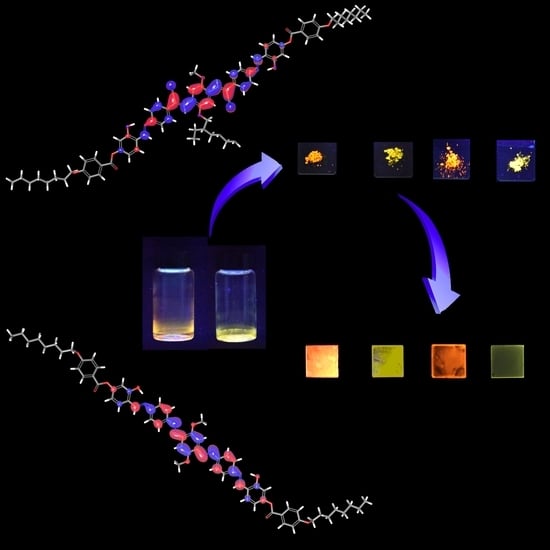
The Effect of Bulky Substituents on Two π-Conjugated Mesogenic Fluorophores. Their Organic Polymers and Zinc-Bridged Luminescent Networks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Thin Films for Optical Measurements and PLQY Setup
2.4. Cyclic Voltammetry
2.5. Theoretical Calculations
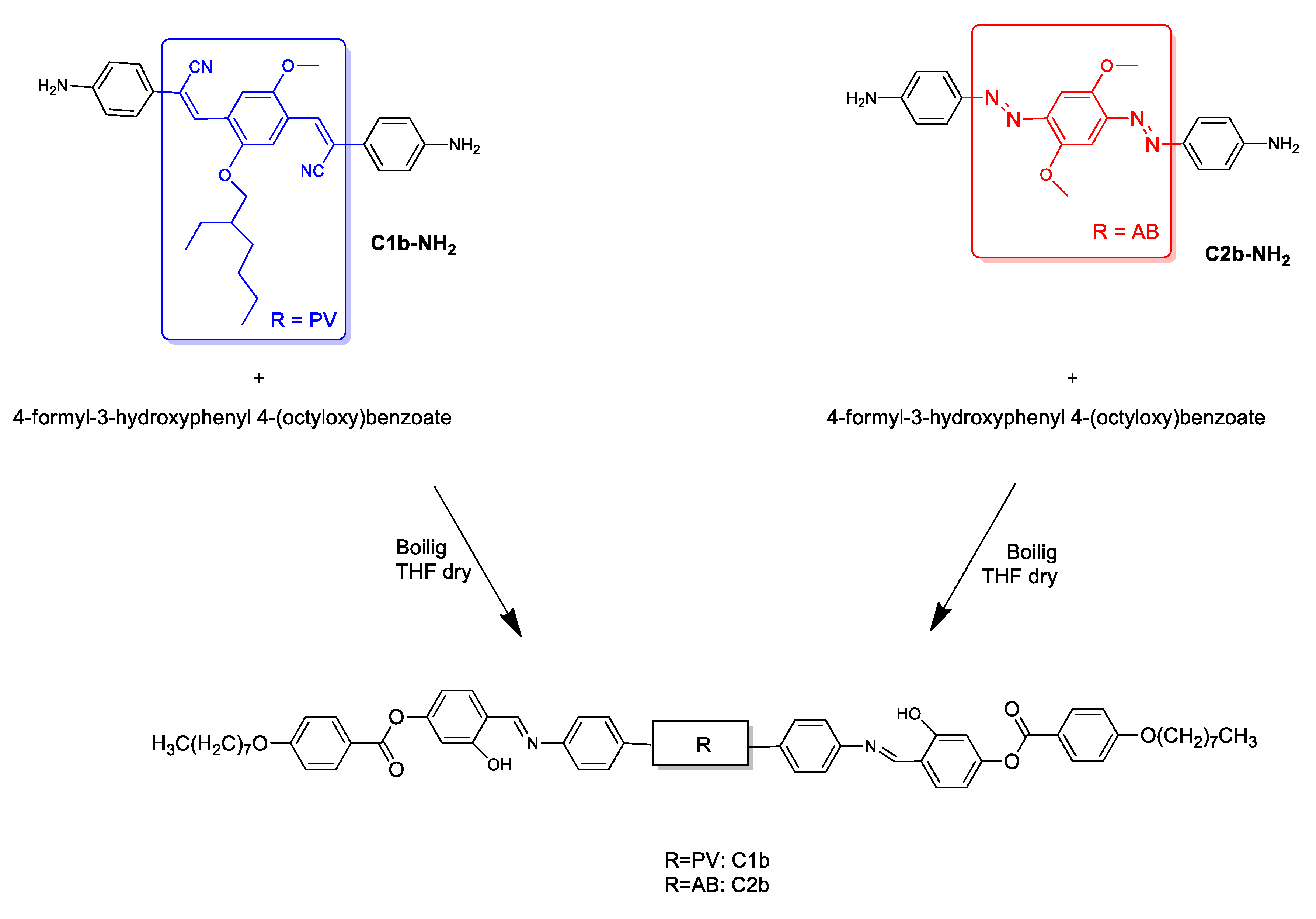
2.6. Synthesis of C1b and C2b
2.7. Synthesis of Organic Polymers
2.8. Synthesis of the Networks
3. Results and Discussion
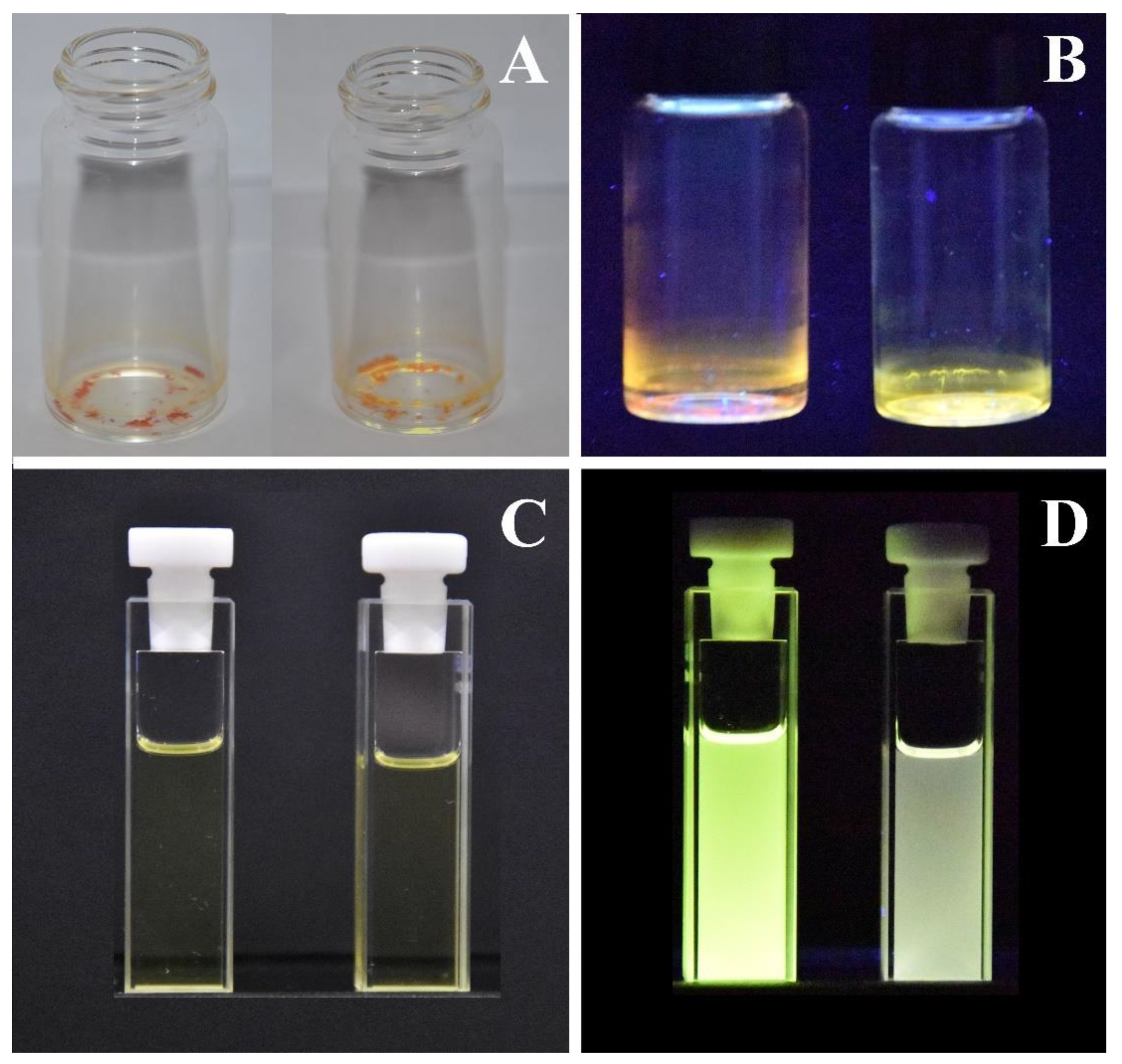
3.1. Synthesis and PL Properties of C1b and C2b
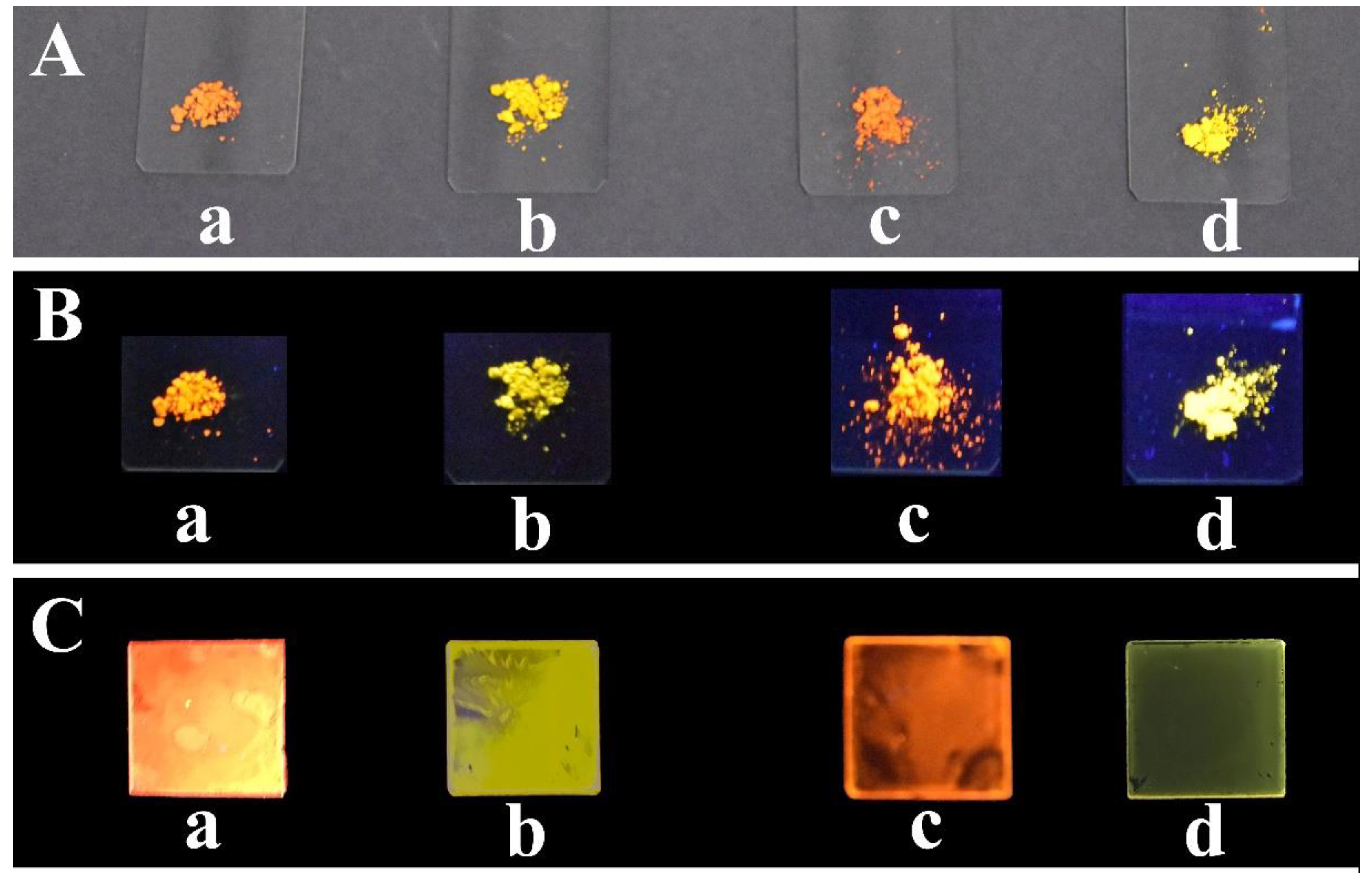
3.2. Synthesis and PL Properties of Organic Polymers
3.3. Synthesis and PL Properties of the Zinc-Bridged Networks
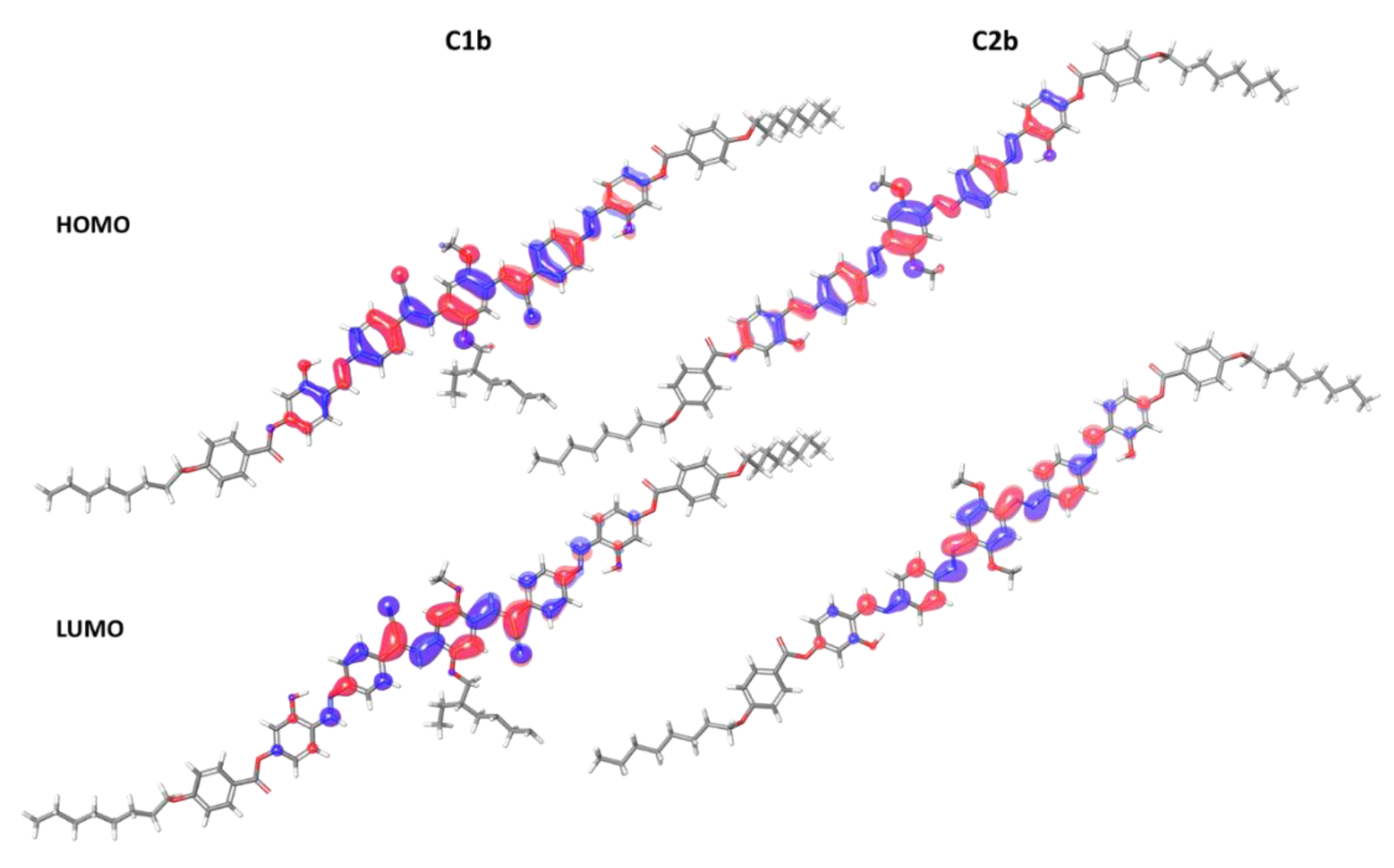
3.4. DFT Analysis
3.5. Electrochemical Bandgaps of Organic Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Okazaki, M.; Takeda, Y.; Data, P.; Pander, P.; Higginbotham, H.; Monkman, A.P.; Minakata, S. Thermally activated delayed fluorescent phenothiazine–dibenzo[a,j]phenazine–phenothiazine triads exhibiting tricolor-changing mechanochromic luminescence. Chem. Sci. 2017, 8, 2677–2686. [Google Scholar] [CrossRef]
- Song, Z.; Lin, T.; Lin, L.; Lin, S.; Fu, F.; Wang, X.; Guo, L. Invisible Security Ink Based on Water-Soluble Graphitic Carbon Nitride Quantum Dots. Angew. Chem. 2016, 55, 2773–2777. [Google Scholar] [CrossRef]
- Tsang, M.K.; Bai, G.; Hao, J. Stimuli responsive upconversion luminescence nanomaterials and films for various applications. Chem. Soc. Rev. 2015, 44, 1585–1607. [Google Scholar] [CrossRef]
- He, Y.; Zhu, Y.; Chen, Z.; He, W.; Wang, X. Remote-control photocycloreversion of dithienylethene driven by strong push-pull azo chromophores. Chem. Commun. (Camb.) 2013, 49, 5556–5558. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lu, J.; Li, H.; Kang, E.T. Nonlinear optical properties and memory effects of the azo polymers carrying different substituents. Dyes Pigments 2011, 88, 18–24. [Google Scholar] [CrossRef]
- Mahimwalla, Z.; Yager, K.G.; Mamiya, J.I.; Shishido, A.; Priimagi, A.; Barrett, C.J. Azobenzene photomechanics: Prospects and potential applications. Polym. Bull. 2012, 69, 967–1006. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Zhou, Y.; Wang, X.; He, Y. Fast Photoinduced Large Deformation of Colloidal Spheres from a Novel 4-arm Azobenzene Compound. ACS Appl. Mater Interfaces 2015, 7, 16889–16895. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, B.; Li, S.; Sinawang, G.; Wang, X.; He, Y. Synthesis and Characterization of Photoprocessable Lignin-Based Azo Polymer. ACS Sustain. Chem. Eng. 2016, 4, 4036–4042. [Google Scholar] [CrossRef]
- Zamanloo, M.R.; Shamkhali, A.N.; Alizadeh, M.; Mansoori, Y.; Imanzadeh, G. A novel barbituric acid-based azo dye and its derived polyamides: Synthesis, spectroscopic investigation and computational calculations. Dyes Pigments 2012, 95, 587–599. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, S.; Wang, Y.; Cui, Z.; Li, N.; Zhu, Y.; Zhang, H.; Jiang, Z. Synthesis, characterization, and photoresponsive behavior of a series of azobenzene-containing side-chain poly(ether sulfone)s with various lengths of flexible spacers. Dyes Pigments 2013, 99, 1117–1123. [Google Scholar] [CrossRef]
- Bruno, A.; Borriello, C.; Luccio, D.T.; Nenna, G.; Sessa, L.; Concilio, S.; Haque, S.A.; Minarini, C. White light-emitting nanocomposites based on an oxadiazole–carbazole copolymer (POC) and InP/ZnS quantum dots. J. Nanopart. Res. 2013, 15, 2085. [Google Scholar] [CrossRef]
- Concilio, S.; Bugatti, V.; Iannelli, P.; Piotto, S.P. Synthesis and Characterization of New Photoluminescent Oxadiazole/Carbazole-Containing Polymers. Int. J. Polym. Sci. 2010, 2010, 1–6. [Google Scholar] [CrossRef]
- Kim, M.; Whang, D.R.; Gierschner, J.; Park, S.Y. A distyrylbenzene based highly efficient deep red/near-infrared emitting organic solid. J. Mater. Chem. C 2015, 3, 231–234. [Google Scholar] [CrossRef]
- Sztanke, K.; Maziarka, A.; Osinka, A.; Sztanke, M. An insight into synthetic Schiff bases revealing antiproliferative activities in vitro. Bioorg. Med. Chem. 2013, 21, 3648–3666. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, C.P.; Das, S.K. Coordination and supramolecular aspects of the metal complexes of chiral N-salicyl-β-amino alcohol Schiff base ligands: Towards understanding the roles of weak interactions in their catalytic reactions. Coord. Chem. Rev. 2013, 257, 1699–1715. [Google Scholar] [CrossRef]
- Esposito, R.; Cucciolito, M.E.; D’Amora, A.; Di Guida, R.; Montagnaro, F.; Ruffo, F. Highly efficient iron(III) molecular catalysts for solketal production. Fuel Process. Technol. 2017, 167, 670–673. [Google Scholar] [CrossRef]
- Das, K.; Mandal, T.N.; Roy, S.; Gupta, S.; Barik, A.K.; Mitra, P.; Rheingold, A.L.; Kar, S.K. Syntheses, characterization, X-ray crystal structures and emission properties of copper(II), zinc(II) and cadmium(II) complexes of pyridyl–pyrazole derived Schiff base ligand Metal selective ligand binding modes. Polyhedron 2010, 29, 2892–2899. [Google Scholar] [CrossRef]
- Gill, R.E.; van Hutten, P.F.; Meetsma, A.; Hadziioannou, G. Synthesis and Crystal Structure of a Cyano-Substituted Oligo(p-phenylenevinylene). Chem. Mater. 1996, 8, 1341–1346. [Google Scholar] [CrossRef]
- Xin, Y.; Shen, W.; Deng, Z.; Zhang, J. Highly Emissive and Color-Tunable Perovskite Cross-linkers for Luminescent Polymer Networks. ACS Appl. Mater Interfaces 2018, 10, 28971–28978. [Google Scholar] [CrossRef]
- Liu, C.; Luo, H.; Shi, G.; Yang, J.; Chi, Z.; Ma, Y. Luminescent network film deposited electrochemically from a carbazole functionalized AIE molecule and its application for OLEDs. J. Mater. Chem. C 2015, 3, 3752–3759. [Google Scholar] [CrossRef]
- Li, M.; Tang, S.; Shen, F.; Liu, M.; Xie, W.; Xia, H.; Liu, L.; Tian, L.; Xie, Z.; Lu, P. Highly luminescent network films from electrochemical deposition of peripheral carbazole functionalized fluorene oligomer and their applications for light-emitting diodes. Chem. Commun. (Camb.) 2006, 28, 3393–3395. [Google Scholar] [CrossRef] [PubMed]
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. Highly efficient dicyano-phenylenevinylene fluorophore as polymer dopant or zinc-driven self-assembling building block. Inorg. Chem. Commun. 2019, 104, 145–149. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. A symmetrical azo-based fluorophore and the derived salen multipurpose framework for emissive layers. Inorg. Chem. Commun. 2019, 104, 186–189. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Das, S. Role of Molecular Packing in Determining Solid-State Optical Properties of pi-Conjugated Materials. J. Phys. Chem. Lett. 2011, 2, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Mutai, T.; Satou, H.; Araki, K. Reproducible on-off switching of solid-state luminescence by controlling molecular packing through heat-mode interconversion. Nat. Mater. 2005, 4, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, B.; Su, J.; Shen, L.; Xie, Y.; Tian, H. Color-tunable solid-state emission of 2,2′-biindenyl-based fluorophores. Angew. Chem. 2011, 50, 11654–11657. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, P.S.; Mothi, E.M.; Moon, D.; Anthony, S.P. Halochromic Isoquinoline with Mechanochromic Triphenylamine: Smart Fluorescent Material for Rewritable and Self-Erasable Fluorescent Platform. ACS Appl. Mater Interfaces 2016, 8, 33034–33042. [Google Scholar] [CrossRef]
- Srujana, P.; Radhakrishnan, T.P. Establishing the Critical Role of Oriented Aggregation in Molecular Solid State Fluorescence Enhancement. Chemistry 2018, 24, 1784–1788. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, J.X.; Zhao, Z.; Chen, Y.; He, X.; Chen, M.; Gong, J.; Sung, H.H.; Williams, I.D.; Lam, J.W.Y.; et al. Multiple yet Controllable Photoswitching in a Single AIEgen System. J. Am. Chem. Soc. 2018, 140, 1966–1975. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Concilio, S.; Nabha, S.; Piotto, S.; Shikler, R.; Tuzi, A.; Panunzi, B. From cadmium(II)-aroylhydrazone complexes to metallopolymers with enhanced photoluminescence. A structural and DFT study. Inorg. Chim. Acta 2017, 458, 129–137. [Google Scholar] [CrossRef]
- Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Shikler, R.; Nabha, S.; Tuzi, A.; Caruso, U.; Piotto, S. Solid-state highly efficient dr mono and poly-dicyano-phenylenevinylene fluorophores. Molecules 2018, 23, 1505. [Google Scholar] [CrossRef] [PubMed]
- Panunzi, B.; Concilio, S.; Diana, R.; Shikler, R.; Nabha, S.; Piotto, S.; Sessa, L.; Tuzi, A.; Caruso, U. Photophysical Properties of Luminescent Zinc(II)‒Pyridinyloxadiazole Complexes and their Glassy Self-Assembly Networks. Eur. J. Inorg. Chem. 2018, 2018, 2709–2716. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Shikler, R.; Nabha, S.; Tuzi, A.; Piotto, S. AIE/ACQ effects in two DR/NIR emitters: A structural and DFT comparative analysis. Molecules 2018, 23, 1947. [Google Scholar] [CrossRef] [PubMed]
- Panunzi, B.; Borbone, F.; Capobianco, A.; Concilio, S.; Diana, R.; Peluso, A.; Piotto, S.; Tuzi, A.; Velardo, A.; Caruso, U. Synthesis, spectroscopic properties and DFT calculations of a novel multipolar azo dye and its zinc(II) complex. Inorg. Chem. Commun. 2017, 84, 103–108. [Google Scholar] [CrossRef]
- Argeri, M.; Borbone, F.; Caruso, U.; Causà, M.; Fusco, S.; Panunzi, B.; Roviello, A.; Shikler, R.; Tuzi, A. Color tuning and noteworthy photoluminescence quantum yields in crystalline mono-/dinuclear ZnII complexes. Eur. J. Inorg. Chem. 2014, 2014, 5916–5924. [Google Scholar] [CrossRef]
- Panunzi, B.; Diana, R.; Tuzi, A.; Carella, A.; Caruso, U. A new donor-acceptor crosslinkable l-shape chromophore for NLO applications. J. Mol. Struct. 2019, 1189, 21–27. [Google Scholar] [CrossRef]
- Diana, R.; Caruso, U.; Concilio, S.; Piotto, S.; Tuzi, A.; Panunzi, B. A real-time tripodal colorimetric/fluorescence sensor for multiple target metal ions. Dyes Pigments 2018, 155, 249–257. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Concilio, S.; Nabha, S.; Panunzi, B.; Piotto, S.; Shikler, R.; Tuzi, A. Mono-, Di-, and Polymeric Pyridinoylhydrazone ZnII Complexes: Structure and Photoluminescent Properties. Eur. J. Inorg. Chem. 2016, 2016, 818–825. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, W.; Hao, X.; Redshaw, C.; Chen, L.; Sun, W.H. 6-Benzhydryl-4-methyl-2-(1H-benzoimidazol-2-yl)phenol ligands and their zinc complexes: Syntheses, characterization and photoluminescence behavior. Inorg. Chim. Acta 2012, 392, 345–353. [Google Scholar] [CrossRef]
- Pallavi, P.; Kumar, V.; Hussain, M.W.; Patra, A. Excited-State Intramolecular Proton Transfer-Based Multifunctional Solid-State Emitter: A Fluorescent Platform with “Write-Erase-Write” Function. ACS Appl. Mater Interfaces 2018, 10, 44696–44705. [Google Scholar] [CrossRef] [PubMed]
- Caruso, U.; Panunzi, B.; Roviello, A.; Sirigu, A. Networks from Liquid Crystalline Segmented Chain Polymers. Macromolecules 1994, 27, 3513–3519. [Google Scholar] [CrossRef]
- de Mello, J.C.; Wittmann, H.F.; Friend, R.H. An improved experimental determination of external photoluminescence quantum efficiency. Adv. Mater. 1997, 9, 230–232. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; Leddy, J.; Zoski, C.G. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980; Volume 2. [Google Scholar]
- Bochevarov, A.D.; Harder, E.; Hughes, T.F.; Greenwood, J.R.; Braden, D.A.; Philipp, D.M.; Rinaldo, D.; Halls, M.D.; Zhang, J.; Friesner, R.A. Jaguar: A high-performance quantum chemistry software program with strengths in life and materials sciences. Int. J. Quantum Chem. 2013, 113, 2110–2142. [Google Scholar] [CrossRef]
- Fetter, A.L.; Walecka, J.D. Quantum Theory of Many-Particle Systems; Dover Publication: New York, NY, USA, 2012. [Google Scholar]
- Marten, B.; Kim, K.; Cortis, C.; Friesner, R.A.; Murphy, R.B.; Ringnalda, M.N.; Sitkoff, D.; Honig, B. New Model for Calculation of Solvation Free Energies: Correction of Self-Consistent Reaction Field Continuum Dielectric Theory for Short-Range Hydrogen-Bonding Effects. J. Phys. Chem. 1996, 100, 11775–11788. [Google Scholar] [CrossRef]
- Piotto, S.; Concilio, S.; Sessa, L.; Diana, R.; Torrens, G.; Juan, C.; Caruso, U.; Iannelli, P. Synthesis and Antimicrobial Studies of New Antibacterial Azo-Compounds Active against Staphylococcus aureus and Listeria monocytogenes. Molecules 2017, 22, 1372. [Google Scholar] [CrossRef] [PubMed]
- Melhuish, W.H. Quantum Efficiencies of Fluorescence of Organic Substances: Effect of Solvent and Concentration of the Fluorescent Solute1. J. Phys. Chem. 1961, 65, 229–235. [Google Scholar] [CrossRef]
- Goes, M.; Verhoeven, J.W.; Hofstraat, H.; Brunner, K. OLED and PLED devices employing electrogenerated, intramolecular charge-transfer fluorescence. Chem. Phys. Chem. 2003, 4, 349–358. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Zhuang, Z.; Wang, Z.; Lin, G.; Shen, P.; Chen, S.; Zhao, Z.; Tang, B.Z. Efficient red AIEgens based on tetraphenylethene: Synthesis, structure, photoluminescence and electroluminescence. J. Mater. Chem. C 2018, 6, 5900–5907. [Google Scholar] [CrossRef]
- Meng, L.C.; Lou, Z.D.; Yang, S.Y.; Hou, Y.B.; Teng, F.; Liu, X.J.; Li, Y.B. White organic light-emitting diodes based on a combined electromer and monomer emission in doubly-doped polymers. Chin. Phys. 2012, 21. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yokota, M.; Hisakado, Y.; Yang, H.; Kajiyama, T. Polymer-stabilized liquid crystal blue phases. Nat. Mater. 2002, 1, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Doane, J.W.; Vaz, N.A.; Wu, B.G.; Žumer, S. Field controlled light scattering from nematic microdroplets. Appl. Phys. Lett. 1986, 48, 269–271. [Google Scholar] [CrossRef]
- Doane, J.W.; West, J.L.; Chidichimo, G.; Vaz, N.A.P.; Wu, B.G.; Golemme, A.; Zumar, S. Light Modulating Materials Dispersed in Solid Resin Matrix and Display Devices from Them. WO 8701822A1, 30 September 1987. [Google Scholar]
- Zhao, D.; Fan, F.; Cheng, J.; Zhang, Y.; Wong, K.S.; Chigrinov, V.G.; Kwok, H.S.; Guo, L.; Tang, B.Z. Light-Emitting Liquid Crystal Displays Based on an Aggregation-Induced Emission Luminogen. Adv. Opt. Mater. 2015, 3, 199–202. [Google Scholar] [CrossRef]
- Kamarudin, M.A.; Khan, A.A.; Said, S.M.; Qasim, M.M.; Wilkinson, T.D. Composite liquid crystal-polymer electrolytes in dye-sensitised solar cells: Effects of mesophase alkyl chain length. Liq. Cryst. 2017, 45, 112–121. [Google Scholar] [CrossRef]
- Sha, J.; Lu, H.; Zhou, M.; Xia, G.; Fang, Y.; Zhang, G.; Qiu, L.; Yang, J.; Ding, Y. Highly polarized luminescence from an AIEE-active luminescent liquid crystalline film. Org. Electron. Phys. Mater. Appl. 2017, 50, 177–183. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Chen, J.; Zhu, W.; Baranoff, E. Recent progress in luminescent liquid crystal materials: Design, properties and application for linearly polarised emission. J. Mater. Chem. C 2015, 3, 7993–8005. [Google Scholar] [CrossRef]
- Zhao, D.; He, H.; Gu, X.; Guo, L.; Wong, K.S.; Lam, J.W.Y.; Tang, B.Z. Circularly Polarized Luminescence and a Reflective Photoluminescent Chiral Nematic Liquid Crystal Display Based on an Aggregation-Induced Emission Luminogen. Adv. Opt. Mater. 2016, 4, 534–539. [Google Scholar] [CrossRef]
- Jing, H.; Lu, L.; Feng, Y.; Zheng, J.F.; Deng, L.; Chen, E.Q.; Ren, X.K. Synthesis, Aggregation-Induced Emission, and Liquid Crystalline Structure of Tetraphenylethylene–Surfactant Complex via Ionic Self-Assembly. J. Phys. Chem. C 2016, 120, 27577–27586. [Google Scholar] [CrossRef]
- Lu, H.; Wu, S.; Hu, J.; Qiu, L.; Wang, X.; Zhang, G.; Hu, J.; Lv, G.; Yang, J. Electrically controllable fluorescence of tristable optical switch based on luminescent molecule-doped cholesteric liquid crystal. Dyes Pigments 2015, 121, 147–151. [Google Scholar] [CrossRef]
- Lu, H.; Xu, C.; Li, Z.; Xia, G.; Jing, S.; Bai, X.; Yang, J.; Qiu, L.; Ding, Y. High-contrast electrically switchable light-emitting liquid crystal displays based on α-cyanostilbenic derivative. Liq. Cryst. 2018, 45, 32–39. [Google Scholar] [CrossRef]
- Concilio, S.; Bugatti, V.; Neitzert, H.C.; Landi, G.; De Sio, A.; Parisi, J.; Piotto, S.; Iannelli, P. Zn-complex based on oxadiazole/carbazole structure: Synthesis, optical and electric properties. Thin Solid Film 2014, 556, 419–424. [Google Scholar] [CrossRef]
- Morvillo, P.; Ricciardi, R.; Nenna, G.; Bobeico, E.; Diana, R.; Minarini, C. Elucidating the origin of the improved current output in inverted polymer solar cells. Sol. Energ. Mater. Sol. Cells 2016, 152, 51–58. [Google Scholar] [CrossRef]
- Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Tuzi, A.; Piotto, S.; Caruso, U. Fluorescence pH-dependent sensing of Zn(II) by a tripodal ligand. A comparative X-ray and DFT study. J. Lumin. 2019, 212, 200–206. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Palma, S.D.; Fusco, S.; Nabha, S.; Panunzi, B.; Shikler, R. High solid state photoluminescence quantum yields and effective color tuning in polyvinylpyridine based zinc(II) metallopolymers. Macromol. Chem. Phys. 2015, 216, 1516–1522. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Roviello, A.; Tingoli, M.; Tuzi, A. Two aminobenzothiazole derivatives for Pd(II) and Zn(II) coordination: Synthesis, characterization and solid state fluorescence. Inorg. Chem. Commun. 2011, 14, 46–48. [Google Scholar] [CrossRef]
- Ji, X.; Yao, Y.; Li, J.; Yan, X.; Huang, F. A Supramolecular Cross-Linked Conjugated Polymer Network for Multiple Fluorescent Sensing. J. Am. Chem. Soc. 2013, 135, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Bredas, J.; Silbey, R.; Boudreaux, D.; Chance, R. Chain-length dependence of electronic and electrochemical properties of conjugated systems: Polyacetylene, polyphenylene, polythiophene, and polypyrrole. J. Am. Chem. Soc. 1983, 105, 6555–6559. [Google Scholar] [CrossRef]
- Mohamed, M.; Eichborn, A.H.; Eichborn, S.H. Measurement and prediction of electronic properties of discotic triphenylenes and phtalocianines. ECS Trans. 2010, 25, 1–10. [Google Scholar]
- Johansson, T.; Mammo, W.; Svensson, M.; Andersson, M.R.; Inganäs, O. Electrochemical bandgaps of substituted polythiophenes. J. Mater. Chem. 2003, 13, 1316–1323. [Google Scholar] [CrossRef]
- D’Andrade, B.W.; Datta, S.; Forrest, S.R.; Djurovich, P.; Polikarpov, E.; Thompson, M.E. Relationship between the ionization and oxidation potentials of molecular organic semiconductors. Org. Electron. 2005, 6, 11–20. [Google Scholar] [CrossRef]
- Morvillo, P.; Diana, R.; Fontanesi, C.; Ricciardi, R.; Lanzi, M.; Mucci, A.; Tassinari, F.; Schenetti, L.; Minarini, C.; Parenti, F. Low band gap polymers for application in solar cells: Synthesis and characterization of thienothiophene-thiophene copolymers. Polym. Chem. 2014, 5, 2391–2400. [Google Scholar] [CrossRef]


| Sample | λabs.sol (nm) a | λem.sol (nm) b | PLQY% c | Solvent | λabs.film (nm) d | λem.film (nm) e | PLQY% f | Matrix f |
|---|---|---|---|---|---|---|---|---|
| C1b | 369 | 533 | 5.00 ± 0.02 | Chloroform | (403)474 j | 613 j | 25 ± 1 j | neat j |
| 373 | 529 | THF | 470 jj | 566 jj | 30 ± 1 jj | PS jj | ||
| 396 | 523 | NMP | 460 jjj | 566 ijjj | 88 ± 5 jjj | PVK jjj | ||
| - | - | - | 460 jv | 571 jv | 50 ± 5 jv | PDLC jv | ||
| C2b | 420 | 534 | 3.00 ± 0.02 | Chloroform | 446 j | 613 j | 22 ± 1 j | neat j |
| 422 | 512 | THF | 429 jj | 566 jj | 10 ± 2 jj | PS jj | ||
| 430 | 511 | NMP | 429 jjj | 566 ijjj | 21 ± 2 jjj | PVK jjj | ||
| - | - | - | 414 jv | 571 jv | 41 ± 5 jv | PDLC jv |
| Compound | λabs.sol (nm) a | λem.sol (nm) b | PLQY% c | λabs.film (nm) d | λem.film (nm) e | PLQY% f |
|---|---|---|---|---|---|---|
| P1b | 321 (365,433) | 532 | 2.00 ± 0.02 | 466 | 606 | 34 ± 2 |
| P2b | 326–420 | 498 | 1.30 ± 0.02 | 448 | 560 | 20 ± 2 |
| co-P1b | 346 (453) | 518–616 | 4.50 ± 0.02 | 444 | 596 | 10 ± 1 |
| co-P2b | 348 (429) | 511 | 3.00 ± 0.02 | 435 | 551 | 14 ± 2 |
| Net-P1b | - | - | - | 460 | 555 | 8.0 ± 1 |
| Net-P2b | - | - | - | 435 | 490 | 7.0 ± 1 |
| Net-co-P1b | - | - | - | 395 | 597 | 9.0 ± 1 |
| Net-co-P2b | - | - | - | 397 | 552 | 10 ± 1 |
| Properties | C1b | C2b |
|---|---|---|
| Oxidation Potential (eV) | 1.27 | 1.26 |
| Reduction Potential (eV) | −0.92 | −1.00 |
| Hole Reorganization Energy (eV) | 0.28 | 0.40 |
| Electron Reorganization Energy (eV) | 0.22 | 0.29 |
| Triplet Energy (eV) | 1.49 | 1.00 |
| λmax (nm) | 516 | 509 |
| Emax (nm) | 565 | 550 |
| Scaled HOMO (eV) | −5.55 | −5.54 |
| Scaled LUMO (eV) | −3.36 | −3.28 |
| HOMO-LUMO (eV) | 2.18 | 2.26 |
| Triplet Stabilization Energy (eV) | 0.35 | 1.04 |
| Hole Extraction Potential (eV) | 5.92 | 5.82 |
| Triplet Reorganization Energy (eV) | 0.62 | 2.05 |
| Electron Extraction Potential (eV) | −2.08 | −2.05 |
| Polymers | ΔEEC (eV) | ΔEopt (eV) | ΔEopt − ΔEtheor (eV) |
|---|---|---|---|
| P1b | 1.9 | 2.9 | 0.7 |
| P2b | 1.8 | 3.0 | 0.7 |
| coP1b | 1.2 | 2.7 | 0.5 |
| coP2b | 1.4 | 2.9 | 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diana, R.; Panunzi, B.; Concilio, S.; Marrafino, F.; Shikler, R.; Caruso, T.; Caruso, U. The Effect of Bulky Substituents on Two π-Conjugated Mesogenic Fluorophores. Their Organic Polymers and Zinc-Bridged Luminescent Networks. Polymers 2019, 11, 1379. https://doi.org/10.3390/polym11091379
Diana R, Panunzi B, Concilio S, Marrafino F, Shikler R, Caruso T, Caruso U. The Effect of Bulky Substituents on Two π-Conjugated Mesogenic Fluorophores. Their Organic Polymers and Zinc-Bridged Luminescent Networks. Polymers. 2019; 11(9):1379. https://doi.org/10.3390/polym11091379
Chicago/Turabian StyleDiana, Rosita, Barbara Panunzi, Simona Concilio, Francesco Marrafino, Rafi Shikler, Tonino Caruso, and Ugo Caruso. 2019. "The Effect of Bulky Substituents on Two π-Conjugated Mesogenic Fluorophores. Their Organic Polymers and Zinc-Bridged Luminescent Networks" Polymers 11, no. 9: 1379. https://doi.org/10.3390/polym11091379
APA StyleDiana, R., Panunzi, B., Concilio, S., Marrafino, F., Shikler, R., Caruso, T., & Caruso, U. (2019). The Effect of Bulky Substituents on Two π-Conjugated Mesogenic Fluorophores. Their Organic Polymers and Zinc-Bridged Luminescent Networks. Polymers, 11(9), 1379. https://doi.org/10.3390/polym11091379