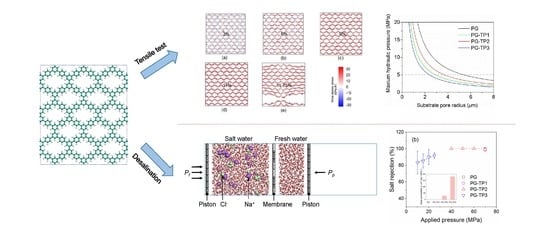
Theoretical Prediction of Mechanical Strength and Desalination Performance of One-Atom-Thick Hydrocarbon Polymer in Pressure-Driven Separation
Abstract
1. Introduction
2. Computational Details
2.1. Tensile Tests
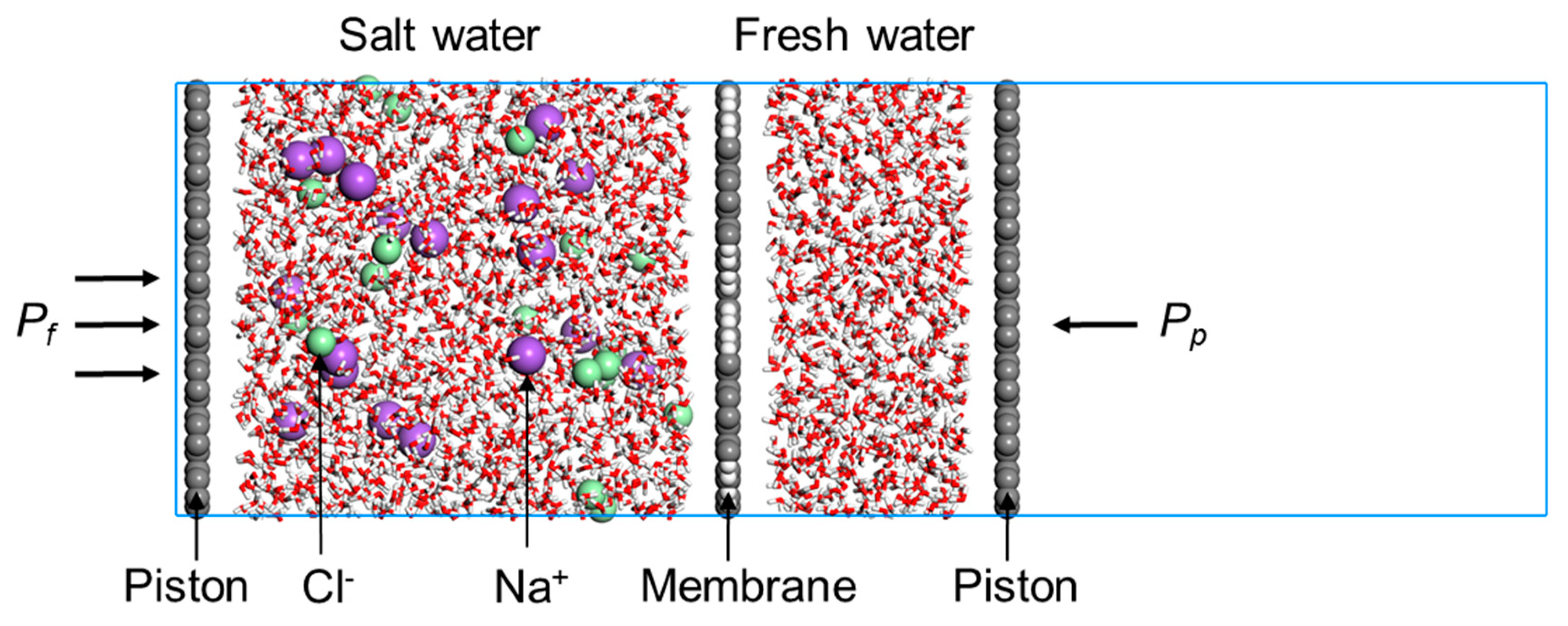
2.2. Reverse Osmosis Desalination
3. Results and Discussion
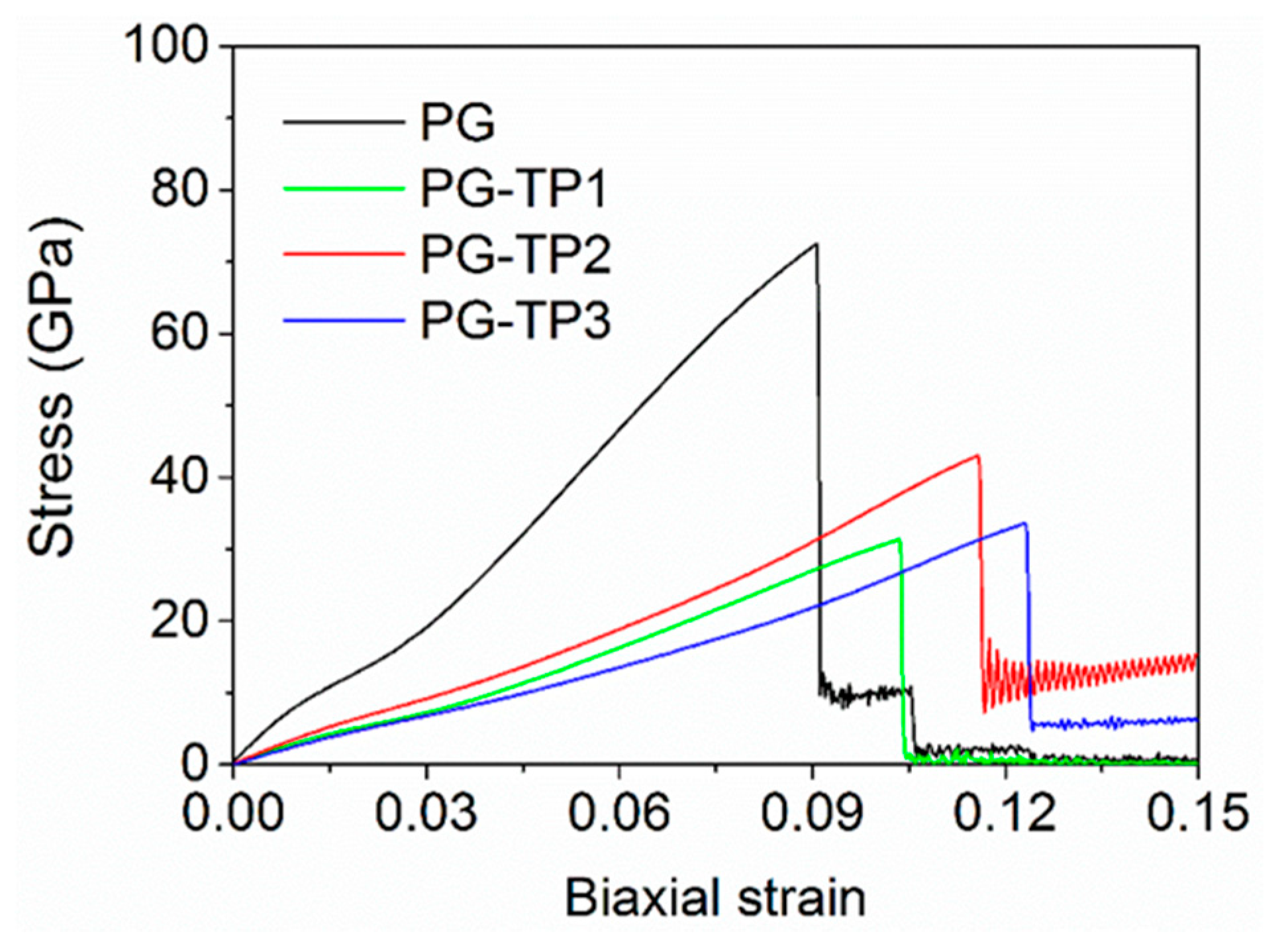
3.1. Mechanical Strength of PG-TPX Membranes
3.1.1. Mechanical Parameters of PG-TPX Membranes
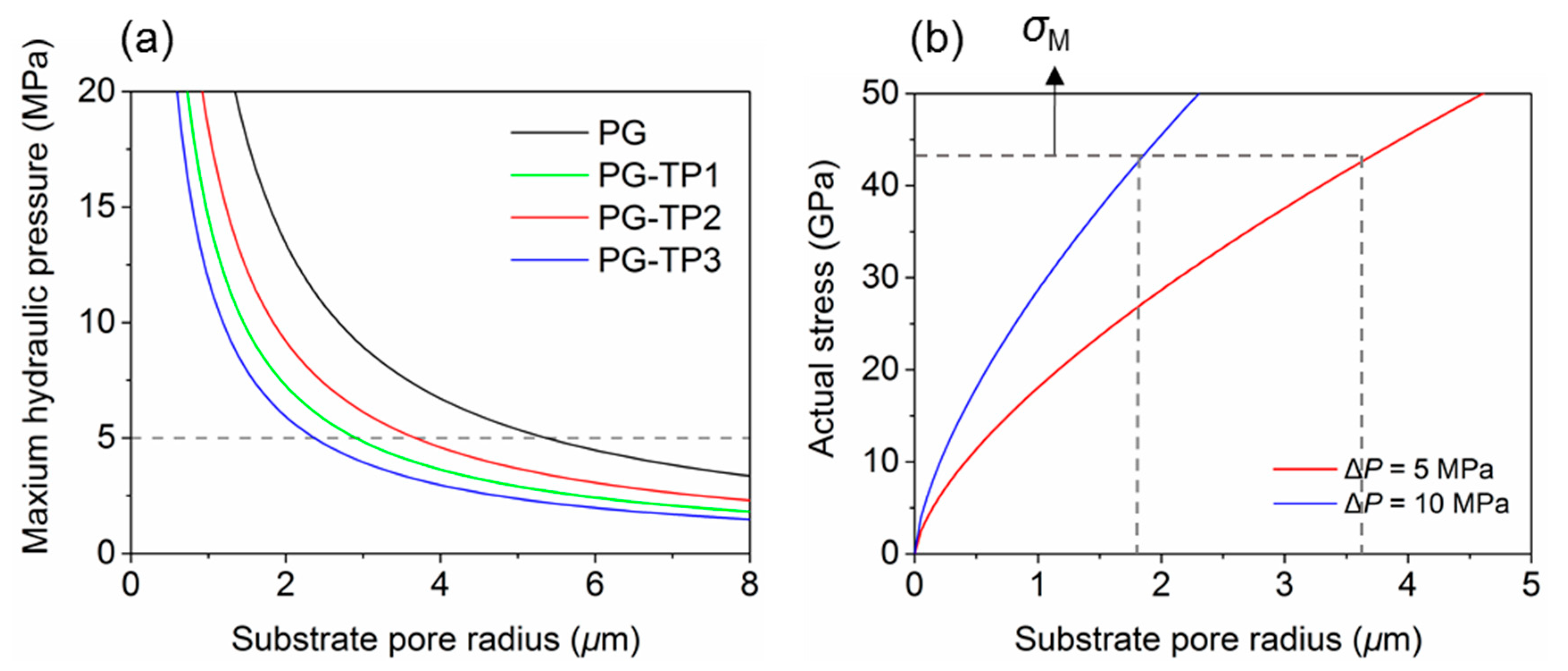
3.1.2. Optimization of Substrate Pore Size
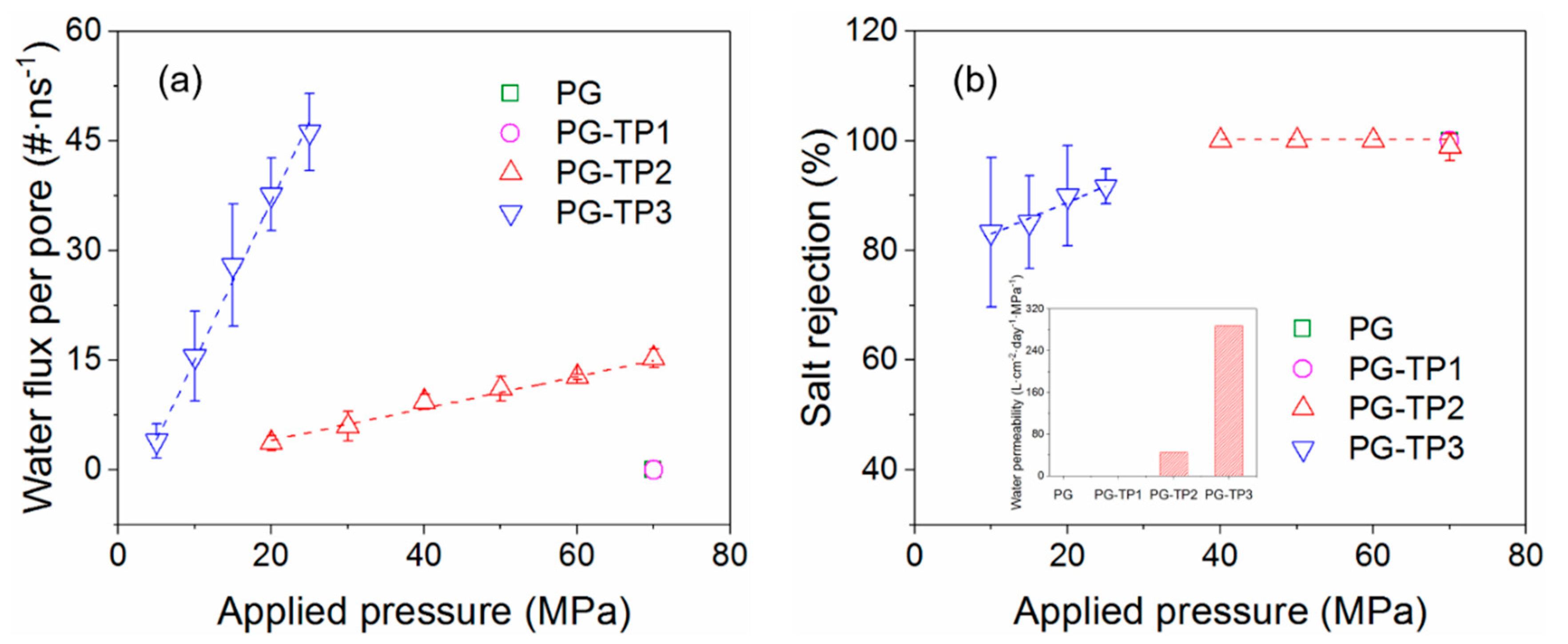
3.2. Desalination Performance of PG-TPX Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Basu, S.; Khan, A.L.; Cano-Odena, A.; Liu, C.; Vankelecom, I.F. Membrane-based technologies for biogas separations. Chem. Soc. Rev. 2010, 39, 750–768. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.; Sholl, D.S. Screening metal-organic framework materials for membrane-based methane/carbon dioxide separations. J. Phys. Chem. C 2007, 111, 14055–14059. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Hosamani, K.M.; Aminabhavi, T.M. Distillery wastewater treatment by the membrane-based nanofiltration and reverse osmosis processes. Water Res. 2006, 40, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, S.; Tao, J.; Gao, C. Preparation, structure characteristics and separation properties of thin-film composite polyamide-urethane seawater reverse osmosis membrane. J. Member. Sci. 2008, 325, 947–956. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Member. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Yuan, L.; Miao, T.; Rong, W. A high-performance and robust membrane with switchable super-wettability for oil/water separation under ultralow pressure. J. Member. Sci. 2017, 543, 123–132. [Google Scholar]
- Fathizadeh, M.; Xu, W.L.; Zhou, F.; Yoon, Y.; Miao, Y. Graphene Oxide: A Novel 2-Dimensional Material in Membrane Separation for Water Purification. Adv. Mater. Interface 2017, 4, 1600918. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Bruggen, B.V.D. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef]
- Lin, L.C.; Choi, J.; Grossman, J.C. Two-dimensional covalent triazine framework as an ultrathin-film nanoporous membrane for desalination. Chem. Commun. 2015, 51, 14921–14924. [Google Scholar] [CrossRef]
- Kadhom, M.; Deng, B. Metal-organic frameworks (MOFs) in water filtration membranes for desalination and other applications. Appl. Mat. Today 2018, 11, 219–230. [Google Scholar] [CrossRef]
- Sun, H.; Tang, B.; Wu, P. Development of hybrid ultrafiltration membranes with improved water separation properties using modified super-hydrophilic metal-organic framework nanoparticles. ACS Appl. Mat. Interfaces 2017, 9, 21473. [Google Scholar] [CrossRef]
- Kadhom, M.; Hu, W.; Deng, B. Thin Film Nanocomposite Membrane Filled with Metal-Organic Frameworks UiO-66 and MIL-125 Nanoparticles for Water Desalination. Membranes 2017, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Yong, P.; Lu, H.; Wang, Z.; Yan, Y. Microstructural optimization of MFI-type zeolite membranes for ethanol–water separation. J. Mater. Chem. A 2014, 2, 16093–16100. [Google Scholar]
- Hsieh, C.T.; Hsu, J.P.; Hsu, H.H.; Lin, W.H.; Juang, R.S. Hierarchical oil–water separation membrane using carbon fabrics decorated with carbon nanotubes. Surf. Coat. Technol. 2016, 286, 148–154. [Google Scholar] [CrossRef]
- Khalid, A.; Al-Juhani, A.A.; Al-Hamouz, O.C.; Laoui, T.; Khan, Z.; Atieh, M.A. Preparation and properties of nanocomposite polysulfone/multi-walled carbon nanotubes membranes for desalination. Desalination 2015, 367, 134–144. [Google Scholar] [CrossRef]
- Postma, H.W.C. Rapid sequencing of individual DNA molecules in graphene nanogaps. Nano Lett. 2010, 10, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yang, J.; Wang, K.; Zhou, Z.; Shen, P. Porous graphene: Properties, preparation and potential applications. Chin. Sci. Bull. 2012, 57, 2948–2955. [Google Scholar] [CrossRef]
- Hauser, A.W.; Schwerdtfeger, P. Nanoporous graphene membranes for efficient 3He/4He separation. J. Phys. Chem. Lett. 2012, 3, 209–213. [Google Scholar] [CrossRef]
- Schrier, J.; McClain, J. Thermally-driven isotope separation across nanoporous graphene. Chem. Phys. Lett. 2012, 521, 118–124. [Google Scholar] [CrossRef]
- Blankenburg, S.; Bieri, M.; Fasel, R.; Müllen, K.; Pignedoli, C.A.; Passerone, D. Porous graphene as an atmospheric nanofilter. Small 2010, 6, 2266–2271. [Google Scholar] [CrossRef]
- Lin, L.-C.; Grossman, J.C. Atomistic understandings of reduced graphene oxide as an ultrathin-film nanoporous membrane for separations. Nat. Commun. 2015, 6, 8335. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Sun, S.; Li, C.; Hu, S.; Lin, L.-C. Rational Design of Two-Dimensional Hydrocarbon Polymer as Ultrathin-Film Nanoporous Membranes for Water Desalination. ACS Appl. Mat. Interfaces 2018, 18, 18778–18786. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Tanugi, D.; Grossman, J.C. Water desalination across nanoporous graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Garaj, S.; Hubbard, W.; Reina, A.; Kong, J.; Branton, D.; Golovchenko, J. Graphene as a subnanometre trans-electrode membrane. Nature 2010, 467, 190. [Google Scholar] [CrossRef]
- Bell, D.C.; Lemme, M.C.; Stern, L.A.; Williams, J.R.; Marcus, C.M. Precision cutting and patterning of graphene with helium ions. Nanotechnology 2009, 20, 455301. [Google Scholar] [CrossRef]
- Bieri, M.; Treier, M.; Cai, J.; Aït-Mansour, K.; Ruffieux, P.; Gröning, O.; Gröning, P.; Kastler, M.; Rieger, R.; Feng, X. Porous graphenes: Two-dimensional polymer synthesis with atomic precision. Chem. Commun. 2009, 45, 6919–6921. [Google Scholar] [CrossRef]
- Kim, M.; Safron, N.S.; Han, E.; Arnold, M.S.; Gopalan, P. Fabrication and characterization of large-area, semiconducting nanoperforated graphene materials. Nano Lett. 2010, 10, 1125–1131. [Google Scholar] [CrossRef]
- Matsuoka, R.; Toyoda, R.; Shiotsuki, R.; Fukui, N.; Wada, K.; Maeda, H.; Sakamoto, R.; Sasaki, S.; Masunaga, H.; Nagashio, K. Expansion of the Graphdiyne Family: A Triphenylene-Cored Analogue. ACS Appl. Mat. Interfaces 2018, 11, 2730–2733. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Shen, P.; Chen, Z. Two-dimensional polyphenylene: Experimentally available porous graphene as a hydrogen purification membrane. Chem. Commun. 2010, 46, 3672–3674. [Google Scholar] [CrossRef]
- Liu, W.; Luo, X.; Bao, Y.; Liu, Y.P.; Ning, G.-H.; Abdelwahab, I.; Li, L.; Nai, C.T.; Hu, Z.G.; Zhao, D. A two-dimensional conjugated aromatic polymer via C–C coupling reaction. Nat. Chem. 2017, 9, 563. [Google Scholar] [CrossRef]
- Solvik, K.; Weaver, J.A.; Brockway, A.M.; Schrier, J. Entropy-driven molecular separations in 2D-nanoporous materials, with application to high-performance paraffin/olefin membrane separations. J. Phys. Chem. C 2013, 117, 17050–17057. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Hoek, E.M. Impacts of support membrane structure and chemistry on polyamide–polysulfone interfacial composite membranes. J. Member. Sci. 2009, 336, 140–148. [Google Scholar] [CrossRef]
- Singh, P.S.; Joshi, S.; Trivedi, J.; Devmurari, C.; Rao, A.P.; Ghosh, P. Probing the structural variations of thin film composite RO membranes obtained by coating polyamide over polysulfone membranes of different pore dimensions. J. Member. Sci. 2006, 278, 19–25. [Google Scholar] [CrossRef]
- Van Duin, A.C.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Budzien, J. Reactive Molecular Dynamics Simulations of Shock through a Single Crystal of Pentaerythritol Tetranitrate. J. Phys. Chem. B 2009, 113, 13142. [Google Scholar] [CrossRef] [PubMed]
- Strachan, A. Shock waves in high-energy materials: The initial chemical events in nitramine RDX. Phys. Rev. Lett. 2003, 91, 098301. [Google Scholar] [CrossRef] [PubMed]
- Braga, C.; Travis, K.P. A configurational temperature Nosé-Hoover thermostat. J. Chem. Phys. 2005, 123, 134101. [Google Scholar] [CrossRef]
- Wong, C.H.; Vijayaraghavan, V. Nanomechanics of free form and water submerged single layer graphene sheet under axial tension by using molecular dynamics simulation. Mat. Sci. Eng. A 2012, 556, 420–428. [Google Scholar] [CrossRef]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A generic force field for molecular simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Rappe, A.K.; Iii, W.A.G. Charge Equilibration for Molecular Dynamics Simulations. J. Phys. Chem. 1991, 95, 3358–3363. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Joung, I.S.; Cheatham, T.E., III. Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J. Phys. Chem. B 2008, 112, 9020–9041. [Google Scholar] [CrossRef] [PubMed]
- Werder, T.; Walther, J.H.; Jaffe, R.; Halicioglu, T.; Koumoutsakos, P. On the water-carbon interaction for use in molecular dynamics simulations of graphite and carbon nanotubes. J. Phys. Chem. B 2003, 107, 1345–1352. [Google Scholar] [CrossRef]
- Cohentanugi, D.; Grossman, J.C. Mechanical strength of nanoporous graphene as a desalination membrane. Nano Lett. 2014, 14, 6171. [Google Scholar] [CrossRef]
- Nakao, S.-I. Determination of pore size and pore size distribution: 3. Filtration membranes. J. Member. Sci. 1994, 96, 131–165. [Google Scholar] [CrossRef]
- Lilov, S.K. Determination of the Effective Kinetic Diameter of the Complex Molecules. Cryst. Res. Technol. 2010, 21, 1299–1302. [Google Scholar] [CrossRef]
- David, C.T.; Grossman, J.C. Water permeability of nanoporous graphene at realistic pressures for reverse osmosis desalination. J. Chem. Phys. 2014, 141, 074704. [Google Scholar]
- Pendergast, M.T.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]


| Membrane Type | Biaxial Tension | Uniaxial Tension | ||
|---|---|---|---|---|
| EM (GPa) | σM (GPa) | εM (%) | v | |
| PG | 839.613 ± 5.751 | 72.520 ± 0.471 | 9.075 ± 0.084 | 0.50 ± 0.01 |
| PG-TP1 | 315.139 ± 4.267 | 31.369 ± 0.249 | 10.350 ± 0.097 | 0.29 ± 0.01 |
| PG-TP2 | 366.552 ± 4.154 | 43.012 ± 0.283 | 11.575 ± 0.106 | 0.51 ± 0.01 |
| PG-TP3 | 269.873 ± 3.967 | 33.368 ± 0.262 | 12.325 ± 0.114 | 0.69 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Shan, F.; Lyu, Q.; Li, C.; Hu, S. Theoretical Prediction of Mechanical Strength and Desalination Performance of One-Atom-Thick Hydrocarbon Polymer in Pressure-Driven Separation. Polymers 2019, 11, 1358. https://doi.org/10.3390/polym11081358
Sun S, Shan F, Lyu Q, Li C, Hu S. Theoretical Prediction of Mechanical Strength and Desalination Performance of One-Atom-Thick Hydrocarbon Polymer in Pressure-Driven Separation. Polymers. 2019; 11(8):1358. https://doi.org/10.3390/polym11081358
Chicago/Turabian StyleSun, Shuangqing, Fei Shan, Qiang Lyu, Chunling Li, and Songqing Hu. 2019. "Theoretical Prediction of Mechanical Strength and Desalination Performance of One-Atom-Thick Hydrocarbon Polymer in Pressure-Driven Separation" Polymers 11, no. 8: 1358. https://doi.org/10.3390/polym11081358
APA StyleSun, S., Shan, F., Lyu, Q., Li, C., & Hu, S. (2019). Theoretical Prediction of Mechanical Strength and Desalination Performance of One-Atom-Thick Hydrocarbon Polymer in Pressure-Driven Separation. Polymers, 11(8), 1358. https://doi.org/10.3390/polym11081358