The Use of Waste from the Production of Rapeseed Oil for Obtaining of New Polyurethane Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of RPU/PIR Foams Modified by Rapeseed Cake
2.3. Methods
3. Results and Discussion
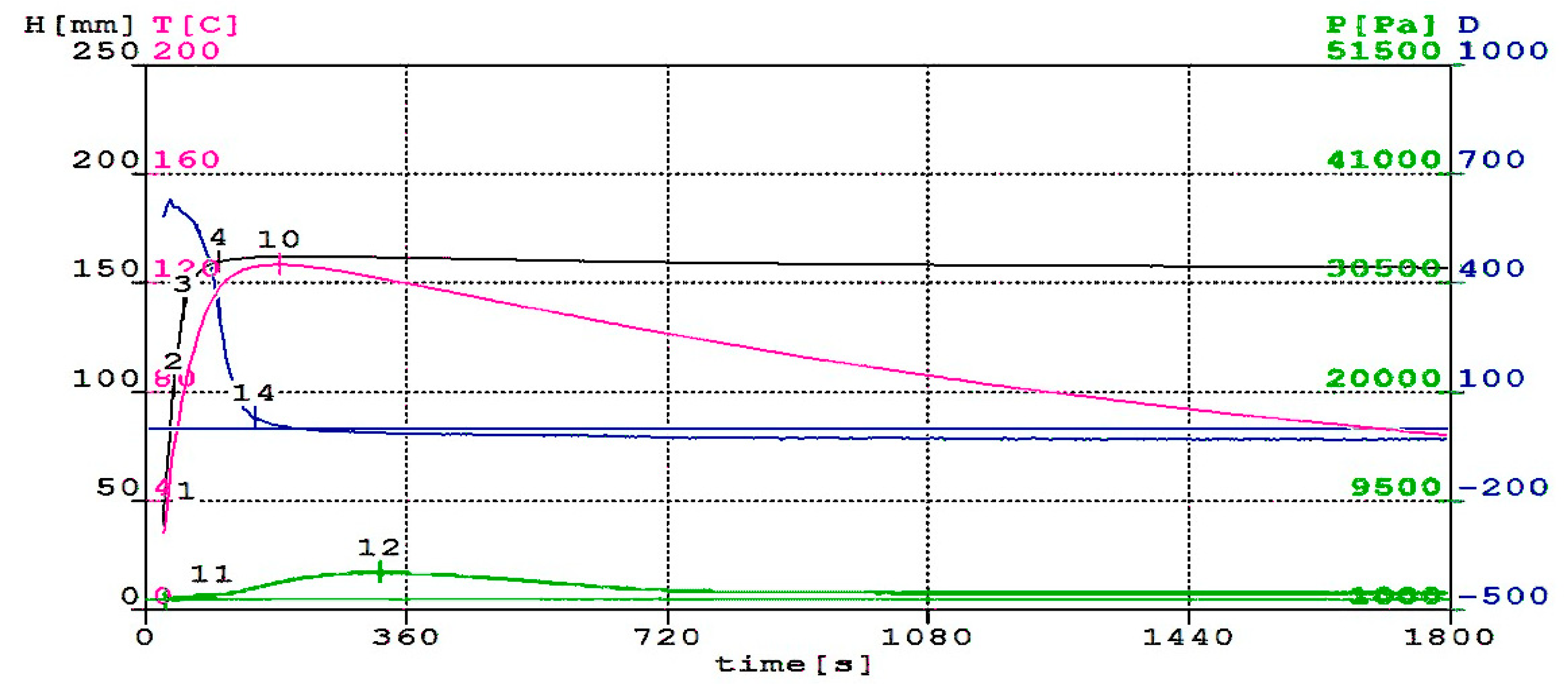
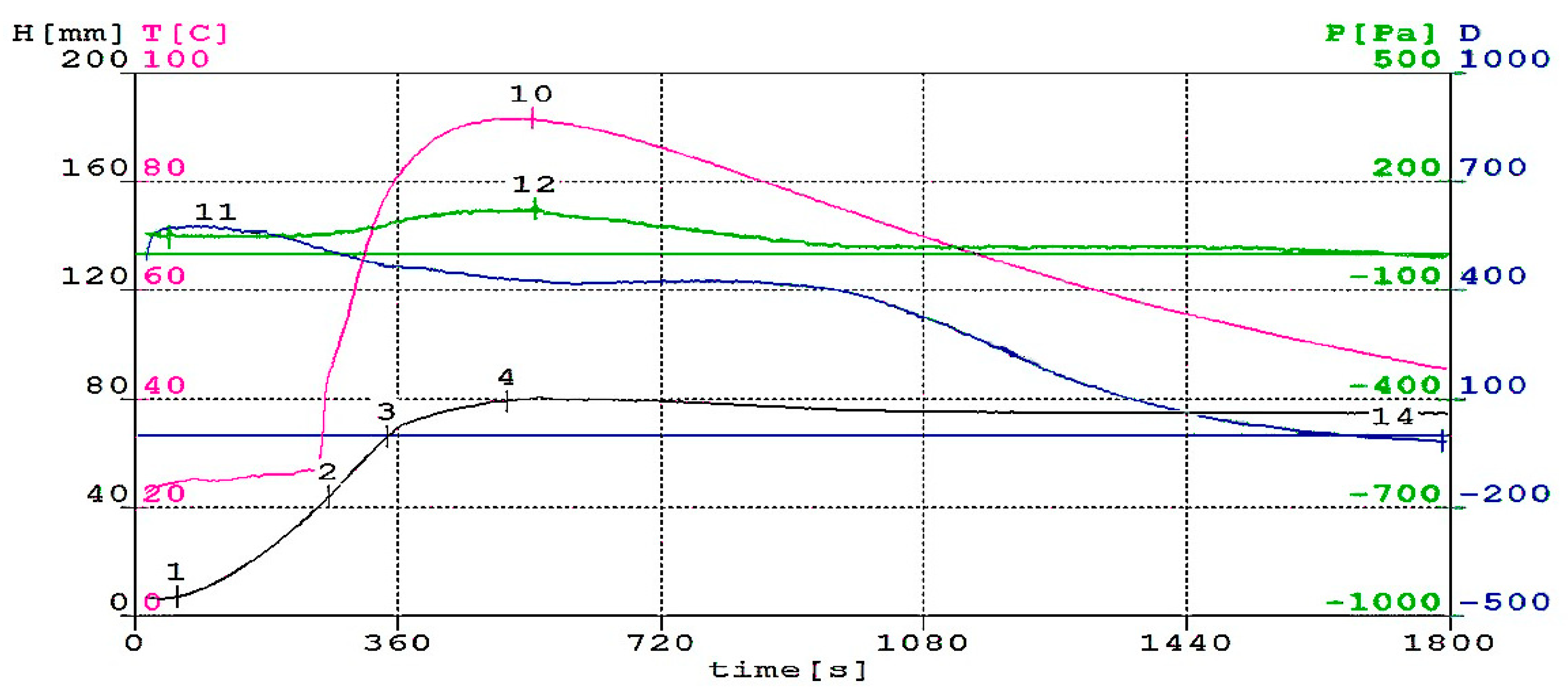
3.1. Foaming Process
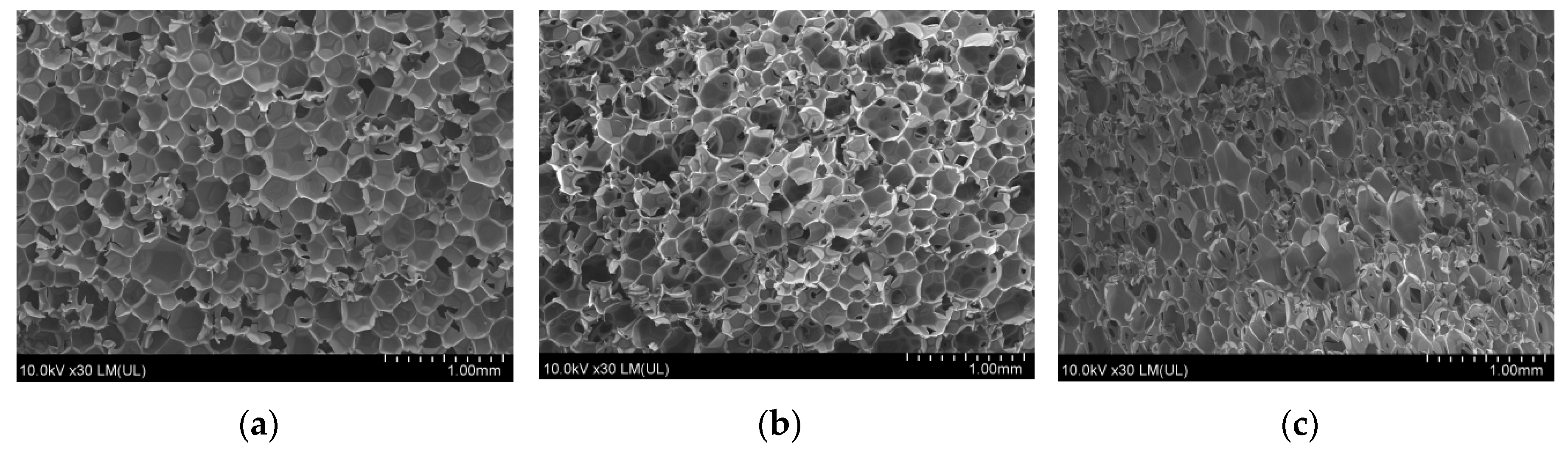
3.2. Cell Structure of RPU/PIR Foams and Thermal Insulation Properties
3.3. Physico-Mechanical Properties
3.4. Aging Resistance Tests
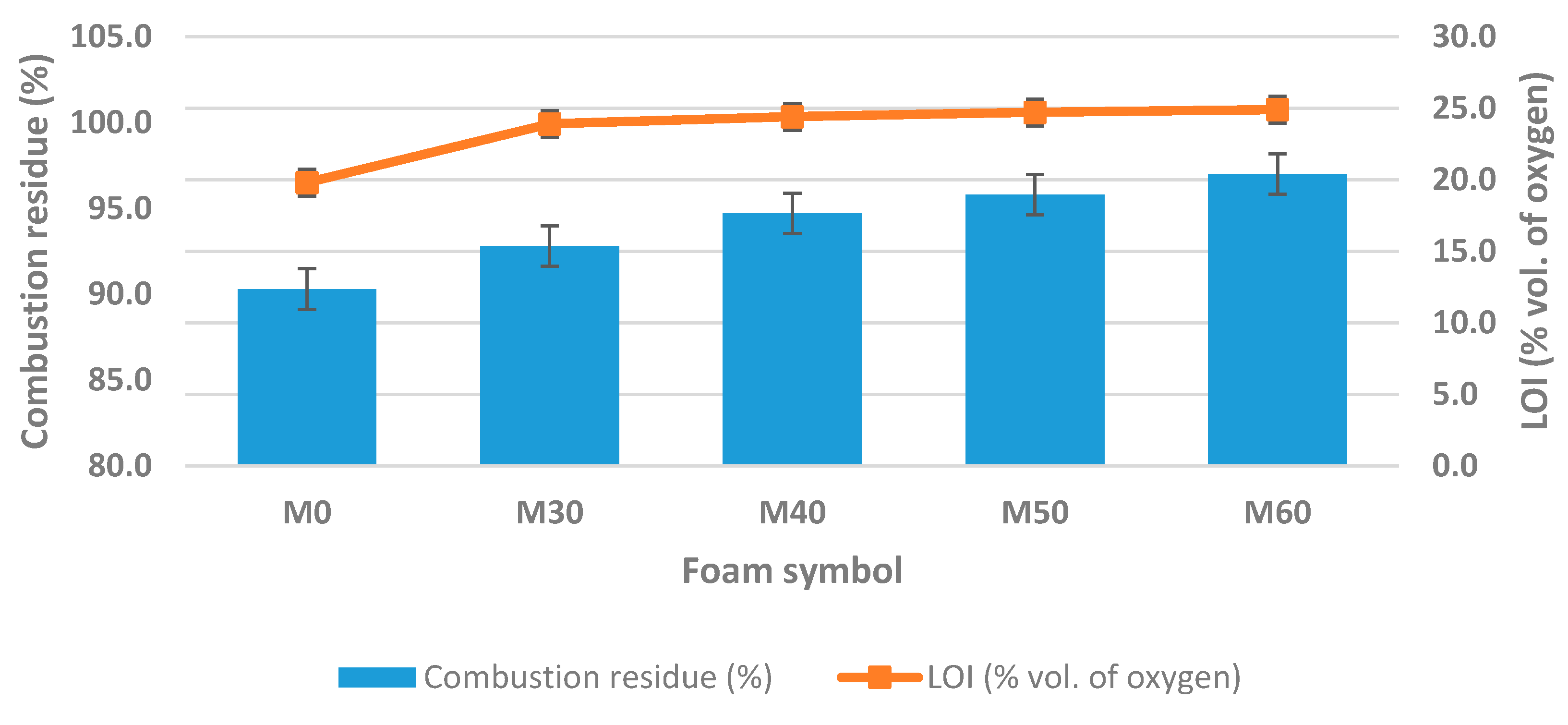
3.5. Flammability and Thermal Resistance
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Svärd, A.; Brännvall, E.; Edlund, U. Rapeseed straw polymeric hemicelluloses obtained by extraction methods based on severity factor. Ind. Crop Prod. 2017, 95, 305–315. [Google Scholar] [CrossRef]
- Carré, P.; Pouzet, A. Rapeseed market, worldwide and in Europe. OCL Oilseeds Fats Crops Lipids 2014, 21. [Google Scholar] [CrossRef]
- Schober, S.; Mittelbach, M. The impact of antioxidants on biodiesel oxidation stability. Eur. J. Lipid. Sci. Technol. 2004, 106, 382–389. [Google Scholar] [CrossRef]
- Ma, H.; Li, S.; Wang, B.; Wang, R.; Tian, S. Transesterification of rapeseed oil for synthesizing biodiesel by K/KOH/γ-Al2O3 as heterogeneous base catalyst. J. Am. Oil Chem. Soc. 2008, 85, 263–270. [Google Scholar] [CrossRef]
- Angelovičová, A.; Angelovič, M. Rapeseed cakes as an important feed raw material for laying hens. Sci. Pap. Anim. Sci. Biotechnol. 2013, 46, 335–338. [Google Scholar]
- Smulikowska, S.; Czerwiński, J.; Mieczkowska, A. Nutritional value of rapeseed expeller cake for broilers: Effect of dry extrusion. J. Anim. Feed Sci. 2006, 15, 447–455. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Mishra, A.S. Glucosinolates in animal nutrition. A review. Anim. Feed Sci. Technol. 2007, 132, 1–27. [Google Scholar] [CrossRef]
- Pohl, F.; Goua, M.; Bermano, G.; Russell, W.R.; Scobbie, L.; Maciel, P.; Lin, P.K.T. Revalorisation of rapeseed pomace extracts: An in vitro study into its anti-oxidant and DNA protective properties. Food Chem. 2018, 239, 323–332. [Google Scholar] [CrossRef]
- Cieślikowski, B.; Juliszewski, T.; Łapczyńska-Kordon, B. Utilisation of bio-fuel technology by-products for power production purposes. Agric. Eng. 2006, 12, 51–57. (In Polish) [Google Scholar]
- Li, S.; Bouzidi, L.; Narine, S.S. Polyols from self-metathesis-generated oligomers of soybean oil and their polyurethane foams. Eur. Polym. J. 2017, 93, 232–245. [Google Scholar] [CrossRef]
- Członka, S.; Bertino, M.F.; Kośny, J.; Strąkowska, A.; Masłowski, M.; Strzelec, K. Linseed oil as a natural modifier of rigid polyurethane foams. Ind. Crop Prod. 2018, 115, 40–51. [Google Scholar] [CrossRef]
- Kattiyaboota, T.; Thongpin, C. Effect of natural oil based polyols on the properties of flexible polyurethane foams blown by distilled water. Energy Procedia 2016, 89, 177–185. [Google Scholar] [CrossRef]
- Kwon, O.; Yang, S.; Kim, D.; Park, J. Characterization of polyurethane foam prepared by using starch as polyol. J. Appl. Polym. Sci. 2006, 103, 1544–1553. [Google Scholar] [CrossRef]
- Tanaka, R.; Hirose, S.; Hatakeyama, H. Preparation and characterization of polyurethane foams using a palm oil-based polyol. Bioresour. Technol. 2008, 99, 3810–3816. [Google Scholar] [CrossRef] [PubMed]
- Prociak, A.; Rokicki, G.; Ryszkowska, J. Polyurethane Materials; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2014. (In Polish) [Google Scholar]
- Pillai, P.K.S.; Li, S.; Bouzidi, L.; Narine, S.S. Metathesized palm oil & novel polyol derivatives: Structure, chemical composition and physical properties. Ind. Crop Prod. 2016, 84, 205–223. [Google Scholar] [CrossRef]
- Beltrán, A.A.; Boyacá, L.A. Production of rigid polyurethane foams from soy-based polyols. Lat. Am. Appl. Res. 2011, 41, 75–80. [Google Scholar]
- Yang, R.; Wang, B.; Li, M.; Zhang, X.; Li, J. Preparation, characterization and thermal degradation behavior of rigid polyurethane foam using a malic acid based polyols. Ind. Crops Prod. 2019, 136, 121–128. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based polyol. Polym. J. 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Fridrihsone, A.; Stirna, U.; Lazdina, B.; Misane, M.; Vilsone, D. Characterization of polyurethane structure and properties based on rapeseed oil derived polyol. Eur. Polym. J. 2013, 49, 1204–1214. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. Odontol. Scand. 2014, 10, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.B.; Boas, C.V.; Schleder, G.R.; Nacas, A.M.; Rosa, D.S.; Santos, D.J. Bio-based polyurethane prepared from Kraft lignin and modified castor oil. Ex. Polym. Lett. 2016, 10, 927–940. [Google Scholar] [CrossRef]
- Pan, X.; Saddler, J.N. Effect of replacing polyol by organosolv and kraft lignin on the property and structure of rigid polyurethane foam. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, K.; Kaddami, H.; Magnin, A.; Dufresne, A.; Ahmad, A. Bio-based polyurethane reinforced with cellulose nanofibers: A comprehensive investigation on the effect of interface. Carbohydr. Polym. 2015, 122, 202–211. [Google Scholar] [CrossRef]
- Park, S.H.; Oh, K.W.; Kim, S.H. Reinforcement effect of cellulose nanowhisker on bio-based polyurethane. Compos. Sci. Technol. 2013, 86, 82–88. [Google Scholar] [CrossRef]
- Gama, N.V.; Soares, B.; Freire, C.S.R.; Silva, R.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Bio-based polyurethane foams toward applications beyond thermal insulation. Mater. Des. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Sanches, A.O.; Ricco, L.H.S.; Malmonge, L.F.; da Silva, M.J.; Sakamoto, W.K.; Malmonge, J.A. Influence of cellulose nanofibrils on soft and hard segments of polyurethane/cellulose nanocomposites and effect of humidity on their mechanical properties. Polym. Test. 2014, 40, 99–105. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Liszkowska, J. Composites of rigid polyurethane-polyisocyanurate foams with oak bark. Polimery 2015, 9, 666–672. [Google Scholar] [CrossRef]
- Bakare, I.O.; Okieimen, F.E.; Pavithran, C.; Abdul Khalil, H.P.S.; Brahmakumar, M. Mechanical and thermal properties of sisal fiber-reinforced rubber seed oil-based polyurethane composites. Mater. Des. 2010, 31, 4274–4280. [Google Scholar] [CrossRef]
- Lee, S.-H.; Wang, S. Biodegradable polymers/bamboo fiber biocomposite with bio-based coupling agent. Compos. Part A 2006, 37, 80–91. [Google Scholar] [CrossRef]
- Ekici, B.; Kentli, A.; Kucuk, H. Improving sound absorption property of polyurethane foams by adding tea-leaf fibers. Arch. Acoust. 2012, 37, 515–520. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryński, B.; Borowicz, M.; Liszkowska, J. New polyurethane materials containing biofiller. Polimery 2015, 9, 586–591. [Google Scholar] [CrossRef]
- Wanasundara, J.P.D. Proteins of Brassicaceae oilseeds and their potential as a plant protein source. Crit. Rev. Food Sci. Nutr. 2011, 51, 635–677. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, J.; Wan, J. Agriculture and crop science in China: Innovation and sustainability. Crop J. 2017, 5, 127–135. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Z.; Li, Z.; Zhang, Q.; Hu, J.; Xiao, Y.; Cai, D.; Wu, J.; King, G.J.; Li, H.; et al. Dissection of the genetic architecture of three seed-quality traits and consequences for breeding in Brassica napus. Plant. Biotechnol. J. 2018, 16, 1336–1348. [Google Scholar] [CrossRef] [PubMed]
- Haberstroh, E.; Zabold, J. Modelling and simulation of the foam formation process. J. Polym. Eng. 2004, 24, 377–390. [Google Scholar] [CrossRef]
- Saha, M.C.; Barua, B.; Mohan, S. Study on the cure kinetic behavior of thermosetting polyurethane solids and foams: Effect of temperature, density and carbon Nanofiber. J. Eng. Mater. Technol. 2011, 133, 011015. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A.; Cabulis, U.; Kirpluks, M.; Ryszkowska, J.; Auguścik, M. Innovative porous polyurethane-polyisocyanurate foams based on rapeseed oil and modified with expandable graphite. Ind. Crop Prod. 2017, 95, 316–323. [Google Scholar] [CrossRef]
- Tu, Y.C.; Fan, H.; Suppes, G.J.; Hsieg, F.-H. Physical properties of water-blown rigid polyurethane foams containing epoxidized soybean oil in different isocyanate indices. J. App. Polym. Sci. 2009, 114, 2577–2583. [Google Scholar] [CrossRef]
- Malewska, E.; Prociak, A. The effect of nanosilica filler on the foaming process and properties of flexible polyurethane foams obtained with rapeseed oil-based polyol. Polimery 2015, 60, 472–480. [Google Scholar] [CrossRef]
- Gómez-Fernández, S.; Ugarte, L.; Calvo-Correas, T.; Peña-Rodríguez, C.; Corcuera, M.A.; Eceiza, A. Properties of flexible polyurethane foams containing isocyanate functionalized kraft lignin. Ind. Crop Prod. 2017, 100, 51–64. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A.; Kirpluks, M.; Cabulis, U. Porous polyurethane composites based on bio-components. Compos. Sci. Technol. 2013, 75, 70–76. [Google Scholar] [CrossRef]
- Yan, D.; Xu, L.; Chen, C.; Tang, J.; Ji, X.; Li, Z. Enhanced mechanical and thermal properties of rigid polyurethane foam composites containing graphene nanosheets and carbon nanotubes. Polym. Int. 2012, 61, 1107–1114. [Google Scholar] [CrossRef]
- Dolomanova, V.; Jens, C.M.R.; Jensen, L.R.; Pyrz, R.; Timmons, A.B. Mechanical properties and morphology of nano-reinforced rigid PU foam. J. Cell. Plast. 2011, 47, 81–93. [Google Scholar] [CrossRef]
- Niyogi, D.; Kumar, R.; Gandhi, K.S. Water blown free rise polyurethane foams. Polym. Eng. Sci. 1999, 39, 199–209. [Google Scholar] [CrossRef]
- Kang, J.W.; Kim, J.M.; Kim, M.S.; Kim, Y.H.; Kim, W.N.; Jang, W.; Shin, D.S. Effects of nucleating agents on the morphological, mechanical and thermal insulating properties of rigid polyurethane foams. Macromol. Res. 2009, 17, 856–862. [Google Scholar] [CrossRef]
- Chang, L. Improving the Mechanical Performance of Wood Fiber Reinforced Bio-Based Polyurethane Foam. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2014; p. 101. [Google Scholar]
- Luo, X.; Mohanty, A.; Misra, M. Lignin as a reactive reinforcing filler for water-blown rigid biofoam composites from soy oil-based polyurethane. Ind. Crop Prod. 2013, 47, 13–19. [Google Scholar] [CrossRef]
- Sung, G.; Kim, J.H. Influence of filler surface characteristics on morphological, physical, acoustic properties of polyurethane composite foams filled with inorganic fillers. Compos. Sci. Technol. 2017, 146, 147–154. [Google Scholar] [CrossRef]
- Kuhn, J.; Ebert, H.-P.; Arduini-Shuster, M.C.; Buttner, D.; Fricke, J. Thermal transport in polystyrene and polyurethane foam insulations. Int. J. Heat Mass Transf. 1992, 35, 1795–1801. [Google Scholar] [CrossRef]
- Kairyte, A.; Vejelis, S. Evaluation of forming mixture composition impact on properties of water blown rigid polyurethane (PUR) foam from rapeseed oil polyol. Ind. Crop Prod. 2015, 66, 210–215. [Google Scholar] [CrossRef]
- Thirumal, M. Effect of foam density on the properties of water blown rigid polyurethane foam. J. Appl. Polym. Sci. 2008, 108, 1810. [Google Scholar] [CrossRef]
- Rojek, P.; Prociak, A. Effect of different rapeseed-oil-based polyols on mechanical properties of flexible polyurethane foams. J. Appl. Polym. Sci. 2012, 125, 2936. [Google Scholar] [CrossRef]
- Hamilton, A.R.; Thomsen, O.T.; Madaleno, L.A.O.; Jensen, L.R.; Rauhe, J.C.M.; Pyrz, R. Evaluation of the anisotropic mechanical properties of reinforced polyurethane foams. Compos. Sci. Technol. 2013, 87, 210–217. [Google Scholar] [CrossRef]
- Członka, S.; Bertino, M.F.; Strzelec, K. Rigid polyurethane foams reinforced with industrial potato protein. Polym. Test. 2018, 68, 135–145. [Google Scholar] [CrossRef]
- Zhang, S.; Xiang, A.; Tian, H.; Varada Rajulu, A. Water-blown castor oil-based polyurethane foams with soy protein as a reactive reinforcing filler. J. Polym. Environ. 2018, 26, 15–22. [Google Scholar] [CrossRef]
- Żabski, L.; Papiński, J. PIR foams—A new type of insulation: Rigid polyurethane foam. Izolacje 2012, 6, 54–60. (In Polish) [Google Scholar]
- Modesti, M.; Adriani, V.; Simioni, F. Chemical and physical blowing agents in structural polyurethane foams: Simulation and characterization. Polym. Eng. Sci. 2000, 40, 2046–2057. [Google Scholar] [CrossRef]
- Borkowski, R. Thermal insulation in industrial applications and environmental protection. In Proceedings of the Izolacje, Warsaw, Poland, 27–28 February 2013. (In Polish). [Google Scholar]
- Paciorek-Sadowska, J. Polyol containing boron atoms as a compound which reduces flammability of rigid polyurethane-polyisocyanurate foam. In Aspects of Polyurethanes; Yilmaz, F., Ed.; InTech: Rijeka, Croatia, 2017; pp. 111–130. [Google Scholar]
- Zarzyka-Niemiec, I. Application of reaction products of parabanic acid and alkylene carbonates to obtaining polyurethane foams. Polym. Int. 2007, 56, 1499–1506. [Google Scholar] [CrossRef]
- Lubczak, J. Polyurethane foams with purine rings. Polimery 2007, 52, 595–600. [Google Scholar] [CrossRef]
- Zarzyka, I.; Naróg, D.; Stagraczyński, R. Polyurethane foams with the contribution of products of hydroxyalkylation of N,N′-bis(2-hydroxyalkyl)oxamides with alkylene carbonates—Obtaining and properties. J. Appl. Polym. Sci. 2012, 124, 755–764. [Google Scholar] [CrossRef]
- Lubczak, J. Hydroxyalkylation of cyclic imides with oxiranes Part I. Kinetics of reaction in presence of triethylamine catalyst. Open J. Phys. Chem. 2012, 2, 88–96. [Google Scholar] [CrossRef]
- Chmiel, E.; Lubczak, J. Synthesis of oligoetherols from mixtures of melamine and boric acid and polyurethane foams formed from these oligoetherols. Polym. Bull. 2019, 76, 2253–2275. [Google Scholar] [CrossRef]
- Chmiel, E.; Oliwa, R.; Lubczak, J. Boron-containing non-flammable polyurethane foams. Polym. Plast. Technol. Eng. 2019, 58, 394–404. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhou, Y.; Hu, L. The study of mechanical behavior and flame retardancy of castor oil phosphate-based rigid polyurethane foam composites containing expanded graphite and triethyl phosphate. Polym. Deg. Stab. 2013, 98, 2784–2794. [Google Scholar] [CrossRef]
- McKenna, S.T.; Hull, T.R. The fire toxicity of polyurethane foams. Fire Sci. Rev. 2016, 5, 3. [Google Scholar] [CrossRef]
- Cullis, C.F.; Hirschler, M.M. The Combustion of Organic Polymers; Clarendon Press: Oxford, UK, 1981. [Google Scholar]
- Cyzio, K.; Lubczak, J. Oligoetherols obtained from uric acid, glycidol, and alkylene carbonates and their use to obtain polyurethane foams of enhanced thermal resistance. Polym.-Plast. Technol. 2017, 56, 13. [Google Scholar] [CrossRef]
- Chmiel, E.; Lubczak, J.; Stagraczyński, R. Modification of polyurethane foams with 1,3,5-triazine ring and boron. Macrom. Res. 2017, 25, 317. [Google Scholar] [CrossRef]
- Morgan, A.B.; Wilkie, C.A.; Nelson, G.L. (Eds.) Fire and Polymers VI: New Advances in Flame Retardant Chemistry and Science; American Chemical Society: Washington, DC, USA, 2012; ISBN 9780841227804. [Google Scholar]
- Modesti, M.; Lorenzetti, A.; Simioni, F.; Checchin, M. Influence of different flame retardants on fire behaviour of modified PIR/PUR polymers. Polym. Deg. Stab. 2001, 74, 475–479. [Google Scholar] [CrossRef]
- Stirna, U.; Cabulis, U.; Beverte, I. Water-blown polyisocyanurate foams from vegetable oil polyols. J. Cell. Plast. 2008, 44, 139–160. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A.; Kirpluks, M.; Cabulis, U. Polyurethane–polyisocyanurate foams modified with hydroxyl derivatives of rapeseed oil. Ind. Crops Prod. 2015, 74, 849–857. [Google Scholar] [CrossRef]


| Parameter | Dry Mass (%) | Proteins (%) | Minerals (%) | Vitamins (%) | Glucosinolates (%) | Fat (%) | Crude Fiber (%) |
|---|---|---|---|---|---|---|---|
| Value | 93.13 | 38.00 | 11.99 | 0.70 | 0.12 | 15.48 | 12.60 |
| Foam Symbol | Rokopol RF-551 (g) | Tegostab 8460 (g) | 33% DABCO (g) | 33% Potassium Acetate (g) | Antiblaze TCMP (g) | Distilled Water (g) | Rapeseed Cake (g) | Purocyn B (g) |
|---|---|---|---|---|---|---|---|---|
| M0 | 66.80 | 5.40 | 3.15 | 7.95 | 54.00 | 3.15 | 0.00 | 250.60 |
| M30 | 66.80 | 5.40 | 3.15 | 7.95 | 54.00 | 3.15 | 95.20 | 250.60 |
| M40 | 66.80 | 5.40 | 3.15 | 7.95 | 54.00 | 3.15 | 127.00 | 250.60 |
| M50 | 66.80 | 5.40 | 3.15 | 7.95 | 54.00 | 3.15 | 158.70 | 250.60 |
| M60 | 66.80 | 5.40 | 3.15 | 7.95 | 54.00 | 3.15 | 190.40 | 250.60 |
| Foam Symbol | Cell Size (μm) | Thickness of Cell Wall (μm) | Content of Closed Cells per Area Unit (cells/mm2) |
|---|---|---|---|
| M0 | 316 ± 15 | 17 ± 2 | 12 ± 1 |
| M30 | 270 ± 14 | 20 ± 2 | 11 ± 1 |
| M60 | 260 ± 12 | 24 ± 2 | 10 ± 1 |
| Foam Symbol | λ (mW/(m·K)) | Closed Cells Content (%) | Absorbability (%) | Water Absorption (%) |
|---|---|---|---|---|
| M0 | 34.1 ± 0.1 | 87.9 ± 0.9 | 10.9 ± 0.2 | 1.5 ± 0.1 |
| M30 | 34.7 ± 0.1 | 82.6 ± 1.1 | 25.6 ± 0.6 | 4.2 ± 0.2 |
| M40 | 34.6 ± 0.1 | 80.8 ± 0.8 | 31.3 ± 0.5 | 4.5 ± 0.2 |
| M50 | 34.9 ± 0.1 | 79.7 ± 1.3 | 45.6 ± 0.9 | 5.8 ± 0.2 |
| M60 | 34.8 ± 0.2 | 79.1 ± 1.2 | 56.6 ± 1.2 | 10.9 ± 0.3 |
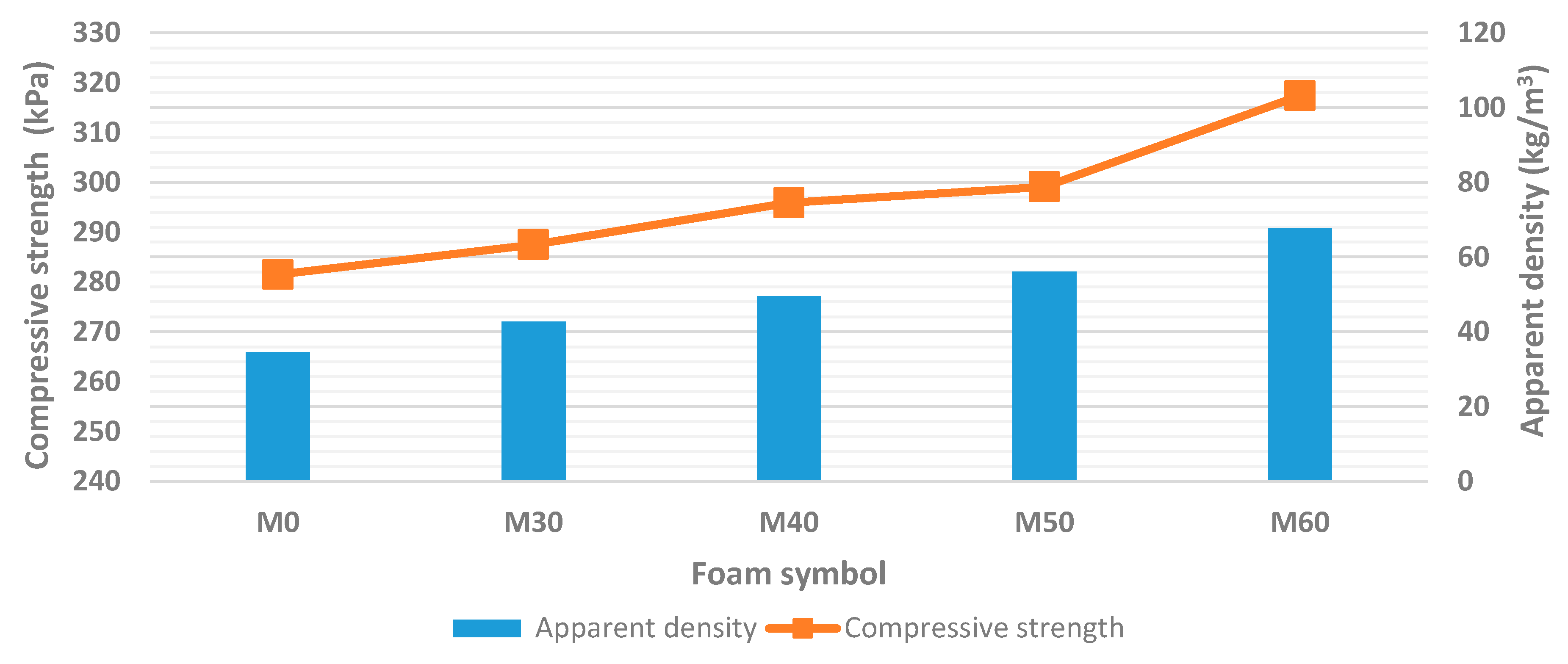
| Foam Symbol | Apparent Density (kg/m3) | Compressive Strength (kPa) | Brittleness (%) |
|---|---|---|---|
| M0 | 34.6 ± 0.4 | 281.5 ± 3.9 | 23.8 ± 0.9 |
| M30 | 42.8 ± 0.7 | 287.5 ± 5.1 | 22.0 ± 0.6 |
| M40 | 49.5 ± 0.8 | 295.9 ± 4.8 | 20.3 ± 0.6 |
| M50 | 56.1 ± 1.1 | 299.1 ± 6.3 | 15.5 ± 0.4 |
| M60 | 67.8 ± 1.2 | 317.4 ± 7.2 | 10.2 ± 0.3 |
| Test | M0 | M30 | M40 | M50 | M60 |
|---|---|---|---|---|---|
| Δl(%) | −2.4 ± 0.1 | +0.9 ± 0.1 | +0.9 ± 0.1 | +0.7± 0.1 | +0.8 ± 0.1 |
| ΔV(%) | +2.8 ± 0.1 | +1.0 ± 0.1 | +0.5 ± 0.1 | +1.0± 0.1 | +1.0 ± 0.1 |
| Δm(%) | 3.1 ± 0.1 | 1.0 ± 0.1 | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.1 ± 0.0 |
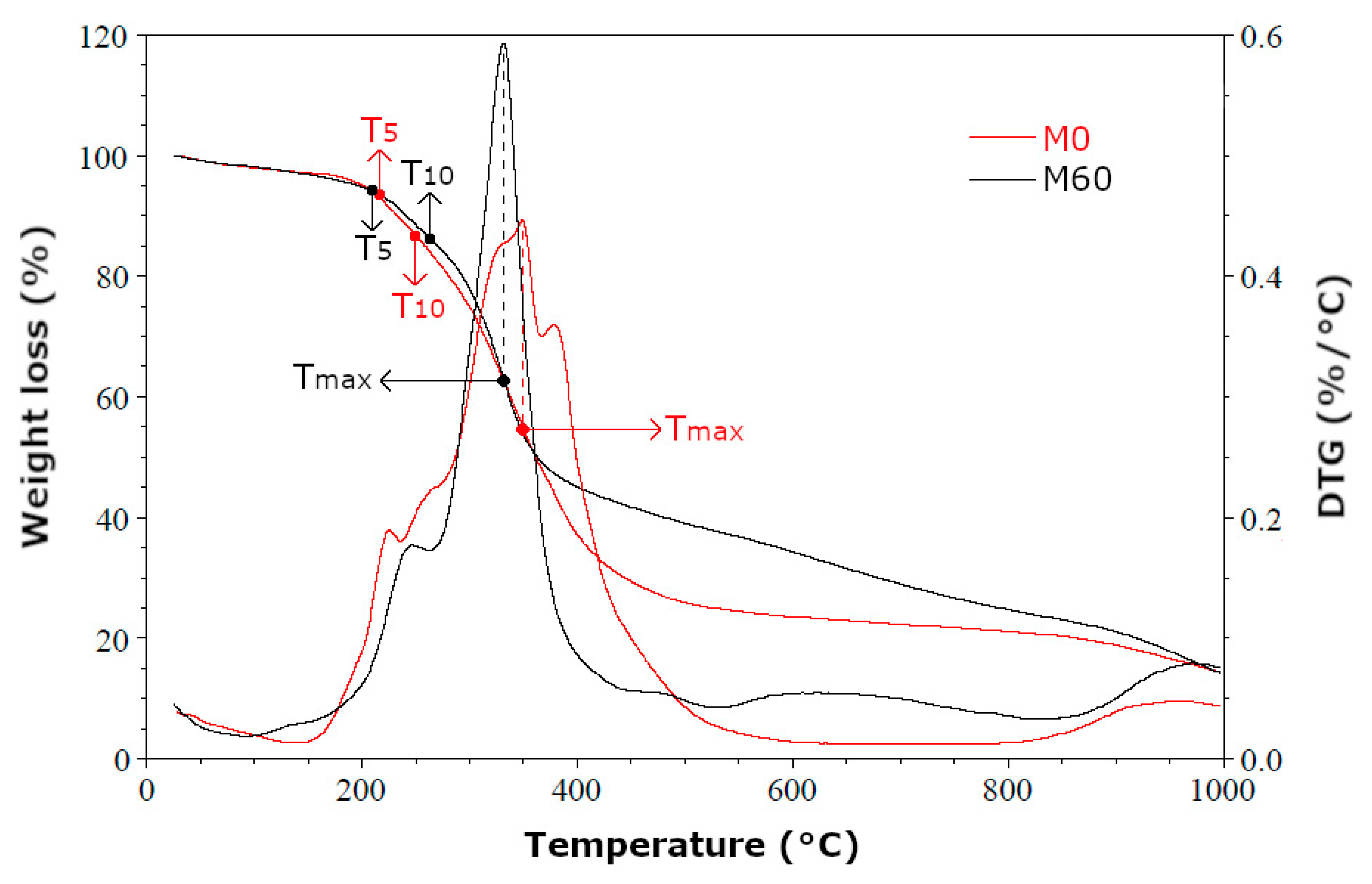
| Foam Symbol | T5 (°C) | T10 (°C) | Tmax (°C) | Highest Weight Loss (%/°C) | Highest Weight Loss Rate (%/min) | Residue (%) |
|---|---|---|---|---|---|---|
| M0 | 206 | 249 | 359 | 0.45 | 4.5 | 15 |
| M60 | 204 | 264 | 347 | 0.59 | 5.9 | 15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paciorek-Sadowska, J.; Borowicz, M.; Isbrandt, M.; Czupryński, B.; Apiecionek, Ł. The Use of Waste from the Production of Rapeseed Oil for Obtaining of New Polyurethane Composites. Polymers 2019, 11, 1431. https://doi.org/10.3390/polym11091431
Paciorek-Sadowska J, Borowicz M, Isbrandt M, Czupryński B, Apiecionek Ł. The Use of Waste from the Production of Rapeseed Oil for Obtaining of New Polyurethane Composites. Polymers. 2019; 11(9):1431. https://doi.org/10.3390/polym11091431
Chicago/Turabian StylePaciorek-Sadowska, Joanna, Marcin Borowicz, Marek Isbrandt, Bogusław Czupryński, and Łukasz Apiecionek. 2019. "The Use of Waste from the Production of Rapeseed Oil for Obtaining of New Polyurethane Composites" Polymers 11, no. 9: 1431. https://doi.org/10.3390/polym11091431
APA StylePaciorek-Sadowska, J., Borowicz, M., Isbrandt, M., Czupryński, B., & Apiecionek, Ł. (2019). The Use of Waste from the Production of Rapeseed Oil for Obtaining of New Polyurethane Composites. Polymers, 11(9), 1431. https://doi.org/10.3390/polym11091431








