Foaming of Polycaprolactone and Its Impregnation with Quercetin Using Supercritical CO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Foaming and Impregnation with scCO2
2.4. Sample Characterization
2.4.1. Scanning Electron Microscopy
2.4.2. X-Ray Diffraction
2.4.3. Differential Scanning Calorimetry
2.5. In Vitro Release Test
3. Results and Discussion
3.1. Analysis of the Design of Experiments
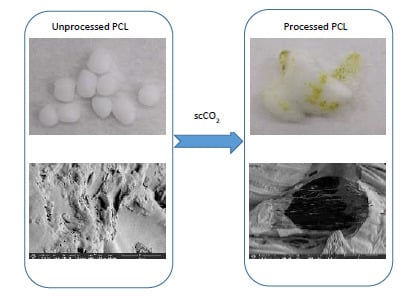
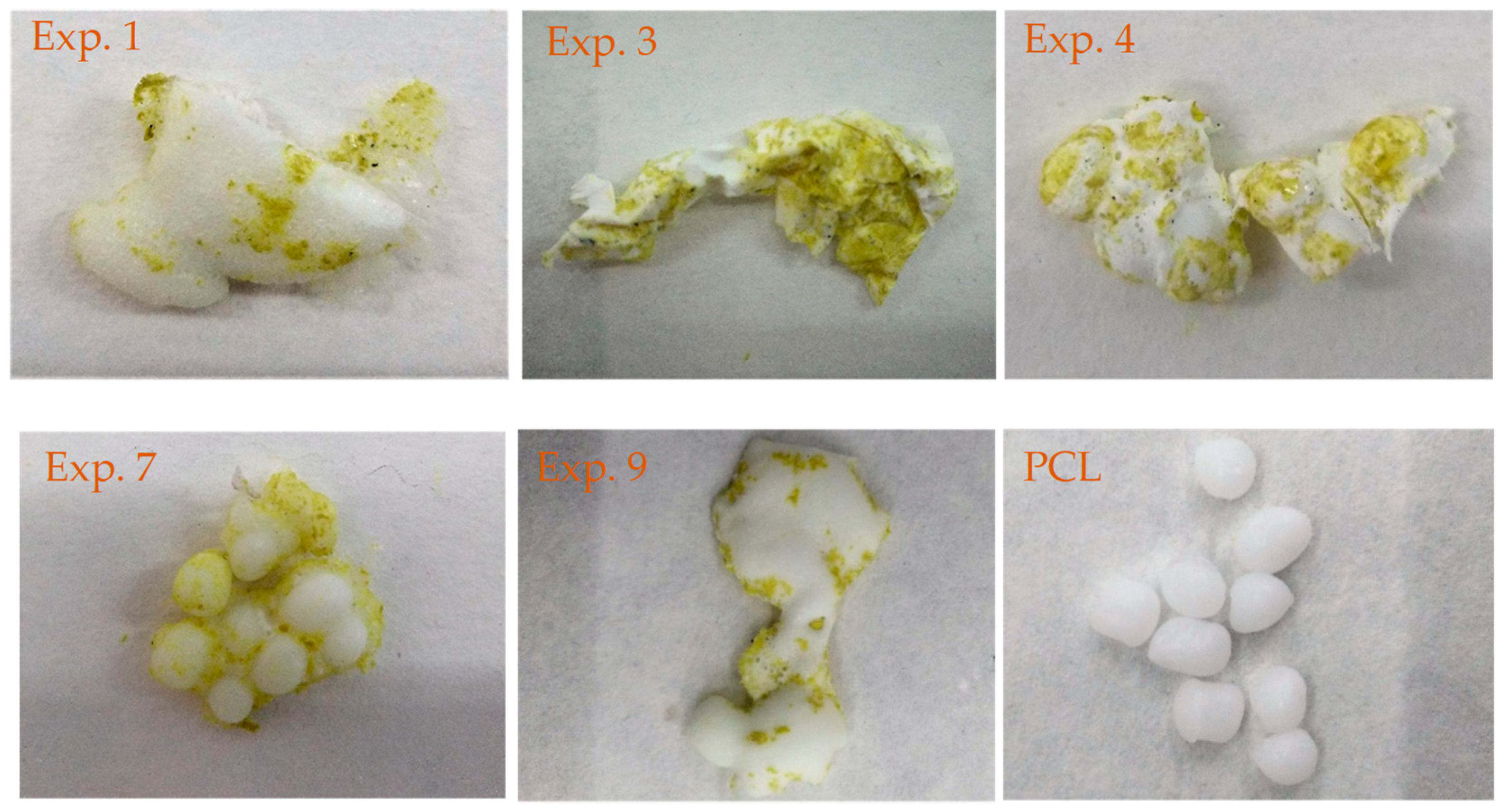
3.2. Foaming and Impregnation Experiments
3.3. Characterization
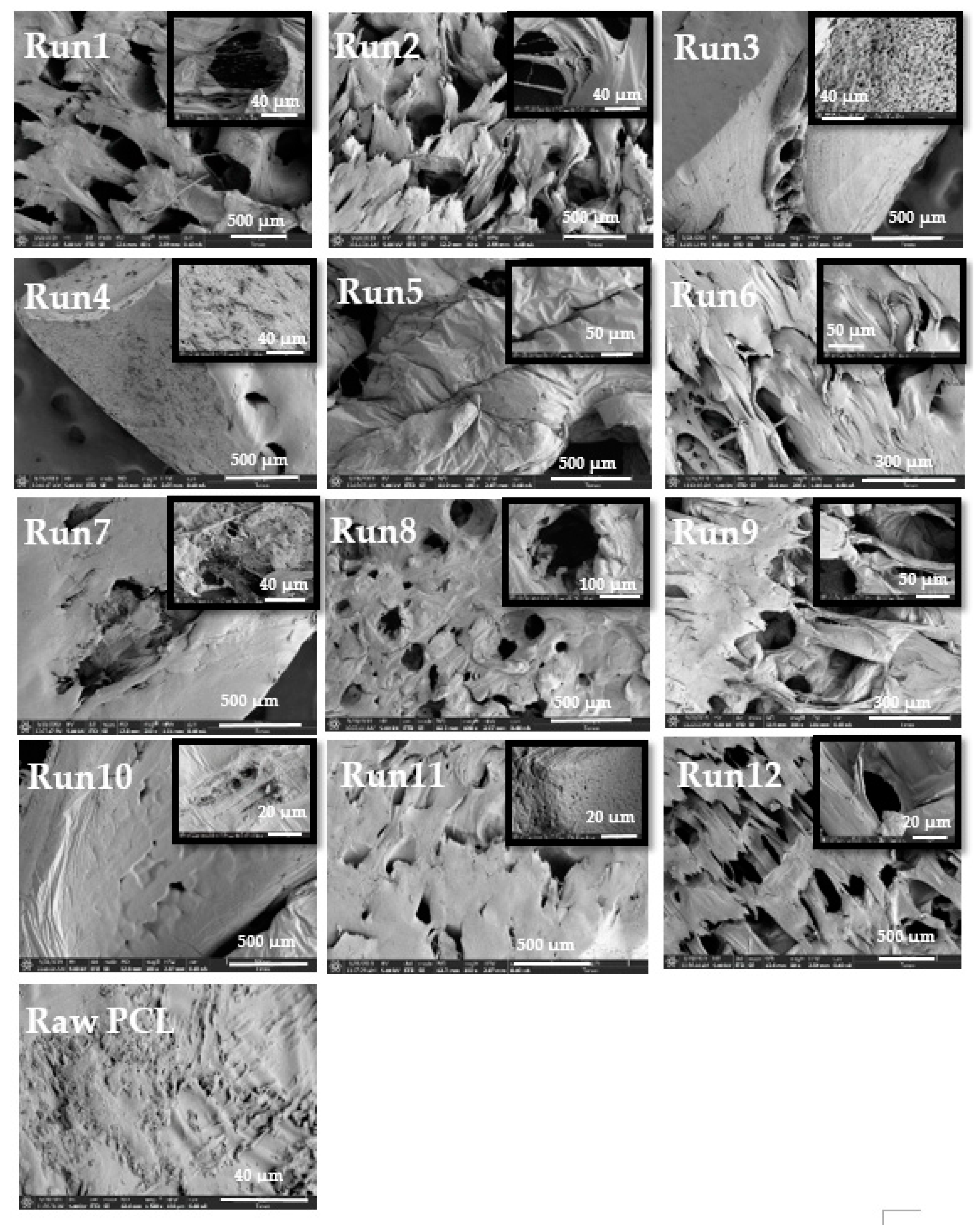
3.3.1. Scanning Electron Microscope (SEM)
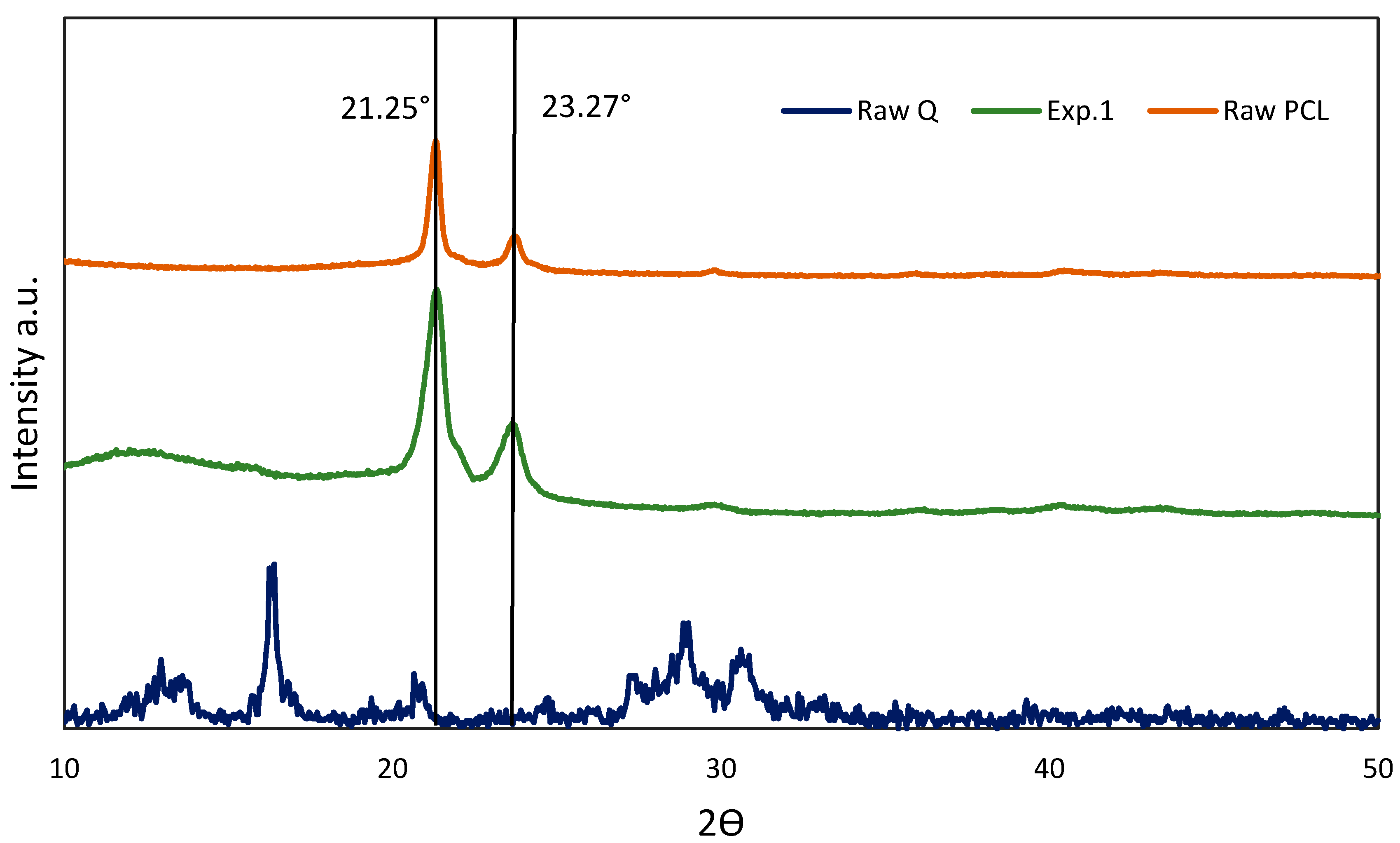
3.3.2. X-ray Diffraction
3.3.3. Differential Scanning Calorimetry
3.4. In Vitro Release Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuang, T.; Chen, F.; Chang, L.; Zhao, Y.; Fu, D.; Gong, X.; Peng, X. Facile Preparation of Open-Cellular Porous Poly(l-lactic acid) Scaffold by Supercritical Carbon Dioxide Foaming for Potential Tissue Engineering Applications. Chem. Eng. J. 2017, 307, 1017–1025. [Google Scholar] [CrossRef]
- Goel, S.K.; Beckman, E.J. Generation of Microcellular Polymeric Foams Using Supercritical Carbon Dioxide. II: Cell Growth and Skin Formation. Polym. Eng. Sci. 1994, 34, 1137–1147. [Google Scholar] [CrossRef]
- Fanovich, M.A.; Jaeger, P. Sorption and Diffusion of Compressed Carbon Dioxide in Polycaprolactone for the Development of Porous Scaffolds. Mater. Sci. Eng. C 2012, 32, 961–968. [Google Scholar] [CrossRef]
- Lian, Z.; Epstein, S.A.; Blenk, C.W.; Shine, A.D. Carbon Dioxide-Induced Melting Point Depression of Biodegradable Semicrystalline Polymers. J. Supercrit. Fluids 2006, 39, 107–117. [Google Scholar] [CrossRef]
- Yoganathan, R.; Mammucari, R.; Foster, N.R. Impregnation of Ibuprofen into Polycaprolactone Using Supercritical Carbon Dioxide. J. Phys. Conf. Ser. 2010, 215, 6–11. [Google Scholar] [CrossRef]
- Takada, M.; Hasegawa, S.; Ohshima, M. Crystallization Kinetics of Poly(l-lactide) in Contact with Pressurized CO2. Polym. Eng. Sci. 2004, 44, 186–196. [Google Scholar] [CrossRef]
- Zhang, Z.; Handa, Y.P. CO2-Assisted Melting of Semicrystalline Polymers. Macromolecules 1997, 30, 8505–8507. [Google Scholar] [CrossRef]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-Caprolactone Microspheres and Nanospheres: An Overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef]
- Moghadam, M.Z.; Hassanajili, S.; Esmaeilzadeh, F.; Ayatollahi, M.; Ahmadi, M. Formation of Porous HPCL/LPCL/HA Scaffolds with Supercritical CO2 Gas Foaming Method. J. Mech. Behav. Biomed. Mater. 2017, 69, 115–127. [Google Scholar] [CrossRef]
- Cotugno, S.; Di Maio, E.; Mensitieri, G.; Iannace, S.; Roberts, G.W.; Carbonell, R.G.; Hopfenberg, H.B. Characterization of Microcellular Biodegradable Polymeric Foams Produced from Supercritical Carbon Dioxide Solutions. Ind. Eng. Chem. Res. 2005, 44, 1795–1803. [Google Scholar] [CrossRef]
- Xu, Q.; Ren, X.; Chang, Y.; Wang, J.; Yu, L.; Dean, K. Generation of Microcellular Biodegradable Polycaprolactone Foams in Supercritical Carbon Dioxide. J. Appl. Polym. Sci. 2004, 94, 593–597. [Google Scholar] [CrossRef]
- Kiran, E. Foaming Strategies for Bioabsorbable Polymers in Supercritical Fluid Mixtures. Part II. Foaming of Poly(ɛ-caprolactone-co-lactide) in Carbon Dioxide and Carbon Dioxide + Acetone Fluid Mixtures and Formation of Tubular Foams via Solution Extrusion. J. Supercrit. Fluids 2010, 54, 308–319. [Google Scholar] [CrossRef]
- Diaz-Gomez, L.; Concheiro, A.; Alvarez-Lorenzo, C.; García-González, C.A. Growth Factors Delivery from Hybrid PCL-Starch Scaffolds Processed Using Supercritical Fluid Technology. Carbohydr. Polym. 2016, 142, 282–292. [Google Scholar] [CrossRef] [PubMed]
- de Matos, M.B.C.; Piedade, A.P.; Alvarez-Lorenzo, C.; Concheiro, A.; Braga, M.E.M.; de Sousa, H.C. Dexamethasone-loaded poly(ɛ-caprolactone)/Silica Nanoparticles Composites Prepared by Supercritical CO2 Foaming/Mixing and Deposition. Int. J. Pharm. 2013, 456, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Goimil, L.; Santos-Rosales, V.; Delgado, A.; Évora, C.; Reyes, R.; Lozano-Pérez, A.A.; Aznar-Cervantes, S.D.; Cenis, J.L.; Gómez-Amoza, J.L.; Concheiro, A.; et al. scCO2-Foamed Silk Fibroin Aerogel/Poly(ε-caprolactone) Scaffolds Containing Dexamethasone for Bone Regeneration. J. CO2 Util. 2019, 31, 51–64. [Google Scholar] [CrossRef]
- Campardelli, R.; Franco, P.; Reverchon, E.; De Marco, I. Polycaprolactone/Nimesulide Patches Obtained by a One-Step Supercritical Foaming + Impregnation Process. J. Supercrit. Fluids 2019, 146, 47–54. [Google Scholar] [CrossRef]
- Zehbe, R.; Zehbe, K. Strontium Doped Poly-ε-Caprolactone Composite Scaffolds Made by Reactive Foaming. Mater. Sci. Eng. C 2016, 67, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Marković, D.; Milovanović, S.; Radovanović, Ž.; Zizovic, I.; Šaponjić, Z.; Radetić, M. Floating Photocatalyst Based on Poly(ε-caprolactone) Foam and TiO2 Nanoparticles for Removal of Textile Dyes. Fibers Polym. 2018, 19, 1219–1227. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Pavlidou, E.; Panayiotou, C. Biodegradable Polymer Foams Prepared with Supercritical CO2-Ethanol Mixtures as Blowing Agents. J. Supercrit. Fluids 2007, 42, 265–272. [Google Scholar] [CrossRef]
- Formica, J.V.; Regelson, W. Review of the Biology of Quercetin and Related Bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.G.; Kakran, M.; Shaal, L.A.; Li, L.; Müller, R.H.; Pal, M.; Tan, L.P. Preparation and Characterization of Quercetin Nanocrystals. J. Pharm. Sci. 2011, 100, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.T.; Meireles, M.A.A. Micronization and Encapsulation of Functional Pigments Using Supercritical Carbon Dioxide. J. Food Process Eng. 2013, 36, 36–49. [Google Scholar] [CrossRef]
- García-Casas, I.; Montes, A.; Pereyra, C.; Martínez, D.L.O.E.J. Generation of Quercetin/Cellulose Acetate Phthalate Systems for Delivery by Supercritical Antisolvent Process. Eur. J. Pharm. Sci. 2017, 100, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.A.; Braga, M.E.M.; Seabra, I.J.; Ferreira, P.; Gil, M.H.; De Sousa, H.C. Development of Natural-Based Wound Dressings Impregnated with Bioactive Compounds and Using Supercritical Carbon Dioxide. Int. J. Pharm. 2011, 408, 9–19. [Google Scholar] [CrossRef] [PubMed]
- García-Casas, I.; Crampon, C.; Montes, A.; Pereyra, C.; Martínez, D.L.O.E.J.; Badens, E. Supercritical CO2 Impregnation of Silica Microparticles with Quercetin. J. Supercrit. Fluids 2019, 143, 157–161. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Shi, J.L.; Li, Y.S.; Chen, H.R.; Shen, W.H.; Dong, X.P. Storage and Release of Ibuprofen Drug Molecules in Hollow Mesoporous Silica Spheres with Modified Pore Surface. Microporous Mesoporous Mater. 2005, 85, 75–81. [Google Scholar] [CrossRef]
- Dahmoune, F.; Spigno, G.; Moussi, K.; Remini, H.; Cherbal, A.; Madani, K. Pistacia lentiscus Leaves as a Source of Phenolic Compounds: Microwave-Assisted Extraction Optimized and Compared with Ultrasound-Assisted and Conventional Solvent Extraction. Ind. Crops Prod. 2014, 61, 31–40. [Google Scholar] [CrossRef]
- Chafer, A.; Fornari, T.; Berna, A.; Stateva, R.P. Solubility of Quercetin in Supercritical CO2 + Ethanol as a Modifier: Measurements and Thermodynamic Modelling. J. Supercrit. Fluids 2004, 32, 89–96. [Google Scholar] [CrossRef]
- Baldwin, D.F.; Park, C.B.; Suh, N.P. A microcellular Processing Study of Poly(ethylene terephthalate) in the Amorphous and Semicrystalline States. Part II: Cell Growth and Process Design. Polym. Eng. Sci. 1996, 36, 1446–1453. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S. Production of Controlled Polymeric Foams by Supercritical CO2. J. Supercrit. Fluids 2007, 40, 144–152. [Google Scholar] [CrossRef]
- Messersmith, P.B.; Giannelis, E.P. Synthesis and Barrier Properties of Poly(ε-caprolactone)-Layered Silicate Nanocomposites. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 1047–1057. [Google Scholar] [CrossRef]
- Fraile, M.; Buratto, R.; Gómez, B.; Martín, Á.; Cocero, M.J. Enhanced Delivery of Quercetin by Encapsulation in Poloxamers by Supercritical Antisolvent Process. Ind. Eng. Chem. Res. 2014, 53, 4318–4327. [Google Scholar] [CrossRef]
- White, L.J.; Hutter, V.; Tai, H.; Howdle, S.M.; Shakesheff, K.M. The Effect of Processing Variables on Morphological and Mechanical Properties of Supercritical CO2 Foamed Scaffolds for Tissue Engineering. Acta Biomater. 2012, 8, 61–71. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Angelopoulou, A.G.; Panayiotou, C. Foaming of Polymers with Supercritical CO2: An Experimental and Theoretical Study. Polymer 2007, 48, 5928–5939. [Google Scholar] [CrossRef]

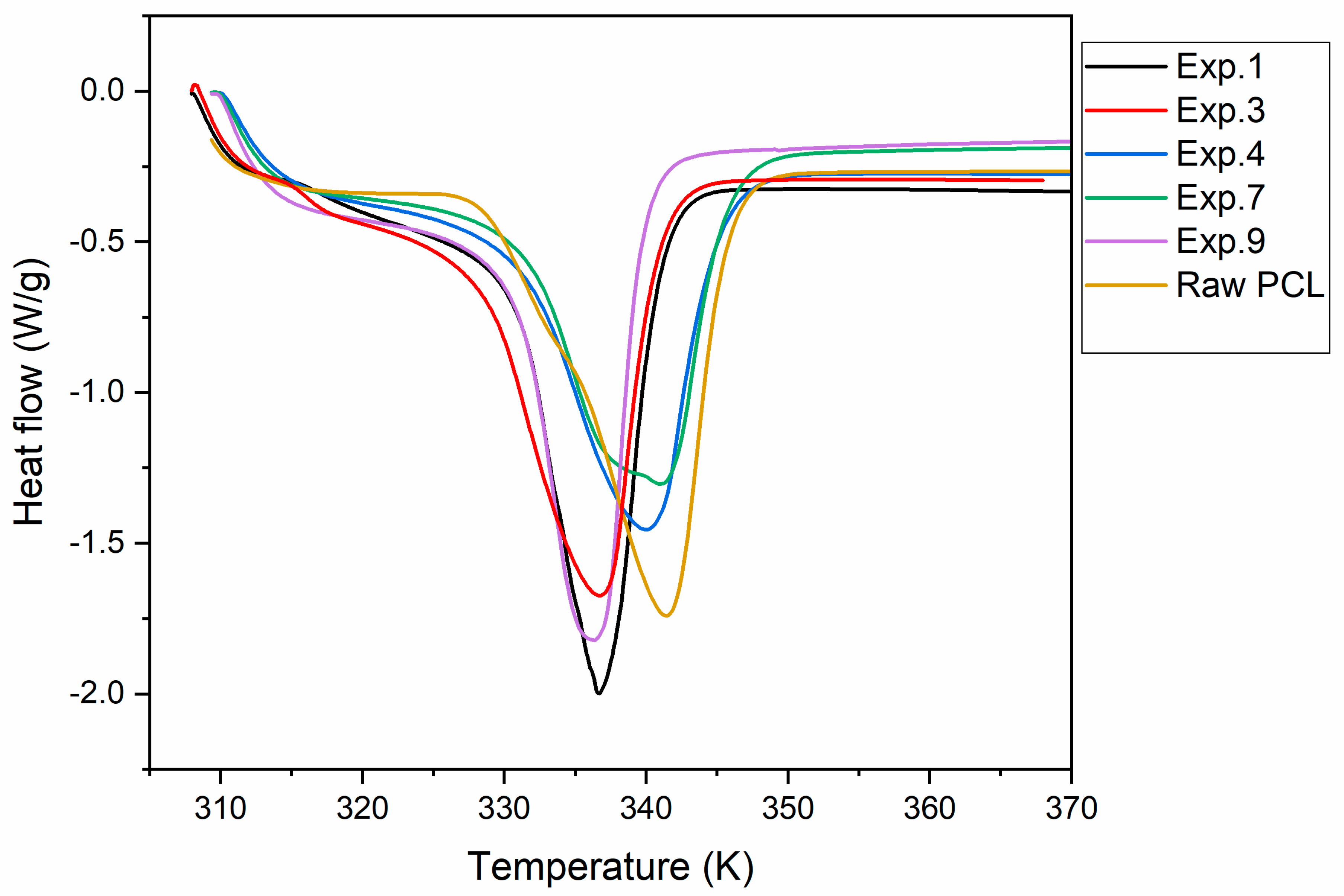
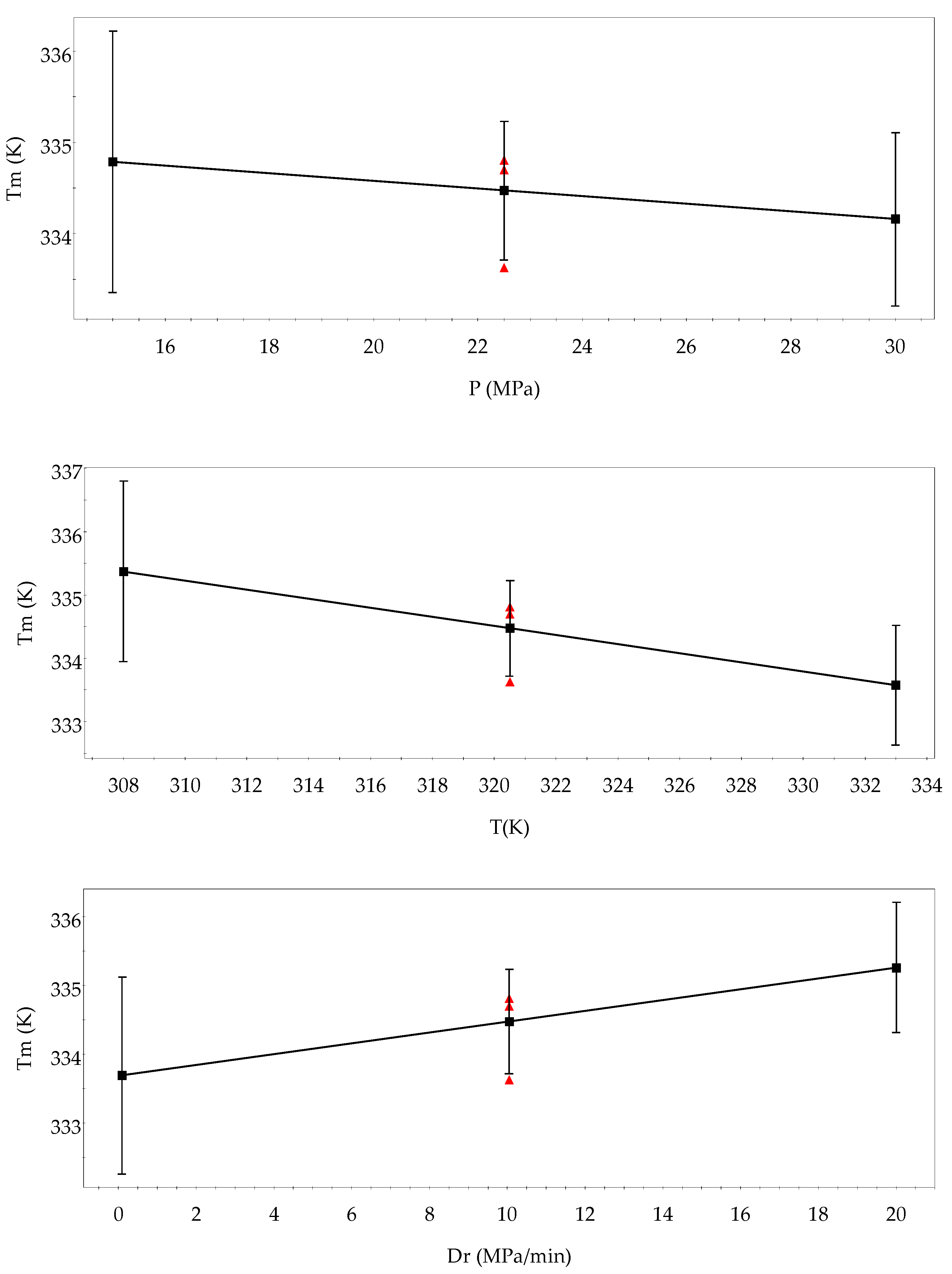
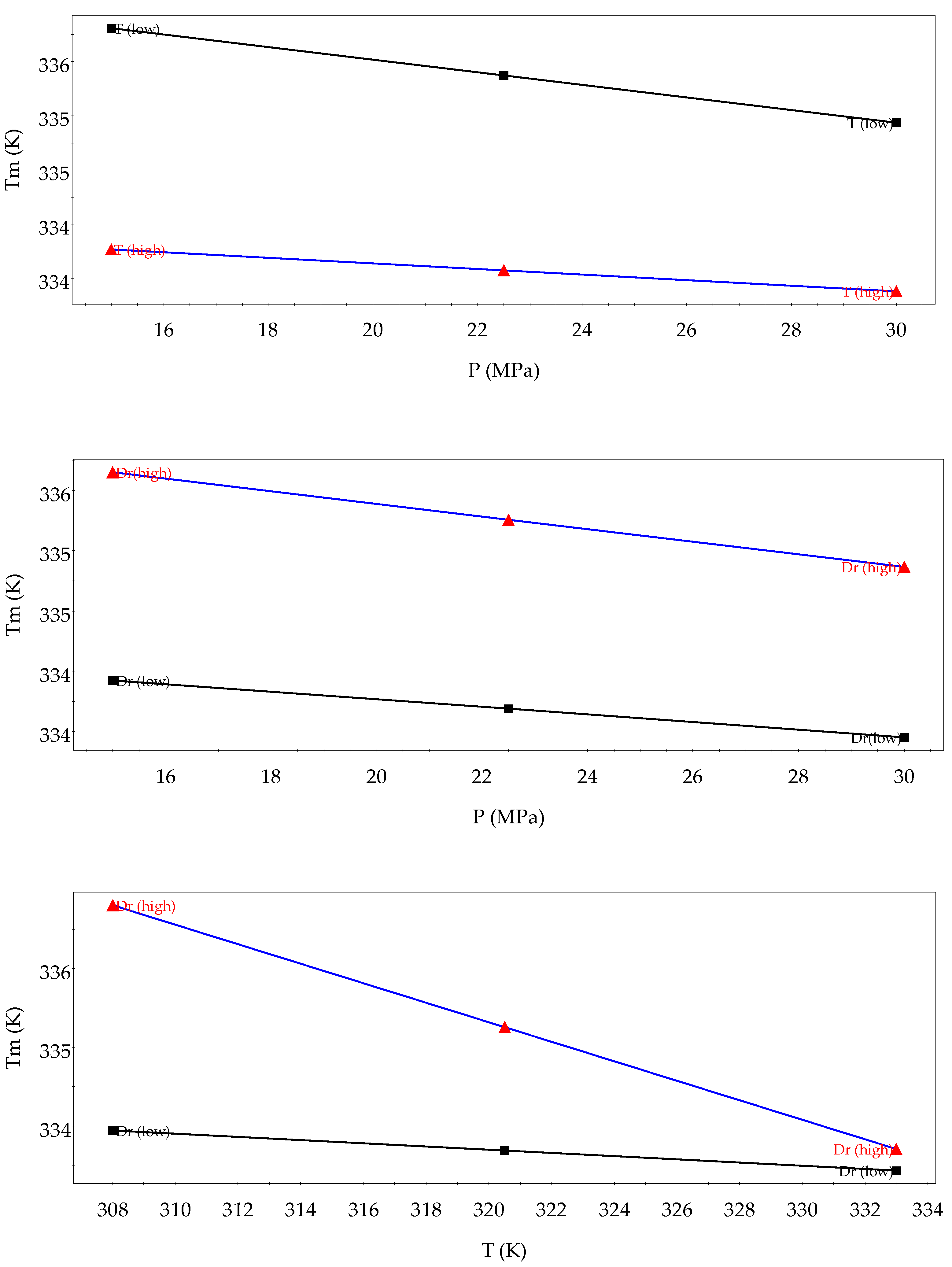

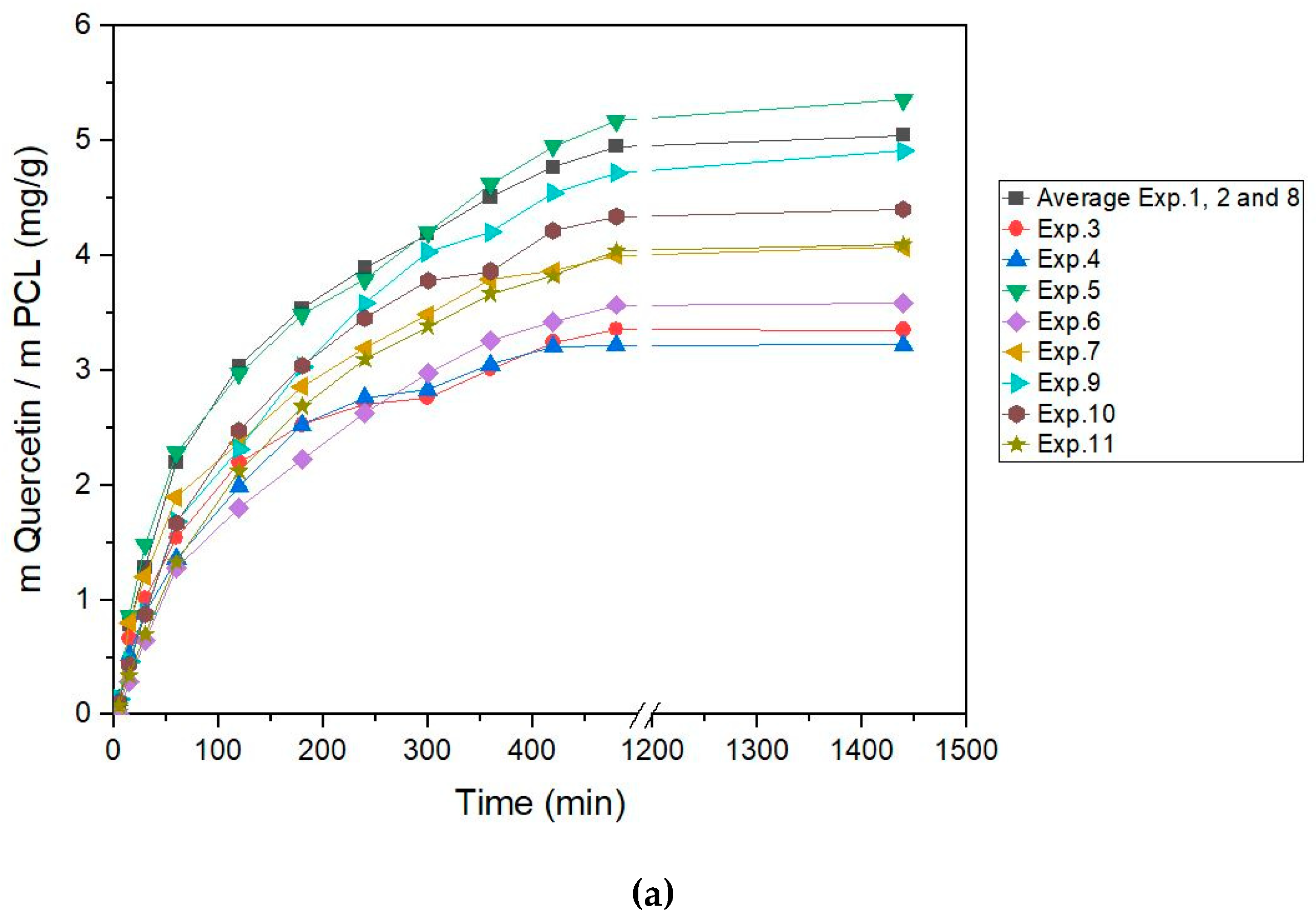

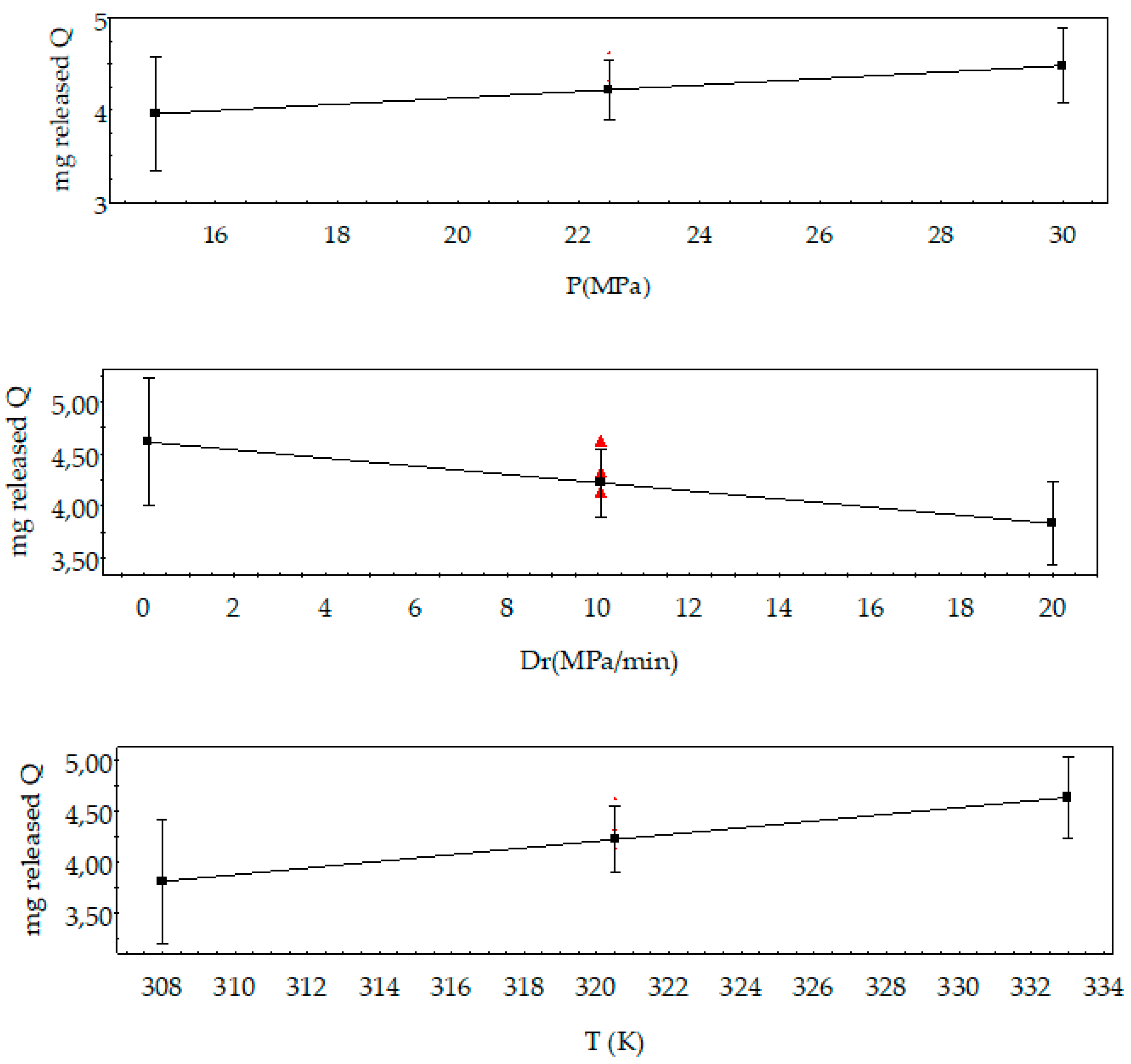
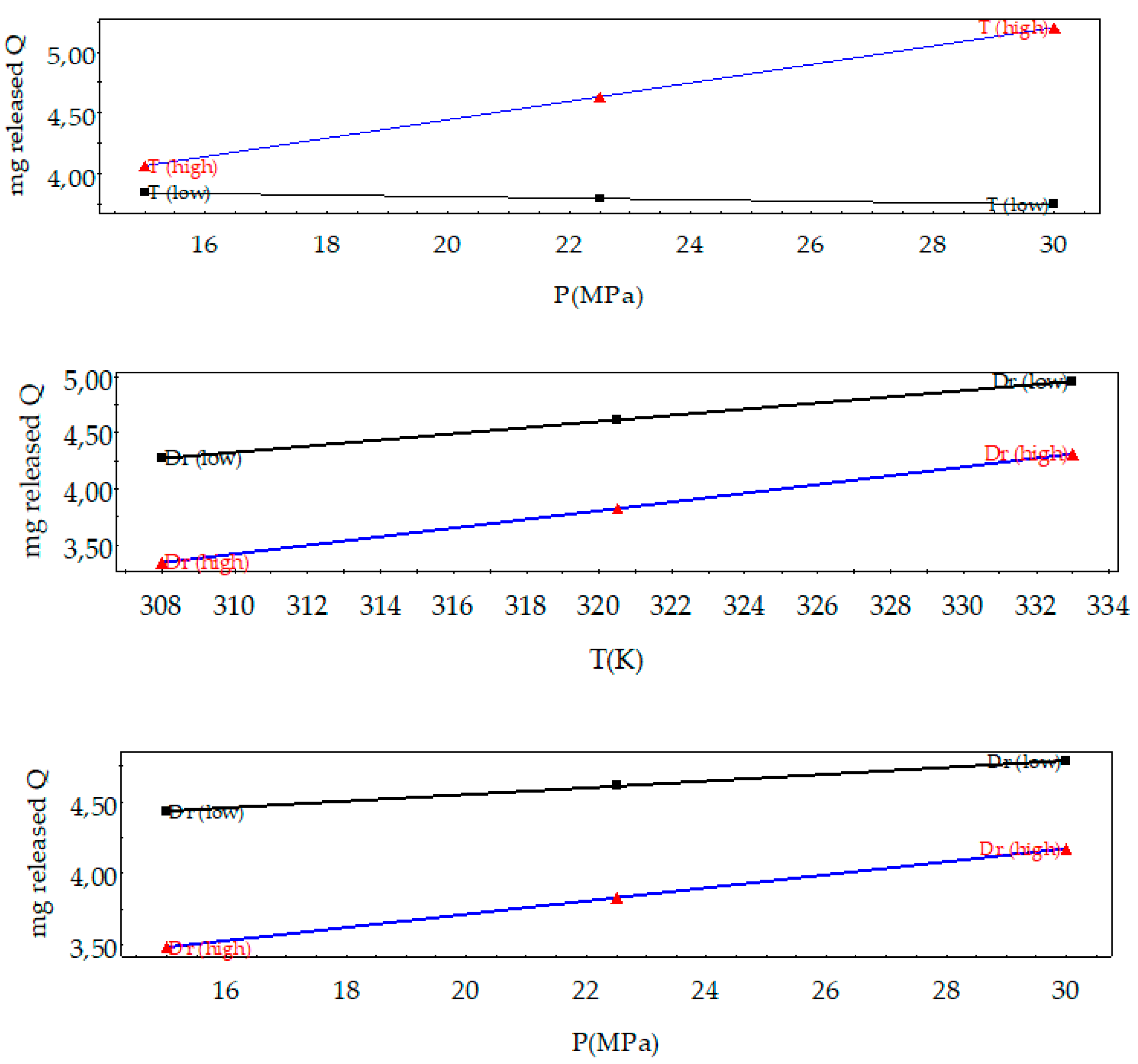

| Factors | Low Level | High Level | Tm Effects | Released Q Effects | Hm Effects |
|---|---|---|---|---|---|
| P (MPa) | 15 | 30 | −0.44 | 0.59 | −0.61 |
| T (K) | 308 | 333 | −2.02 | 0.90 | −1.90 |
| Dr (MPa min−1) | 0.10 | 20 | 1.31 | −0.75 | 1.42 |
| Variables | DF | SS | MS | R2 | AdjR2 | Q2 | Significance F | Lack of Fit p | |
|---|---|---|---|---|---|---|---|---|---|
| Tm | Model | 9 | 16.86 | 1.87 | 0.95 | 0.84 | 0.53 | 8.72 | 0.048 |
| Residual | 3 | 0.91 | 0.30 | ||||||
| Error | 2 | 0.85 | 0.42 | ||||||
| Released Q | Model | 9 | 4.17 | 0.46 | 0.95 | 0.84 | 0.57 | 9.08 | 0.049 |
| Residual | 3 | 0.22 | 0.07 | ||||||
| Error | 2 | 0.12 | 0.06 | ||||||
| Hm | Model | 9 | 379.17 | 42.13 | 0.49 | −0.52 | 0.04 | 0.48 | 0.79 |
| Residual | 3 | 191.68 | 63.90 | ||||||
| Error | 2 | 88.78 | 44.40 |
| Experiments | P (MPa) | T (K) | Dr (MPa min−1) | Tm (K) | mg Released Q/g PCL | Hm (J/g) |
|---|---|---|---|---|---|---|
| 1 | 22.5 | 320.5 | 2.5 | 334.70 | 4.63 | 85.07 |
| 2 | 22.5 | 320.5 | 2.5 | 333.63 | 4.33 | 73.50 |
| 3 | 30 | 308 | 20 | 336.70 | 3.35 | 87.39 |
| 4 | 15 | 308 | 20 | 337.10 | 3.22 | 83.69 |
| 5 | 30 | 333 | 0.1 | 333.48 | 5.36 | 90.58 |
| 6 | 15 | 333 | 20 | 333.91 | 3.58 | 87.77 |
| 7 | 15 | 308 | 0.1 | 338.32 | 3.35 | 84.57 |
| 8 | 22.5 | 320.5 | 2.5 | 334.81 | 4.14 | 85.01 |
| 9 | 30 | 333 | 20 | 333.23 | 4.91 | 96.52 |
| 10 | 15 | 333 | 0.1 | 333.61 | 4.40 | 80.10 |
| 11 | 30 | 308 | 0.1 | 333.74 | 4.10 | 92.69 |
| Coefficient (Tm) | p (Tm) | Coefficient (Released Q) | p (Released Q) | |
|---|---|---|---|---|
| Constant | 61.40 | 6.26 × 10−8 | 4.25 | 2.18 × 10−5 |
| P | −0.19 | 0.43 | 0.26 | 0.08 |
| T | −0.88 | 0.02 | 0.39 | 0.03 |
| Dr | 0.57 | 0.04 | −0.33 | 0.04 |
| P·T | −5.71 × 10−3 | 0.98 | 0.23 | 0.09 |
| P Dr | −0.04 | 0.86 | 0.06 | 0.55 |
| T·Dr | −0.57 | 0.06 | 0.05 | 0.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Casas, I.; Montes, A.; Valor, D.; Pereyra, C.; Martínez de la Ossa, E.J. Foaming of Polycaprolactone and Its Impregnation with Quercetin Using Supercritical CO2. Polymers 2019, 11, 1390. https://doi.org/10.3390/polym11091390
García-Casas I, Montes A, Valor D, Pereyra C, Martínez de la Ossa EJ. Foaming of Polycaprolactone and Its Impregnation with Quercetin Using Supercritical CO2. Polymers. 2019; 11(9):1390. https://doi.org/10.3390/polym11091390
Chicago/Turabian StyleGarcía-Casas, Ignacio, Antonio Montes, Diego Valor, Clara Pereyra, and Enrique J. Martínez de la Ossa. 2019. "Foaming of Polycaprolactone and Its Impregnation with Quercetin Using Supercritical CO2" Polymers 11, no. 9: 1390. https://doi.org/10.3390/polym11091390
APA StyleGarcía-Casas, I., Montes, A., Valor, D., Pereyra, C., & Martínez de la Ossa, E. J. (2019). Foaming of Polycaprolactone and Its Impregnation with Quercetin Using Supercritical CO2. Polymers, 11(9), 1390. https://doi.org/10.3390/polym11091390