despite the use of the tissue from two different types of animals. This confirms the fact that rat and mouse urothelium have very similar structures and similar responses to PLL. Additionally, we can expose that ex vivo experiments performed with Ussing chambers may replace in vivo experiments when the main focus of the research is the influence of a substance on urothelial permeability at different treatment conditions. In view of this, the concept of our study supports the basic principles of the 3Rs in the use of animals for research purposes.