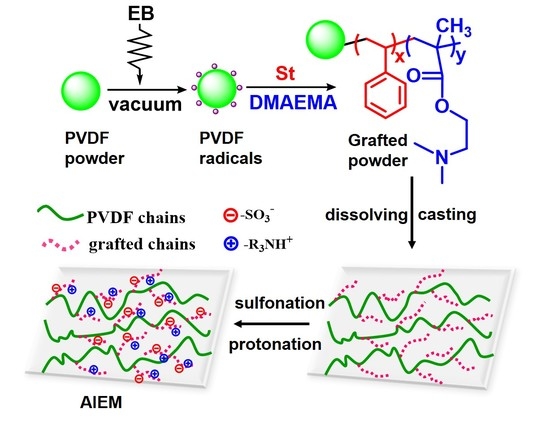
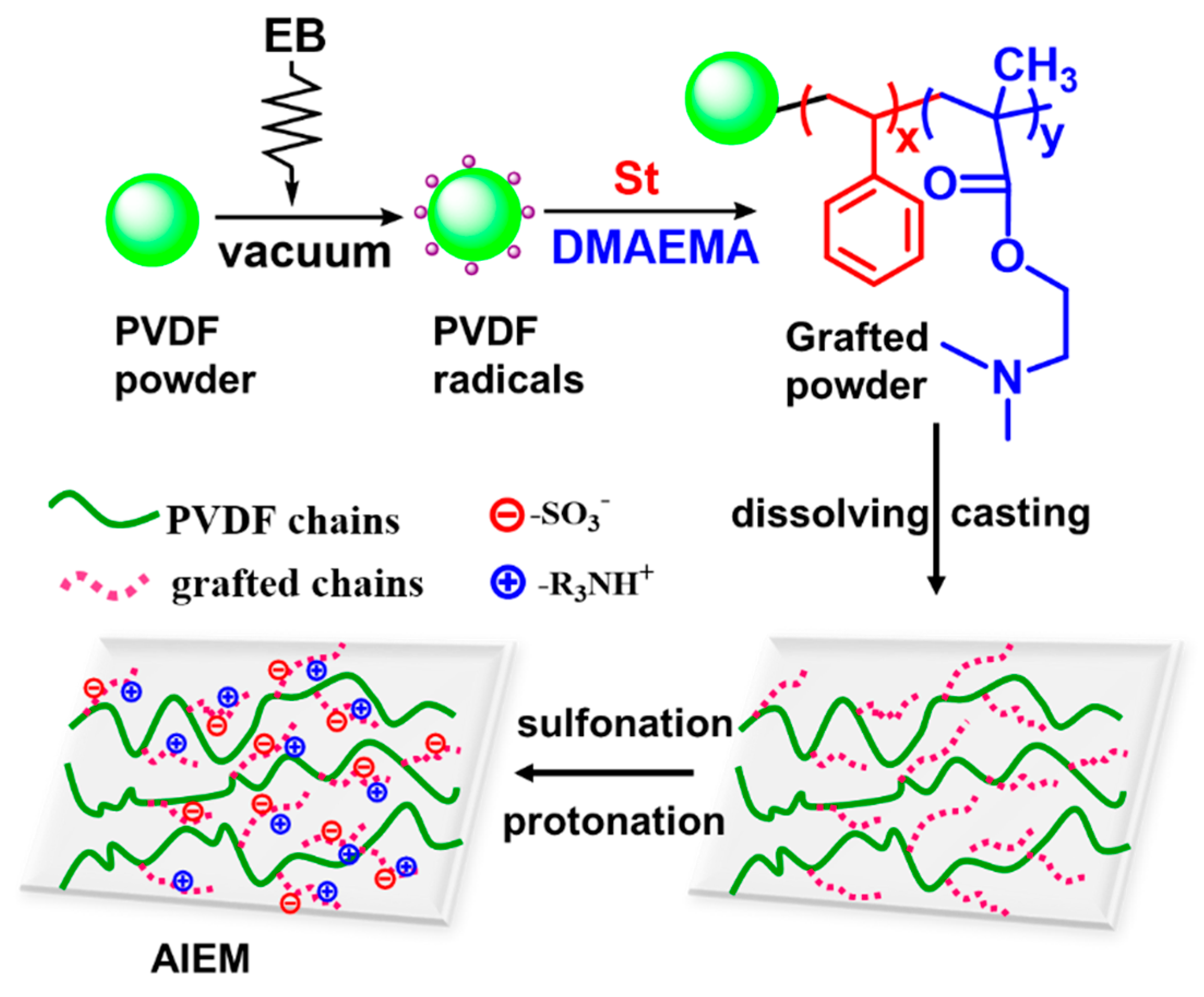
Amphoteric Ion Exchange Membranes Prepared by Preirradiation-Induced Emulsion Graft Copolymerization for Vanadium Redox Flow Battery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preirradiation-Induced Emulsion Graft Copolymerization
2.3. Preparation of the AIEM
2.4. Characterization of the Grafted Powders and the AIEM
2.5. Water Uptake (WU)
2.6. Ion Exchange Capacity (IEC)
2.7. Ionic Conductivity
2.8. V (IV) Permeability
2.9. Open Circuit Voltage of VRFB Assembled with AIEM
3. Results and Discussion
3.1. Preirradiation-Induced Emulsion Graft Copolymerization of St and DMAEMA into PVDF and the Preparation of AIEM
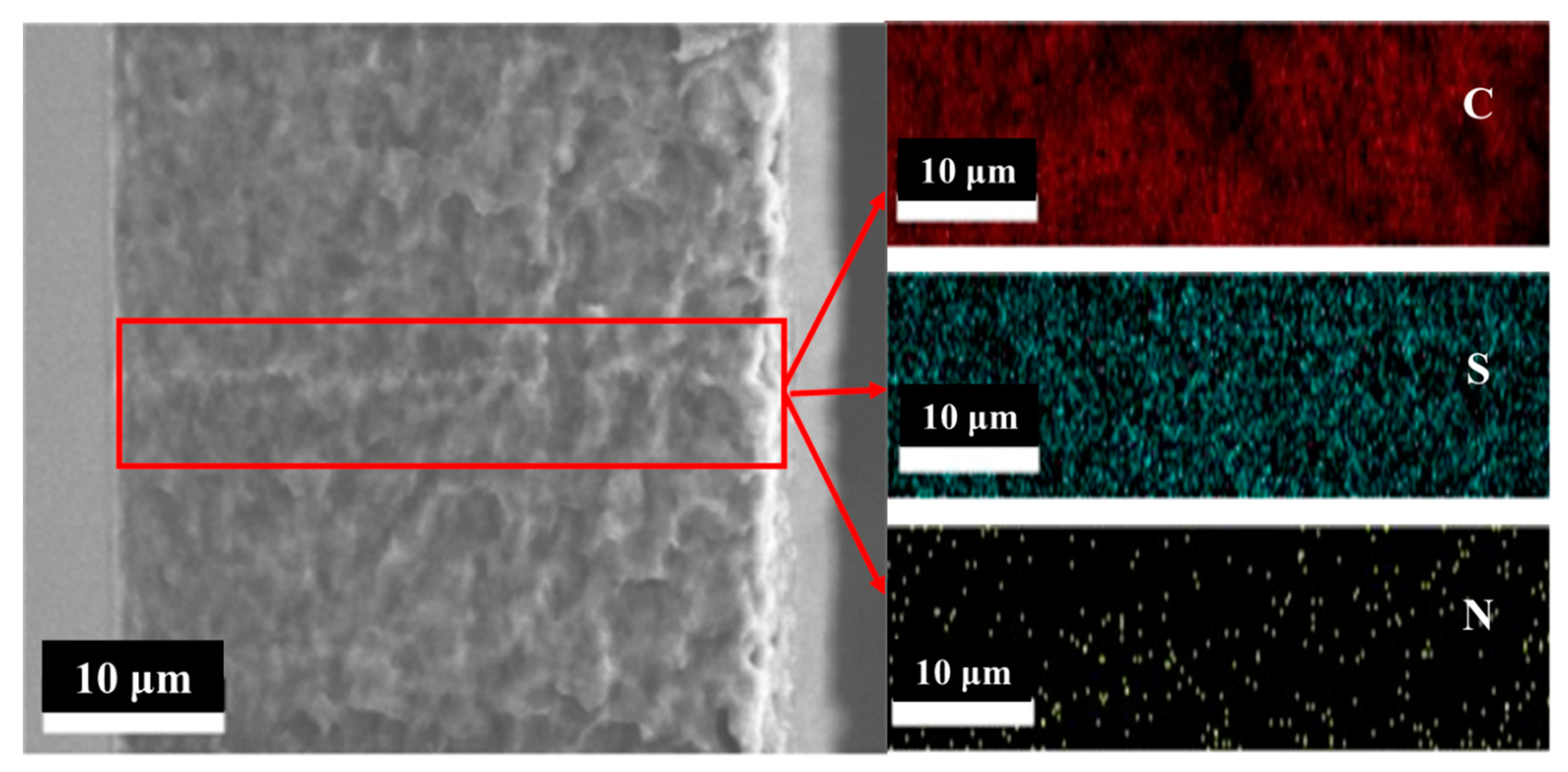
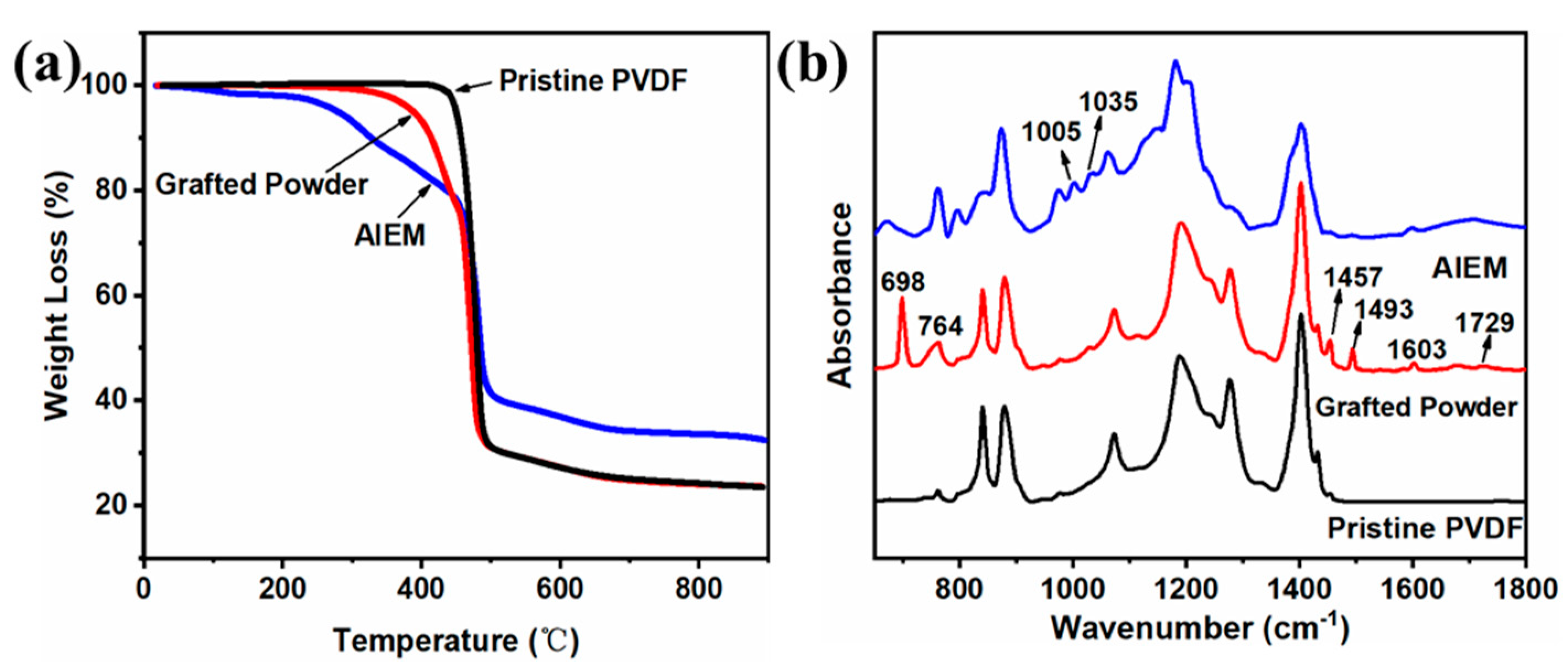
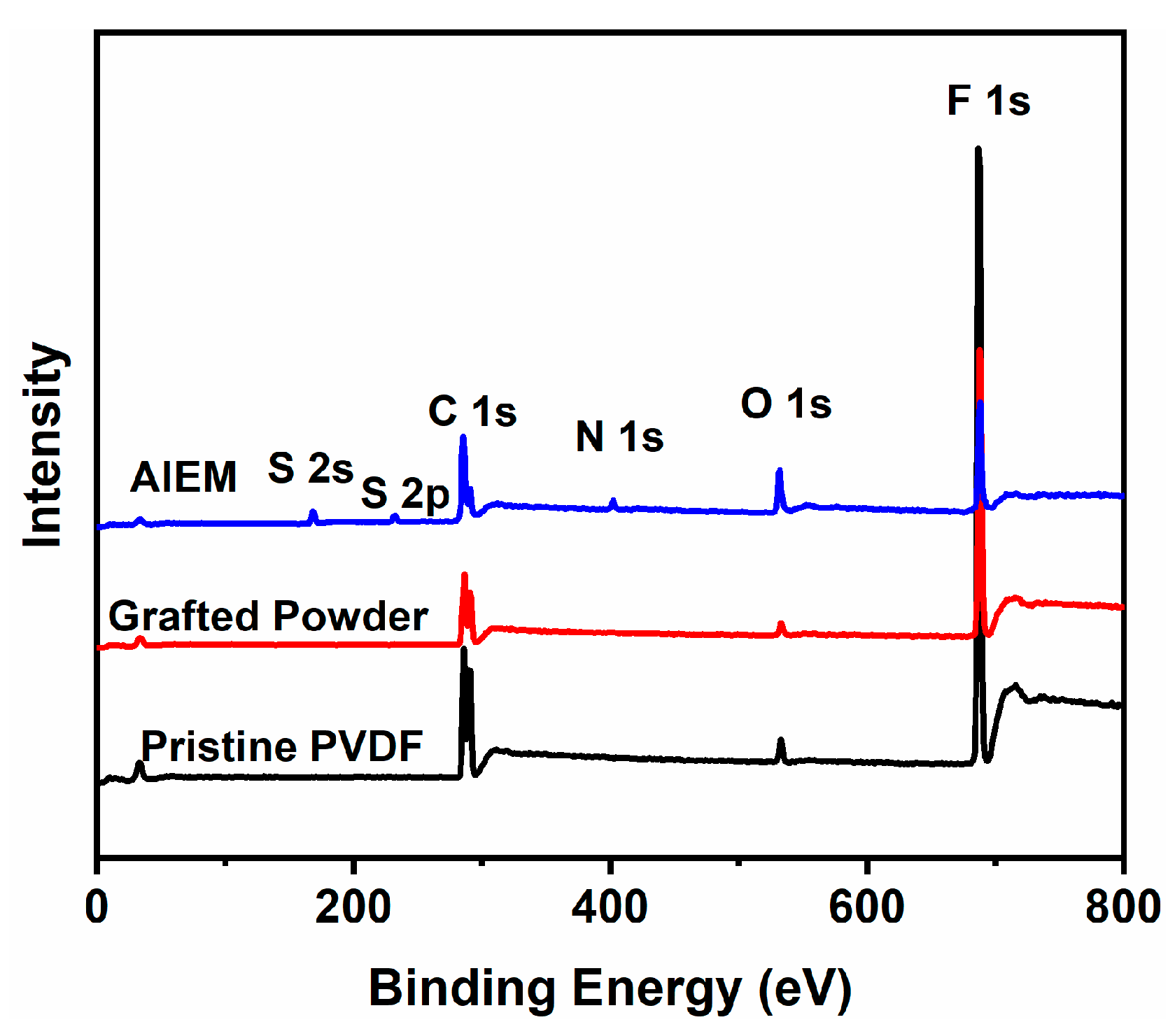
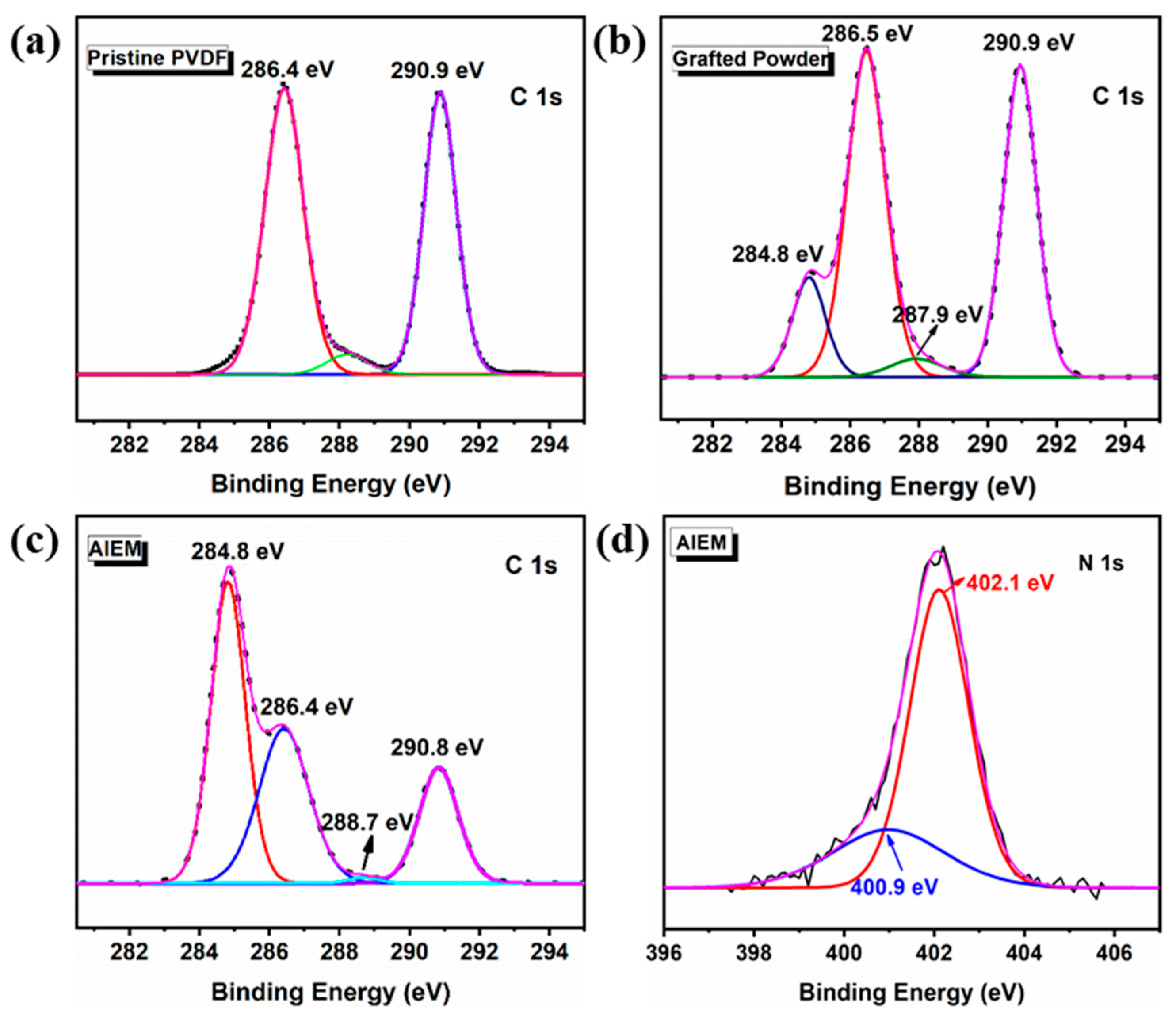
3.2. Characterization of the Grafted Powders and the AIEMs
3.3. Performance of the AIEM for VRFBs
3.3.1. WU, IEC, and Ionic Conductivity
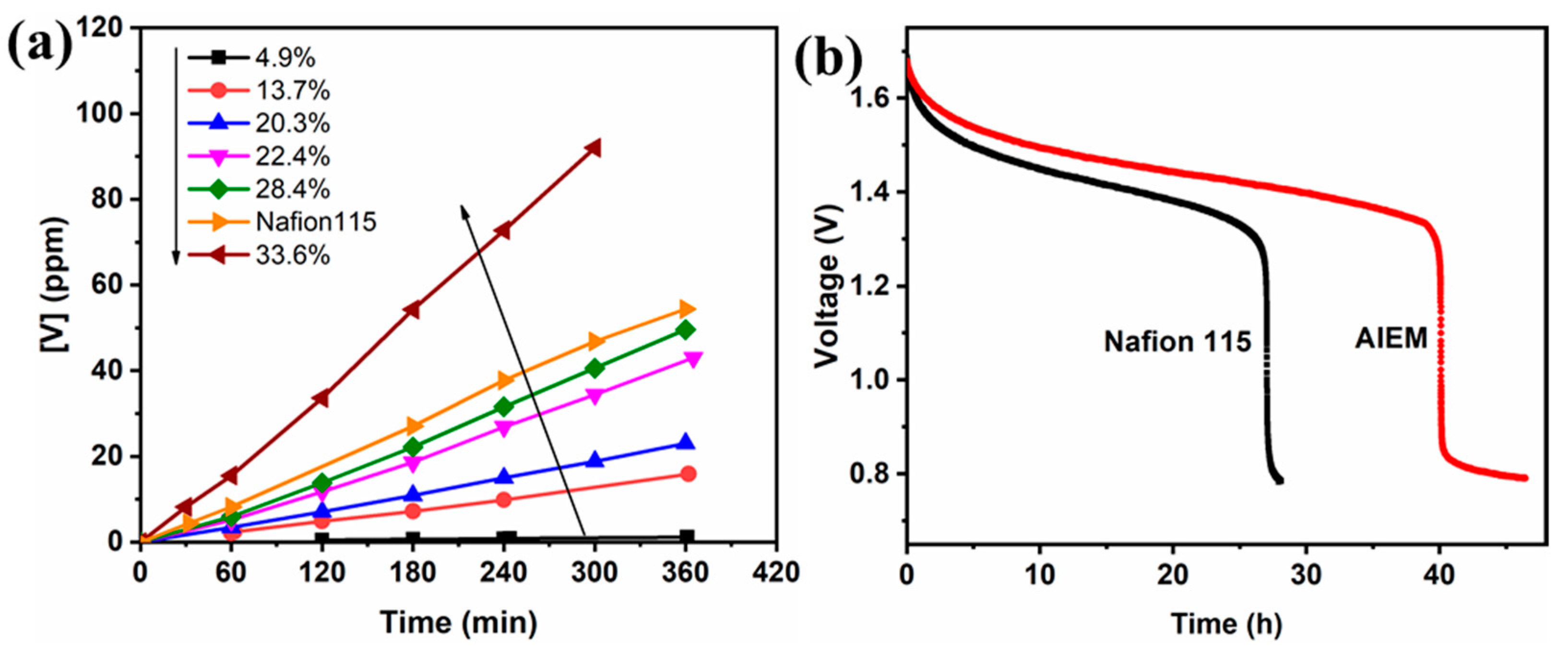
3.3.2. Crossover of Vanadium Ions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhao, T.S.; An, L.; Zeng, Y.K.; Wei, L. Modeling of ion transport through a porous separator in vanadium redox flow batteries. J. Power Sources 2016, 327, 67–76. [Google Scholar] [CrossRef]
- Chae, I.; Luo, T.; Moon, G.H.; Ogieglo, W.; Kang, Y.S.; Wessling, M. Ultra-high proton/vanadium selectivity for hydrophobic polymer membranes with intrinsic nanopores for redox flow battery. Adv. Energy Mater. 2016, 6, 1600517. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, L.T.; Yu, L.H.; Qiu, X.P.; Xi, J.Y. A comparative study of Nafion series membranes for vanadium redox flow batteries. J. Membr. Sci. 2016, 510, 18–26. [Google Scholar] [CrossRef]
- Li, W.Y.; Zhang, Z.Y.; Tang, Y.B.; Bian, H.D.; Ng, T.W.; Zhang, W.J.; Lee, C.S. Graphene-nanowall-decorated carbon felt with excellent electrochemical activity toward VO2+/VO2+ couple for all vanadium redox flow battery. Adv. Sci. 2016, 3, 1500276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Zeng, Y.K.; Zhu, X.B.; Wei, L.; Zhao, T.S. A high-performance dual-scale porous electrode for vanadium redox flow batteries. J. Power Sources 2016, 325, 329–336. [Google Scholar] [CrossRef]
- Teng, X.; Sun, C.; Dai, J.; Liu, H.; Su, J.; Li, F. Solution casting Nafion/polytetrafluoroethylene membrane for vanadium redox flow battery application. Electrochim. Acta 2017, 88, 725–734. [Google Scholar] [CrossRef]
- Teng, X.; Zhao, Y.; Xi, J.; Wu, Z.; Qiu, X.; Chen, L. Nafion/organic silica modified TiO2 composite membrane for vanadium redox flow battery via in situ sol–gel reactions. J. Membr. Sci. 2009, 341, 149–154. [Google Scholar] [CrossRef]
- Aziz, M.A.; Shanmugam, S. Zirconium oxide nanotube–Nafion composite as high performance membrane for all vanadium redox flow battery. J. Power Sources 2017, 337, 36–44. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Peng, S.; Zhang, D.; Yan, X.; Wu, X.; Gong, X.; Wang, Q.; He, G. Electrospinning fiberization of carbon nanotube hybrid sulfonated poly (ether ether ketone) ion conductive membranes for a vanadium redox flow battery. J. Membr. Sci. 2019, 583, 93–102. [Google Scholar] [CrossRef]
- Ling, L.; Xiao, M.; Han, D.; Ren, S.; Wang, S.; Meng, Y. Porous composite membrane of PVDF/Sulfonic silica with high ion selectivity for vanadium redox flow battery. J. Membr. Sci. 2019, 585, 230–237. [Google Scholar] [CrossRef]
- Shukla, G.; Shahi, V.K. Amine functionalized graphene oxide containing C16 chain grafted with poly(ether sulfone) by DABCO coupling: Anion exchange membrane for vanadium redox flow battery. J. Membr. Sci. 2019, 575, 109–117. [Google Scholar] [CrossRef]
- Wang, T.; Moon, S.J.; Hwang, D.-S.; Park, H.; Lee, J.; Kim, S.; Lee, Y.M.; Kim, S. Selective ion transport for a vanadium redox flow battery (VRFB) in nano-crack regulated proton exchange membranes. J. Membr. Sci. 2019, 583, 16–22. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, X.; Gao, L.; Hu, L.; Ruan, X.; Zheng, W.; He, G. Novel triple tertiary amine polymer-based hydrogen bond network inducing highly efficient proton-conducting channels of amphoteric membranes for high-performance vanadium redox flow battery. ACS Appl. Mater. Interfaces 2019, 11, 5003–5014. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Liu, B.; Shi, J.; Zhang, J.; Shi, H. An ultra-high ion selective hybrid proton exchange membrane incorporated with zwitterion-decorated graphene oxide for vanadium redox flow batteries. J. Mater. Chem. A 2019, 7, 12669–12680. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Liu, B.; Wang, H.; Shi, H. Sulfonated polysulfone proton exchange membrane influenced by a varied sulfonation degree for vanadium redox flow battery. J. Membr. Sci. 2019, 584, 173–180. [Google Scholar] [CrossRef]
- Cui, Z.; Drioli, E.; Lee, Y.M. Recent progress in fluoropolymers for membranes. Prog. Polym. Sci. 2014, 39, 164–198. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.T.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Clochard, M.C.; Bègue, J.; Lafon, A.; Caldemaison, D.; Bittencourt, C.; Pireaux, J.J.; Betz, N. Tailoring bulk and surface grafting of poly(acrylic acid) in electron-irradiated PVDF. Polymer 2004, 45, 8683–8694. [Google Scholar] [CrossRef]
- Gubler, L. Polymer design strategies for radiation-grafted fuel cell membranes. Adv. Energy Mater. 2014, 4, 1300827. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Peng, J.; Qiu, J.; Xu, L.; Li, J.; Zhai, M. Designing a new process to prepare amphoteric ion exchange membrane with well-distributed grafted chains for vanadium redox flow battery. J. Membr. Sci. 2012, 419–420, 1–8. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, J.; Chen, J.; Peng, J.; Xu, L.; Zhai, M.; Li, J.; Wei, G. Amphoteric ion exchange membrane synthesized by radiation-induced graft copolymerization of styrene and dimethylaminoethyl methacrylate into PVDF film for vanadium redox flow battery applications. J. Membr. Sci. 2009, 334, 9–15. [Google Scholar] [CrossRef]
- Hu, G.; Wang, Y.; Ma, J.; Qiu, J.; Peng, J.; Li, J.; Zhai, M. A novel amphoteric ion exchange membrane synthesized by radiation-induced grafting α-methylstyrene and N,N-dimethylaminoethyl methacrylate for vanadium redox flow battery application. J. Membr. Sci. 2012, 407–408, 184–192. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, C.H.; Peng, J.; Wang, Y.; Ma, J.; Qiu, J.Y.; Li, J.Q.; Zhai, M.L. Facile synthesis of amphoteric ion exchange membrane by radiation grafting of sodium styrene sulfonate and N,N-dimethylaminoethyl methacrylate for vanadium redox flow battery. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 5194–5202. [Google Scholar] [CrossRef]
- Nasef, M.M.; Saidi, H.; Dahlan, K.Z.M. Single-step radiation induced grafting for preparation of proton exchange membranes for fuel cell. J. Membr. Sci. 2009, 339, 115–119. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, J.Y.; Peng, J.; Xu, L.; Li, J.Q.; Zhai, M.L. Study on the chemical stability of the anion exchange membrane of grafting dimethylaminoethyl methacrylate. J. Membr. Sci. 2011, 376, 70–77. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Ben youcef, H.; Wallasch, F.; Gubler, L. Radiation grafted ETFE-graft-poly(α-methylstyrenesulfonic acid-co-methacrylonitrile) membranes for fuel cell applications. J. Membr. Sci. 2013, 447, 228–235. [Google Scholar] [CrossRef]
- Jetsrisuparb, K.; Ben youcef, H.; Wokaun, A.; Gubler, L. Radiation grafted membranes for fuel cells containing styrene sulfonic acid and nitrile comonomers. J. Membr. Sci. 2014, 450, 28–37. [Google Scholar] [CrossRef]
- Xie, K.; Dong, Z.; Wang, Y.; Qi, W.; Zhai, M.; Zhao, L. Facile preparation of EVOH-based amphoteric ion exchange membrane using radiation grafting technique: A preliminary investigation on its application for vanadium redox flow battery. Polymers 2019, 11, 843. [Google Scholar] [CrossRef]
- Madrid, J.F.; Abad, L.V.; Yamanobe, T.; Seko, N. Effects of chain transfer agent on the electron beam-induced graft polymerization of glycidyl methacrylate in emulsion phase. Colloid Polym. Sci. 2017, 295, 1007–1016. [Google Scholar] [CrossRef]
- Madrid, J.F.; Ueki, Y.; Seko, N. Abaca/polyester nonwoven fabric functionalization for metal ion adsorbent synthesis via electron beam-induced emulsion grafting. Radiat. Phys. Chem. 2013, 90, 104–110. [Google Scholar] [CrossRef]
- Ting, T.M.; Nasef, M.M.; Sithambaranathan, P. Kinetic investigations of emulsion- and solvent-mediated radiation induced graft copolymerization of glycidyl methacrylate onto nylon-6 fibres. J. Radioanal. Nucl. Chem. 2017, 311, 843–857. [Google Scholar] [CrossRef]
- Jiang, B.; Yu, L.; Wu, L.; Mu, D.; Liu, L.; Xi, J.; Qiu, X. Insights into the impact of the Nafion membrane pretreatment process on vanadium flow battery performance. ACS Appl. Mater. Interfaces 2016, 8, 12228–12238. [Google Scholar] [CrossRef] [PubMed]
- Matsusaka, N.; Suzuki, T.; Okubo, M. Effect of partitioning of monomer and emulsifier in aqueous media on particle formation in emulsion homopolymerization of hydrophobic and hydrophilic monomers with a nonionic emulsifier. Polym. J. 2012, 45, 153. [Google Scholar] [CrossRef]
- Ma, J.; Wang, S.; Peng, J.; Yuan, J.; Yu, C.; Li, J.; Ju, X.; Zhai, M. Covalently incorporating a cationic charged layer onto Nafion membrane by radiation-induced graft copolymerization to reduce vanadium ion crossover. Eur. Polym. J. 2013, 49, 1832–1840. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, L.; Zhai, M.; Ni, J.; Zhou, H.; Peng, J.; Li, J.; Wei, G. Pre-irradiation grafting of styrene and maleic anhydride onto PVDF membrane and subsequent sulfonation for application in vanadium redox batteries. J. Power Sources 2008, 177, 617–623. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J.; Li, J.; Zhai, M. PVDF based ion exchange membrane prepared by radiation grafting of ethyl styrenesulfonate and sequent hydrolysis. Radiat. Phys. Chem. 2017, 130, 252–258. [Google Scholar] [CrossRef]
- Dong, L.; Liu, X.; Xiong, Z.; Sheng, D.; Zhou, Y.; Lin, C.; Yang, Y. Design of UV-absorbing PVDF membrane via surface-initiated AGET ATRP. Appl. Surf. Sci. 2018, 435, 680–686. [Google Scholar] [CrossRef]
- Ding, Y.; Shen, X.; Zeng, J.; Wang, X.; Peng, L.; Zhang, P.; Zhao, J. Pre-irradiation grafted single lithium-ion conducting polymer electrolyte based on poly(vinylidene fluoride). Solid State Ion. 2018, 323, 16–24. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, D.K.; Lee, K.J.; Kim, J.H. Single-step synthesis of proton conducting poly(vinylidene fluoride) (PVDF) graft copolymer electrolytes. Eur. Polym. J. 2008, 44, 932–939. [Google Scholar] [CrossRef]
- Yang, X.; Deng, B.; Liu, Z.; Shi, L.; Bian, X.; Yu, M.; Li, L.; Li, J.; Lu, X. Microfiltration membranes prepared from acryl amide grafted poly(vinylidene fluoride) powder and their pH sensitive behaviour. J. Membr. Sci. 2010, 362, 298–305. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Yu, J.-Z.; Xu, Y.-Y.; Xi, Z.-Y.; Zhu, B.-K. Surface modification of PVDF porous membranes via poly(DOPA) coating and heparin immobilization. Colloids Surf. B 2009, 69, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Hester, J.F.; Banerjee, P.; Won, Y.Y.; Akthakul, A.; Acar, M.H.; Mayes, A.M. ATRP of amphiphilic graft copolymers based on PVDF and their use as membrane additives. Macromolecules 2002, 35, 7652–7661. [Google Scholar] [CrossRef]
- Hester, J.F.; Banerjee, P.; Mayes, A.M. Preparation of protein-resistant surfaces on poly(vinylidene fluoride) membranes via surface segregation. Macromolecules 1999, 32, 1643–1650. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M.C.; Tibirna, C.M.; Tuchilus, C.; Debarnot, D.; Pâslaru, E.; Poncin-Epaillard, F. Microwave plasma activation of a polyvinylidene fluoride surface for protein immobilization. J. Phys. D Appl. Phys. 2011, 44, 475303. [Google Scholar] [CrossRef]
- Bai, M.-Y.; Tsai, J.-C. Preparation of electrospun EDTA/PVDF blend nonwoven mats and their use in removing heavy metal ions from electropolishing electrolyte. Fibers Polym. 2014, 15, 2265–2271. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X.G. Water transport in polymer electrolyte membrane fuel cells. Prog. Energy Combust. Sci. 2011, 37, 221–291. [Google Scholar] [CrossRef]
- Devanathan, R. Recent developments in proton exchange membranes for fuel cells. Energy Environ. Sci. 2008, 1, 101–119. [Google Scholar] [CrossRef]
- Xie, Y.J.; Liu, B.; Chen, Z.; Han, X.C.; Liu, B.J.; Zhang, H.B.; Pang, J.H.; Jiang, Z.H. Graft fluorinated poly(arylene ether ketone)s containing highly dense sulfonic-acid-functionalized pendants for proton exchange membranes by C-N coupling. Polymer 2017, 131, 84–94. [Google Scholar] [CrossRef]
- Elgammal, R.A.; Tang, Z.J.; Sun, C.N.; Lawton, J.; Zawodzinski, T.A. Species uptake and mass transport in membranes for vanadium redox flow batteries. Electrochim. Acta 2017, 237, 1–11. [Google Scholar] [CrossRef]
- Yan, X.M.; Zhang, C.M.; Dong, Z.W.; Jiang, B.W.; Dai, Y.; Wu, X.M.; He, G.H. Amphiprotic side-chain functionalization constructing highly proton/vanadium-selective transport channels for high-performance membranes in vanadium redox flow batteries. ACS Appl. Mater. Interfaces 2018, 10, 32247–32255. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.Y.; Li, M.Y.; Ni, J.F.; Zhai, M.L.; Peng, J.; Xu, L.; Zhou, H.H.; Li, J.Q.; Wei, G.S. Preparation of ETFE-based anion exchange membrane to reduce permeability of vanadium ions in vanadium redox battery. J. Membr. Sci. 2007, 297, 174–180. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, L.H.; Wang, J.S.; Liu, L.; Liang, F.; Xi, J.Y. Rational use and reuse of Nafion 212 membrane in vanadium flow batteries. RSC Adv. 2017, 7, 19425–19433. [Google Scholar] [CrossRef]
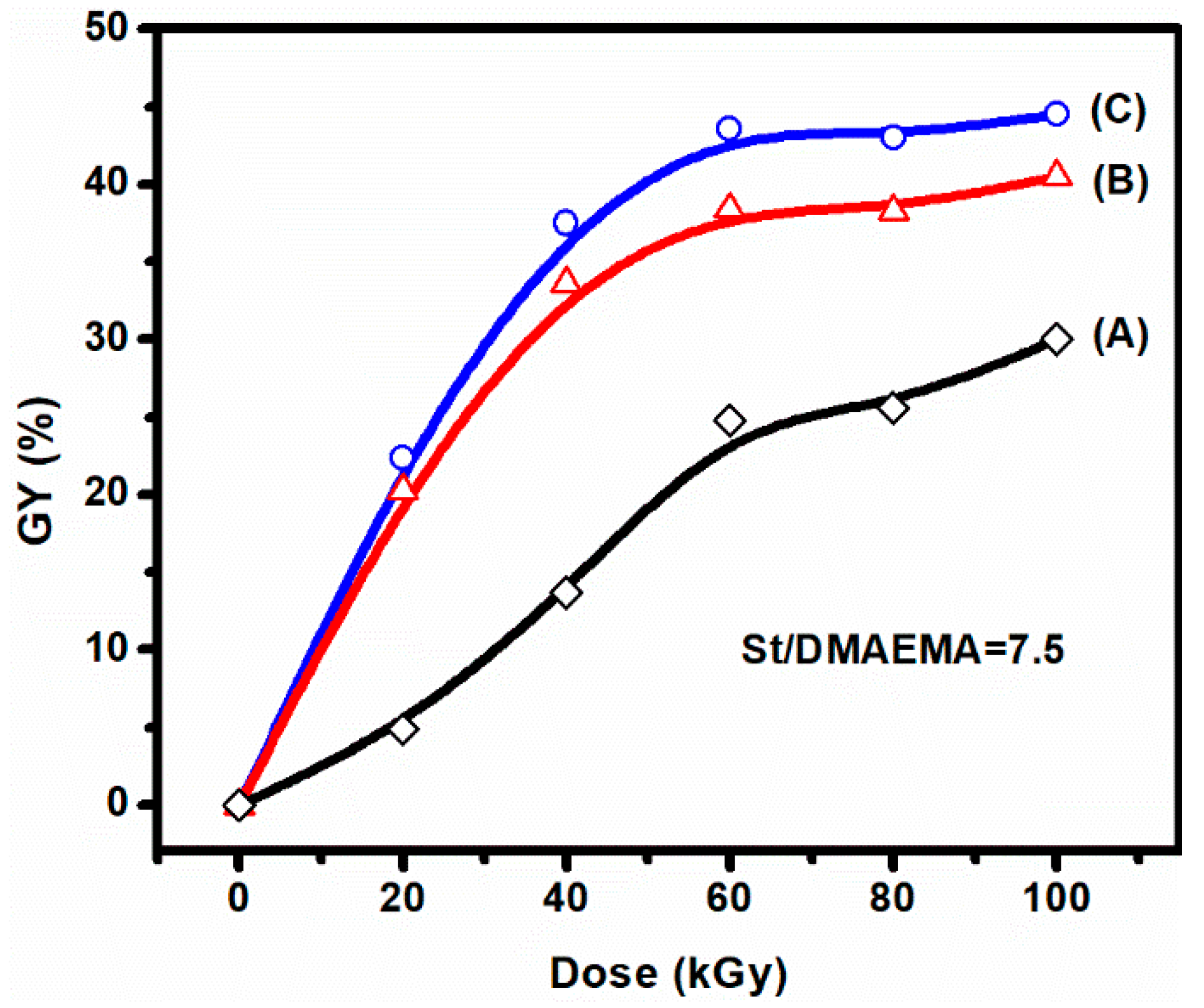
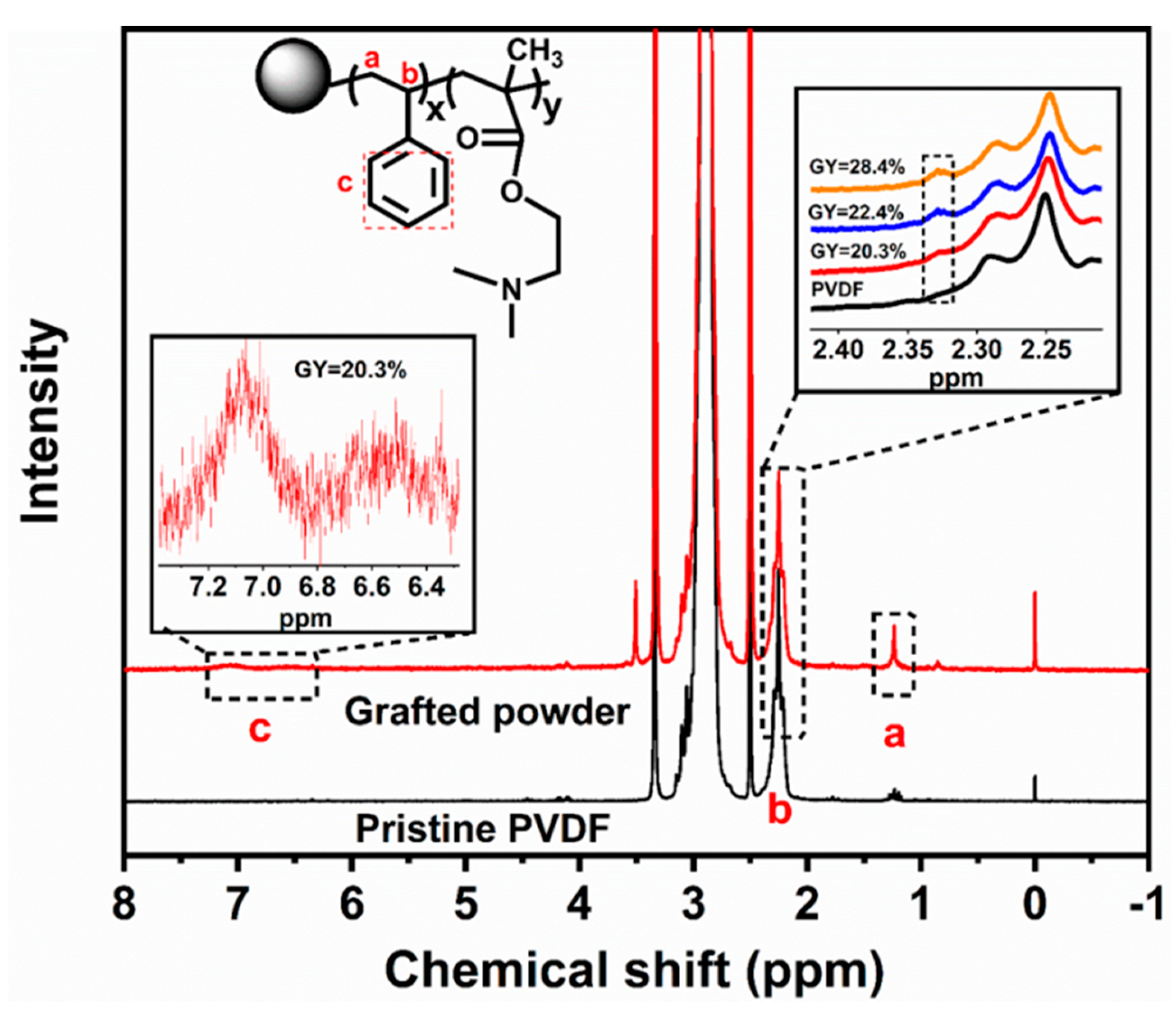
| DMAEMA/St in the Feed | Weight Percent (%) | GY (%) | DMAEMA/St in the Grafted Powder | ||
|---|---|---|---|---|---|
| C | H | N | |||
| 0.13 | 43.93 | 3.74 | 0.13 | 13.7 | 0.09 |
| 0.13 | 47.14 | 4.08 | 0.19 | 20.3 | 0.10 |
| 0.13 | 47.53 | 4.13 | 0.22 | 22.4 | 0.10 |
| 0.13 | 47.47 | 4.24 | 0.25 | 28.4 | 0.10 |
| 0.13 | 50.64 | 4.59 | 0.27 | 33.6 | 0.09 |
| Sample | GY (%) | WU (%) | IEC (mmol g−1) | σ (mS cm−1) |
|---|---|---|---|---|
| AIEM | 4.9 | 15.2 ± 0.1 | 0.37 ± 0.005 | 7.4 ± 0.1 |
| 13.7 | 28.0 ± 0.9 | 0.59 ± 0.002 | 22.3 ± 0.6 | |
| 20.3 | 40.6 ± 0.4 | 0.81 ± 0.007 | 64.7 ± 0.9 | |
| 22.4 | 44.8 ± 0.9 | 0.85 ± 0.010 | 68.0 ± 1.7 | |
| 28.4 | 47.7 ± 0.8 | 1.26 ± 0.004 | 94.8 ± 2.6 | |
| 33.6 | 56.0 ± 1.0 | 1.43 ± 0.040 | 114.0 ± 5.7 | |
| Nafion 115 | - | 26.5 ± 0.3 | 0.90 ± 0.01 | 83.1 ± 1.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Chen, X.; Wang, Y.; Peng, J.; Zhao, L.; Du, J.; Zhai, M. Amphoteric Ion Exchange Membranes Prepared by Preirradiation-Induced Emulsion Graft Copolymerization for Vanadium Redox Flow Battery. Polymers 2019, 11, 1482. https://doi.org/10.3390/polym11091482
Cui Y, Chen X, Wang Y, Peng J, Zhao L, Du J, Zhai M. Amphoteric Ion Exchange Membranes Prepared by Preirradiation-Induced Emulsion Graft Copolymerization for Vanadium Redox Flow Battery. Polymers. 2019; 11(9):1482. https://doi.org/10.3390/polym11091482
Chicago/Turabian StyleCui, Yu, Xibang Chen, Yicheng Wang, Jing Peng, Long Zhao, Jifu Du, and Maolin Zhai. 2019. "Amphoteric Ion Exchange Membranes Prepared by Preirradiation-Induced Emulsion Graft Copolymerization for Vanadium Redox Flow Battery" Polymers 11, no. 9: 1482. https://doi.org/10.3390/polym11091482
APA StyleCui, Y., Chen, X., Wang, Y., Peng, J., Zhao, L., Du, J., & Zhai, M. (2019). Amphoteric Ion Exchange Membranes Prepared by Preirradiation-Induced Emulsion Graft Copolymerization for Vanadium Redox Flow Battery. Polymers, 11(9), 1482. https://doi.org/10.3390/polym11091482