Simonkolleite Coating on Poly(Amino Acids) to Improve Osteogenesis and Suppress Osteoclast Formation in Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Zn–PAA
2.2. Ion Release
2.3. Biocompatibility In Vitro
2.3.1. Cell Culture
2.3.2. BMSCs Proliferation and Morphology
2.4. Osteogensis of BMSCs
2.5. Osteoclast Differentiation of RAW264.7
2.6. Antibacterial Activity
2.7. Statistical analysis
3. Results
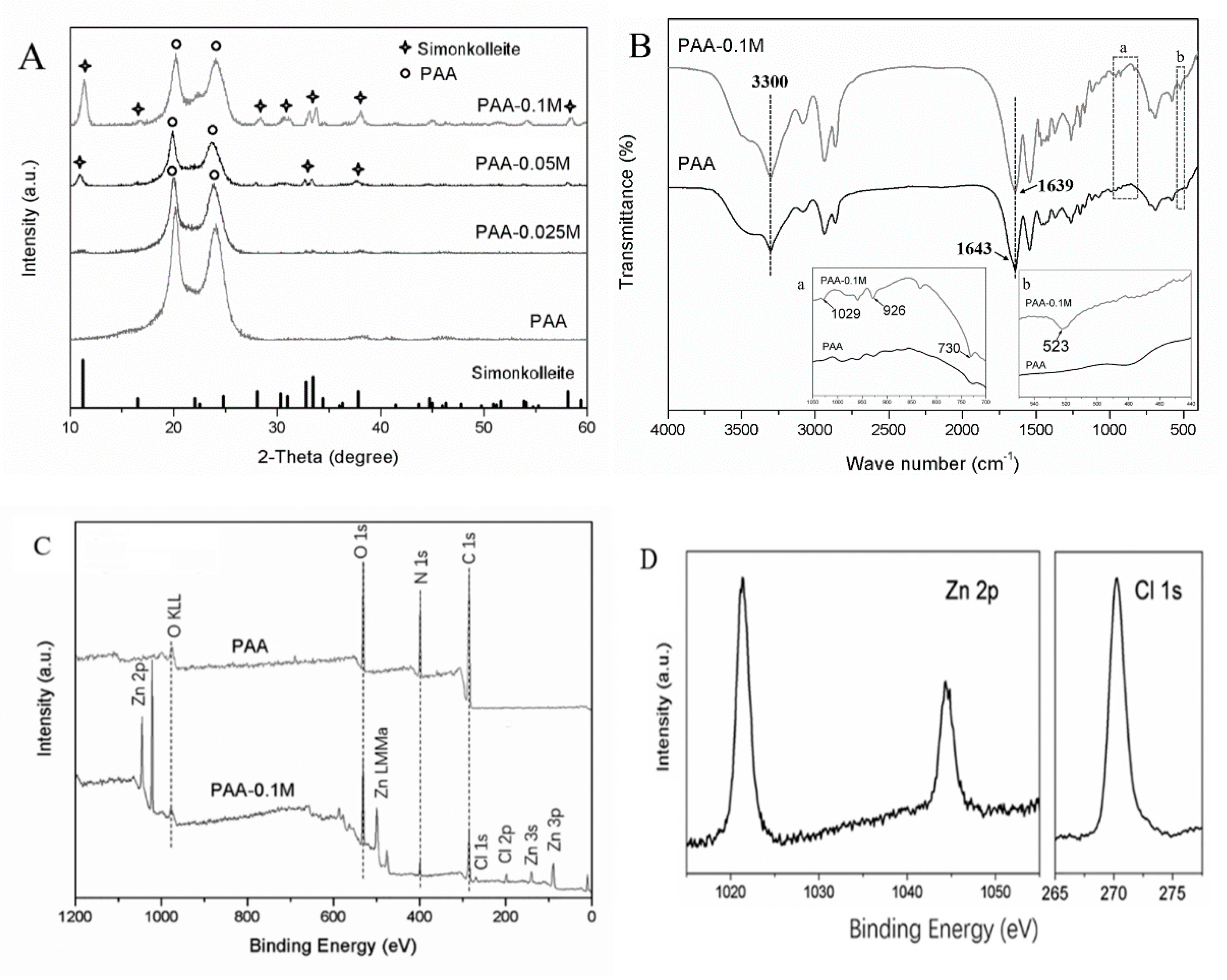
3.1. Characterization of PAA and Zn-PAA
3.2. Morphology and Composition of PAA and Zn–PAA
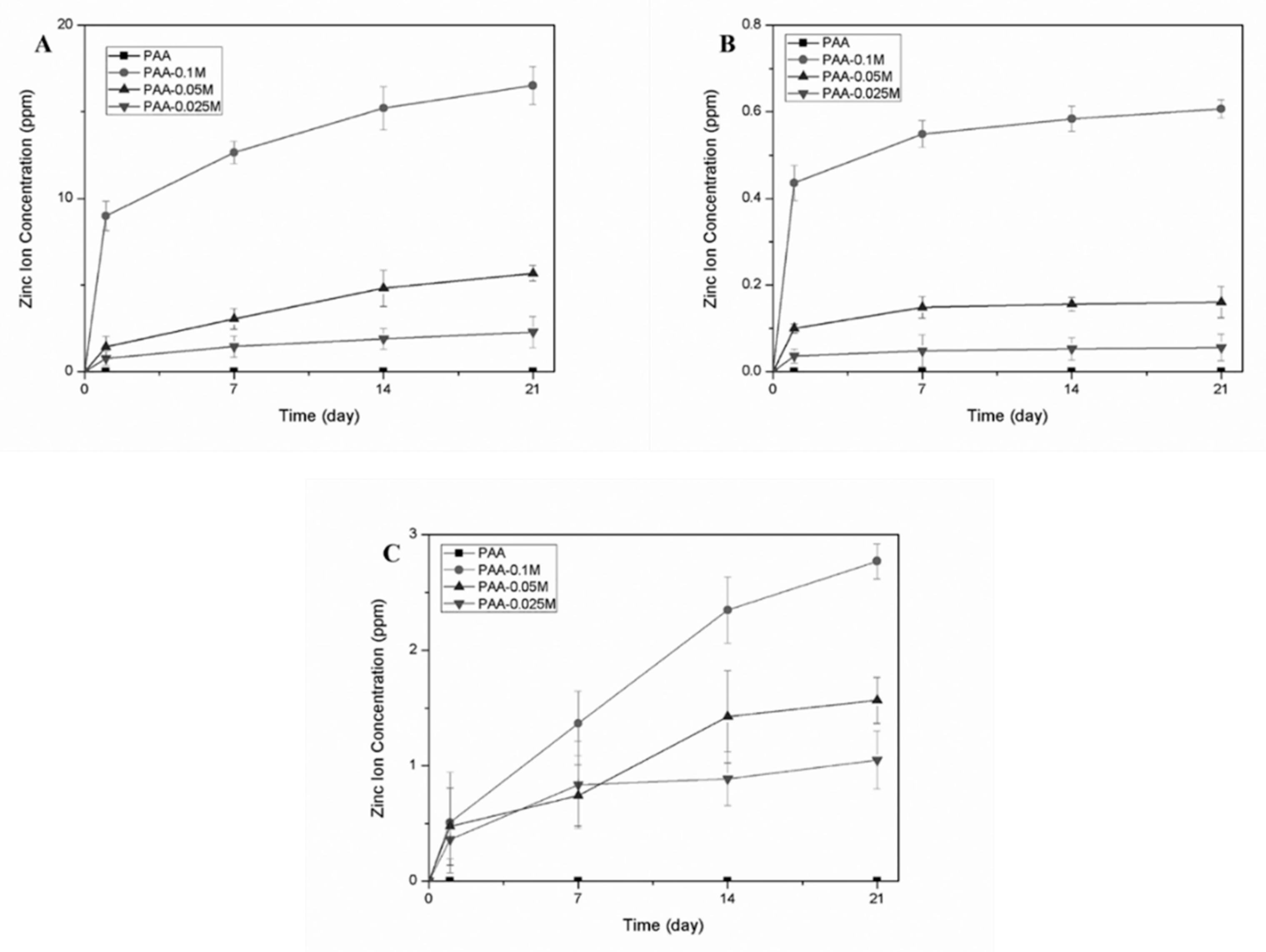
3.3. Zinc Ion Release in Different Solutions
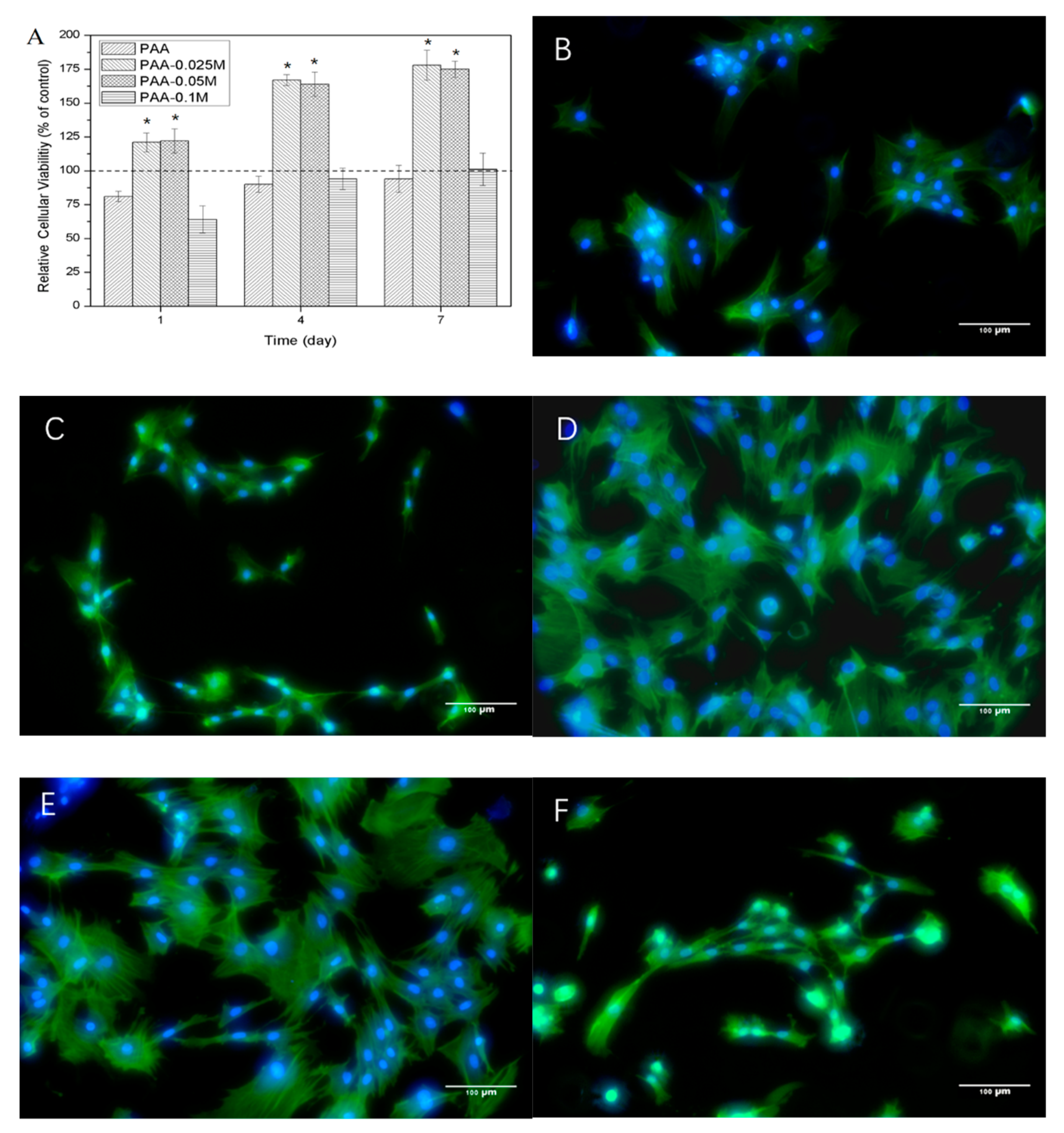
3.4. Cytocompatibility of Zn–PAA
3.5. Osteogenic Differentiation of BMSCs
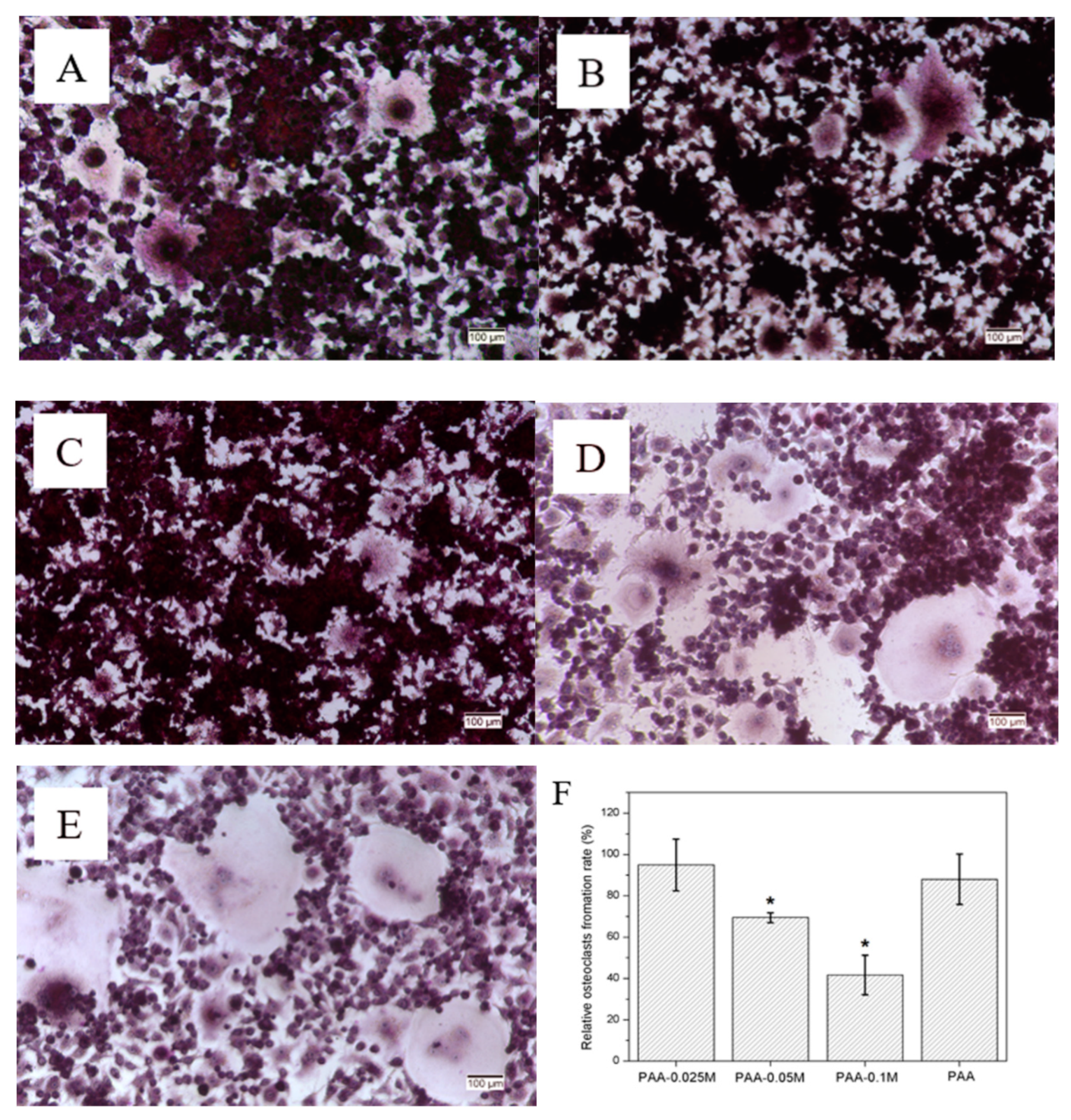
3.6. Osteoclast Differentiation of RAW264.7
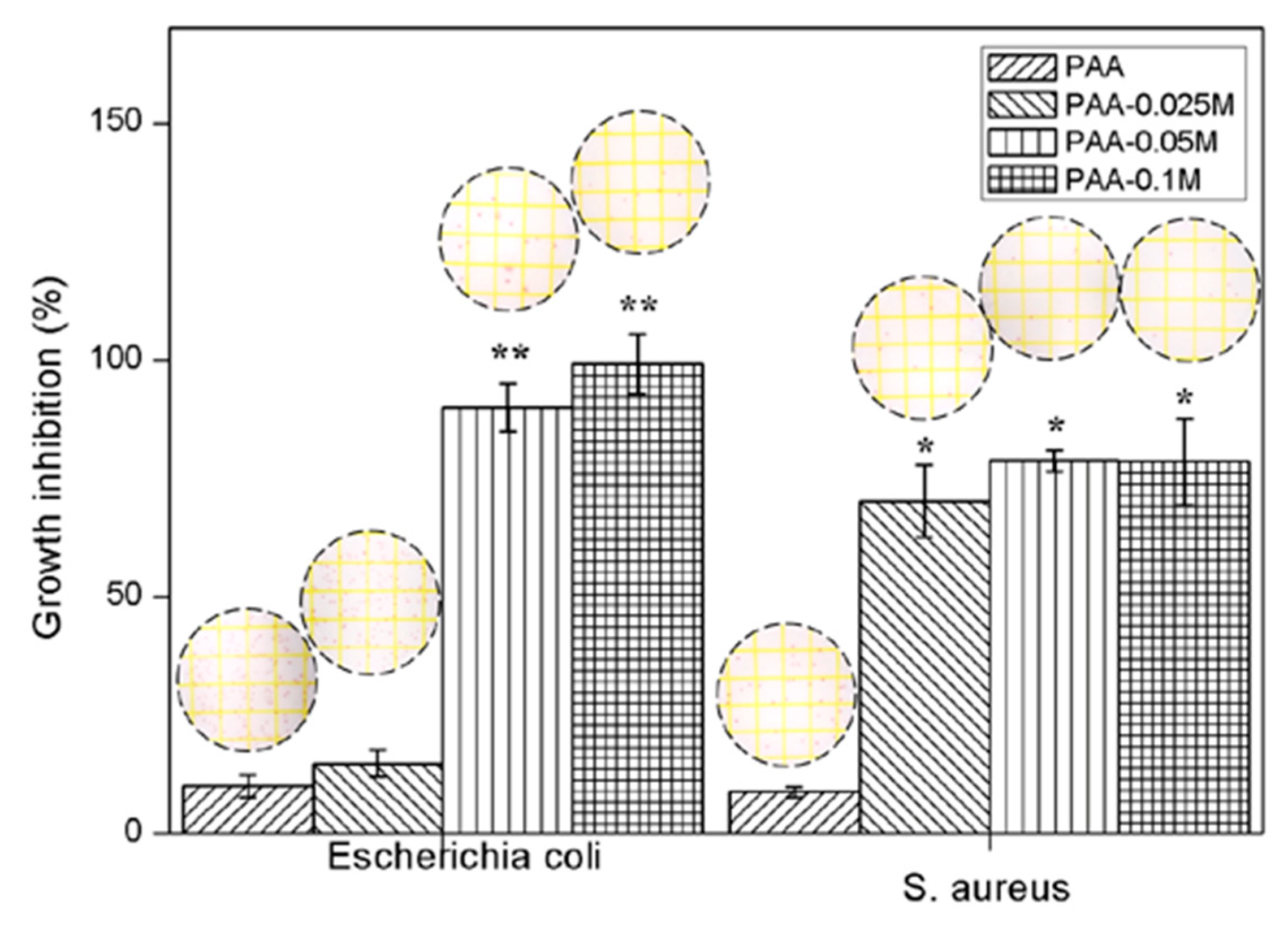
3.7. Antibacterial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, T.Y.; Su, W.T.; Chen, P.H. Comparing the effects of chitosan scaffolds containing various divalent metal phosphates on osteogenic differentiation of stem cells from human exfoliated deciduous teeth. Biol. Trace Elem. Res. 2018, 185, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Solomons, N.W. Update on zinc biology. Ann. Nutr. Metab. 2013, 62, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, A.; Matsunaga, T.; Ishizaki, H.; Yamada, S.; Hayashi, Y. Zinc as an essential trace element in the acceleration of matrix vesicles-mediated mineral deposition. Microsc. Res. Tech. 2011, 74, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Barbieri, D.; Davison, N.; Yan, Y.; Bruijn, J.D.D.; Yuan, H. Zinc in calcium phosphate mediates bone induction: In vitro and in vivo model. Acta Biomater. 2014, 10, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yamaguchi, R. Action of zinc on bone metabolism in rats: Increases in alkaline phosphatase activity and DNA content. Biochem. Pharmacol. 1986, 35, 773–777. [Google Scholar] [CrossRef]
- Yamada, Y.; Ito, A.; Kojima, H.; Sakane, M.; Miyakawa, S.; Uemura, T.; LeGeros, R.Z. Inhibitory effect of Zn2+ in zinc-containing beta-tricalcium phosphate on resorbing activity of mature osteoclasts. J. Biomed. Mater. Res. A 2008, 84, 344–352. [Google Scholar] [CrossRef]
- Khadeer, M.A.; Sahu, S.N.; Bai, G.; Abdulla, S.; Gupta, A. Expression of the zinc transporter ZIP1 in osteoclasts. Bone 2005, 37, 296–304. [Google Scholar] [CrossRef]
- Ito, A.; Ojima, K.; Naito, H.; Ichinose, N.; Tateishi, T. Preparation, solubility, and cytocompatibility of zinc-releasing calcium phosphate ceramics. J. Biomed. Mater. Res. A 2015, 50, 178–183. [Google Scholar] [CrossRef]
- Prasad, A.S.; Abbasi, A.; Ortega, J. Zinc deficiency in man: Studies in sickle cell disease. Prog Clin Biol Res 1977, 14, 211–239. [Google Scholar]
- Kawamura, H.; Ito, A.; Muramatsu, T.; Miyakawa, S.; Ochiai, N.; Tateishi, T. Long-term implantation of zinc-releasing calcium phosphate ceramics in rabbit femora. J. Biomed. Mater. Res. A 2010, 65, 468–474. [Google Scholar] [CrossRef]
- Aina, V.; Perardi, A.; Bergandi, L.; Malavasi, G.; Menabue, L.; Morterra, C.; Ghigo, D. Cytotoxicity of zinc-containing bioactive glasses in contact with human osteoblasts. Chem.-Biol. Interact. 2007, 167, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-T.; Kang, B.S.; Ren, F.; Tien, L.-C.; Sadik, P.; Norton, D.; Pearton, S.; Lin, J. Hydrogen-selective sensing at room temperature with ZnO nanorods. Appl. Phys. Lett. 2005, 86, 243503. [Google Scholar] [CrossRef]
- Volovitch, P.; Allely, C.; Ogle, K. Understanding corrosion via corrosion product characterization: I. Case study of the role of Mg alloying in Zn–Mg coating on steel. Corros. Sci. 2009, 51, 1251–1262. [Google Scholar] [CrossRef]
- Cao, J.; Henry, P.R.; Ammerman, C.B.; Miles, R.D.; Littell, R.C. Relative Bioavailability of Basic Zinc Sulfate and Basic Zinc Chloride for Chicks. J. Appl. Poult. Res. 2000, 9, 513–517. [Google Scholar] [CrossRef]
- Gorodylova, N.; Cousy, S.; Šulcová, P.; Svoboda, L. Thermal transformation of layered zinc hydroxide chloride. J. Therm. Anal. Calorim. 2017, 127, 675–683. [Google Scholar] [CrossRef]
- Alves, M.M.; Marques, L.M.; Nogueira, I.; Santos, C.F.; Salazar, S.B.; Eugénio, S.; Mira, N.P.; Montemor, M.F. In silico, in vitro and antifungal activity of the surface layers formed on zinc during this biomaterial degradation. Appl. Surf. Sci. 2018, 447, 401–407. [Google Scholar] [CrossRef]
- Batal, A.B.; Parr, T.M.; Baker, D.H. Zinc bioavailability in tetrabasic zinc chloride and the dietary zinc requirement of young chicks fed a soy concentrate diet. Poult. Sci. 2001, 80, 87–90. [Google Scholar] [CrossRef]
- Roske, C.W.; Lefler, J.W.; Müller, A.M. Complex nanomineral formation utilizing kinetic control by PLAL. J. Colloid Interf. Sci 2017, 489, 68–75. [Google Scholar] [CrossRef]
- Khamlich, S.; Bello, A.; Fabiane, M.; Ngom, B.D.; Manyala, N. Hydrothermal synthesis of simonkolleite microplatelets on nickel foam-graphene for electrochemical supercapacitors. J. Sol. State Electr. 2013, 17, 2879–2886. [Google Scholar] [CrossRef]
- Zhang, W. Hydrothermal synthesis of zinc hydroxide chloride sheets and their conversion to ZnO. Chem. Mat. 2007, 19, 2329–2334. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, H.; Wang, P.; Liu, P.Z.; Yan, Y.G. Improved cell adhesion of poly(amino acid) surface by cyclic phosphonate modification for bone tissue engineering. J. Appl. Polym. Sci. 2018, 135, 46226. [Google Scholar] [CrossRef]
- Wang, P.; Liu, P.; Xu, F.; Fan, X.; Yuan, H.; Li, H.; Yan, Y. Controlling the degradation of dicalcium phosphate/calcium sulfate/poly(amino acid) biocomposites for bone regeneration. Polym. Comp. 2016, 39, E122–E131. [Google Scholar] [CrossRef]
- Wu, Y.N.; Ding, Z.W.; Ren, H.H.; Ji, M.Z.; Yan, Y.G. Preparation, characterization and In vitro biological evaluation of a novel pearl powder/poly-amino acid composite as a potential substitute for bone repair and reconstruction. Polymers 2019, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Liu, P.; Peng, H.; Luo, X.; Yuan, H.; Zhang, J.; Yan, Y. Biocompatibility evaluation of dicalcium phosphate/calcium sulfate/poly (amino acid) composite for orthopedic tissue engineering in vitro and in vivo. J. Biomater. Sci. Polym. Ed. 2016, 27, 1170–1186. [Google Scholar]
- Song, C.C.; Yang, X.B.; Lei, Y.S.; Zhang, Z.; Smith, W.L.; Yan, J.L.; Kong, L.B. Evaluation of efficacy on RANKL induced osteoclast from RAW264.7 cells. J. Cell. Phys. 2019, 234, 11969–11975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.X.; Yu, Z.Z.; Yang, M.; Ma, J.; Mai, Y.W. Multiple melting and crystallization of nylon-66/montmorillonite nanocomposites. J. Polym. Sci. B 2003, 41, 2861–2869. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Sokolova, E. Simonkolleite, Zn5(OH)8Cl2(H2O), a decorated interrupted-sheet structure of the form [M φ2]4. Can. Mineral. 2002, 40, 939–946. [Google Scholar] [CrossRef]
- Autengruber, R.; Luckeneder, G.; Hassel, A.W. Corrosion of press-hardened galvanized steel. Corros. Sci. 2012, 63, 12–19. [Google Scholar] [CrossRef]
- Prosek, T.; Thierry, D.; Taxén, C.; Maixner, J. Effect of cations on corrosion of zinc and carbon steel covered with chloride deposits under atmospheric conditions. Corros. Sci. 2007, 49, 2676–2693. [Google Scholar] [CrossRef]
- Prestat, M.; Holzer, L.; Lescop, B.; Rioual, S.; Zaubitzer, C.; Diler, E.; Thierry, D. Microstructure and spatial distribution of corrosion products anodically grown on zinc in chloride solutions. Electr. Commun. 2017, 81, 56–60. [Google Scholar] [CrossRef]
- He, J.F.; Hu, J.M.; Mo, X.; Hao, Q.; Fan, Z.L.; He, G.N.; Wang, Y.Z.; Li, W.; He, Q.Y. Novel photocatalyst nitrogen-doped simonkolleite Zn5(OH)8Cl2 center dot H2O with vis-up-conversion photoluminescence and effective visible-light photocatalysis. Appl. Phys. A 2019, 125, 3. [Google Scholar] [CrossRef]
- Thu, H.E.; Hussain, Z.; Mohamed, I.N.; Shuid, A.N. Eurycoma longifolia, a promising suppressor of RANKL-induced differentiation and activation of osteoclasts: An in vitro mechanistic evaluation. J. Ayurveda Integr. Med. 2019, 10, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Yamaguchi, M. Stimulatory effect of β-alanyl-L-histidinato zinc on cell proliferation is dependent on protein synthesis in osteoblastic MC3T3-E1 cells. Mol. Cell. Biochem. 1993, 122, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Togari, A.; Arakawa, S.; Arai, M.; Matsumoto, S. Alteration of in-vitro bone metabolism and tooth formation by Zinc. Gen. Pharmacol.-Vasc. Syst. 1993, 24, 1133–1140. [Google Scholar] [CrossRef]
- Sithole, J.; Ngom, B.D.; Khamlich, S.; Manikanadan, E.; Manyala, N.; Saboungi, M.L.; Knoessen, D.; Nemutudi, R.; Maaza, M. Simonkolleite nano-platelets: Synthesis and temperature effect on hydrogen gas sensing properties. Appl. Surf. Sci. 2012, 258, 7839–7843. [Google Scholar] [CrossRef]
- O’Shea, J.N.; Schnadt, J.; Bruhwiler, P.A.; Hillesheimer, H.; Martensson, N.; Patthey, L.; Krempasky, J.; Wang, C.K.; Luo, Y.; Agren, H. Hydrogen-bond induced surface core-level shift in isonicotinic acid. J. Phys. Chem. B 2001, 105, 1917–1920. [Google Scholar] [CrossRef]
- Venezia, A.M.; Duca, D.; Floriano, M.A.; Deganello, G.; Rossi, A. Chemical effect on the XPS spectra of the valence band and on O KLL and Pd MNN Auger spectra in pumice-supported catalysts. Surf. Interface Anal. 1992, 18, 619–622. [Google Scholar] [CrossRef]
- Zaveri, T.D.; Dolgova, N.V.; Byung Hwan, C.; Jiyeon, L.; Joey, W.; Lele, T.P.; Fan, R.; Keselowsky, B.G. Contributions of surface topography and cytotoxicity to the macrophage response to zinc oxide nanorods. Biomaterials 2010, 31, 2999–3007. [Google Scholar] [CrossRef]
- Wenhua, S.; Jinyang, Z.; Jing, G.; Jinhua, Z.; Feng, D.; Liying, L.; Zengtian, S. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar]
- Ito, A.; Kawamura, H.; Otsuka, M.; Ikeuchi, M.; Ohgushi, H.; Ishikawa, K.; Onuma, K.; Kanzaki, N.; Yu, S.; Ichinose, N. Zinc-releasing calcium phosphate for stimulating bone formation. Mater. Sci. Eng. C 2002, 22, 21–25. [Google Scholar] [CrossRef]
- Karsenty, G. Central control of bone formation. Adv. Nephrol. Necker Hosp. 2001, 31, 119–133. [Google Scholar] [PubMed]
- Roodman, G.D. Mechanisms of disease: Mechanisms of bone metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Park, B.; Yoon, D.S.; Kwon, S.H.; Shin, D.M.; Lee, J.W.; Lee, H.G.; Shim, J.H.; Park, J.H.; Lee, J.M. Zinc inhibits osteoclast differentiation by suppression of Ca2+-Calcineurin-NFATc1 signaling pathway. Cell Commun. Signal. 2013, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Weitzmann, M.N. Zinc stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-kappa B activation. Mol. Cell. Biochem. 2011, 355, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.M.; Xu, L.Z.; Xie, N.; Li, K.; Xi, Y.H.; Liu, X.L.; Zheng, X.B.; Chen, X.S.; Ye, X.J.; Wei, D.X. Optimal Zn-modified Ca-Si-based ceramic nanocoating with Zn ion release for osteoblast promotion and osteoclast inhibition in bone tissue engineering. J. Nanomater. 2017, 2017, 9. [Google Scholar] [CrossRef]
- Collin-Osdoby, P.; Osdoby, P. RANKL-mediated osteoclast formation from murine RAW 264.7 cells. Methods Mol. Biol. (Clifton N.J.) 2012, 816, 187–202. [Google Scholar]
- Abe, E.; Mocharla, H.; Yamate, T.; Taguchi, Y.; Manolagas, S.C. Meltrin-alpha, a fusion protein involved in multinucleated giant cell and osteoclast formation. Calcif. Tissue Int. 1999, 64, 508–515. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Wronski, T.J.; Hollinger, J.O.; Einhorn, T.A. Application of histomorphometric methods to the study of bone repair. J. Bone Miner. Res. 2010, 20, 1715–1722. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloid Surf. A-Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Stanic, V.; Dimitrijevic, S.; Antic-Stankovic, J.; Mitric, M.; Jokic, B.; Plecas, I.B.; Raicevic, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Fang, M.; Chen, J.H.; Xu, X.L.; Yang, P.H.; Hildebrand, H.F. Antibacterial activities of inorganic agents on six bacteria associated with oral infections by two susceptibility tests. Int. J. Antimicrob. Agents 2006, 27, 513–517. [Google Scholar] [CrossRef] [PubMed]


| Samples/Elements | C (at %) | O (at %) | Zn (at %) | Cl (Atomic %) |
|---|---|---|---|---|
| PAA–0.1M | 79.76 ± 0.42 | 18.94 ± 0.03 | 0.97 ± 0.01 | 0.34 ± 0.03 |
| PAA–0.05M | 79.69 ± 0.31 | 19.84 ± 0.06 | 0.33 ± 0.01 | 0.12 ± 0.02 |
| PAA–0.025M | 78.12 ± 0.19 | 20.91 ± 0.14 | 0.17 ± 0.01 | 0.08 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Chen, X.; Wang, X.; Xiong, Y.; Yan, Y.; Tan, Z.; Yang, X.; Li, Y. Simonkolleite Coating on Poly(Amino Acids) to Improve Osteogenesis and Suppress Osteoclast Formation in Vitro. Polymers 2019, 11, 1505. https://doi.org/10.3390/polym11091505
Li S, Chen X, Wang X, Xiong Y, Yan Y, Tan Z, Yang X, Li Y. Simonkolleite Coating on Poly(Amino Acids) to Improve Osteogenesis and Suppress Osteoclast Formation in Vitro. Polymers. 2019; 11(9):1505. https://doi.org/10.3390/polym11091505
Chicago/Turabian StyleLi, Shuyang, Xingtao Chen, Xiaomei Wang, Yi Xiong, Yonggang Yan, Zhi Tan, Xiaoyu Yang, and Yuanye Li. 2019. "Simonkolleite Coating on Poly(Amino Acids) to Improve Osteogenesis and Suppress Osteoclast Formation in Vitro" Polymers 11, no. 9: 1505. https://doi.org/10.3390/polym11091505
APA StyleLi, S., Chen, X., Wang, X., Xiong, Y., Yan, Y., Tan, Z., Yang, X., & Li, Y. (2019). Simonkolleite Coating on Poly(Amino Acids) to Improve Osteogenesis and Suppress Osteoclast Formation in Vitro. Polymers, 11(9), 1505. https://doi.org/10.3390/polym11091505