Redox-Active Gel Electrolyte Combined with Branched Polyaniline Nanofibers Doped with Ferrous Ions for Ultra-High-Performance Flexible Supercapacitors
Abstract
1. Introduction
2. Experimental
2.1. Preparation of PANI/Fe2+ and PANI Materials
2.2. Preparation of H2SO4/Fe2+/3+/Poly(Vinyl Alcohol) (PVA) Gel Electrolyte
2.3. Characterization
2.4. Electrochemical Measurements
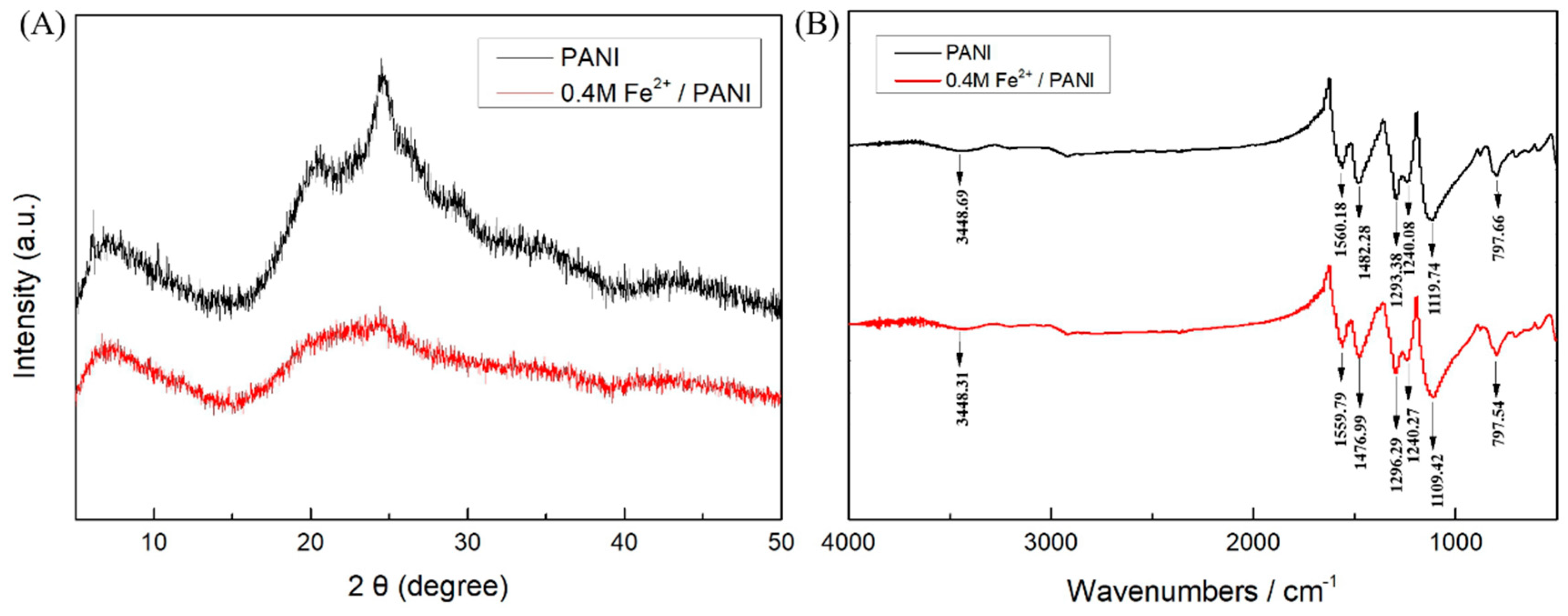
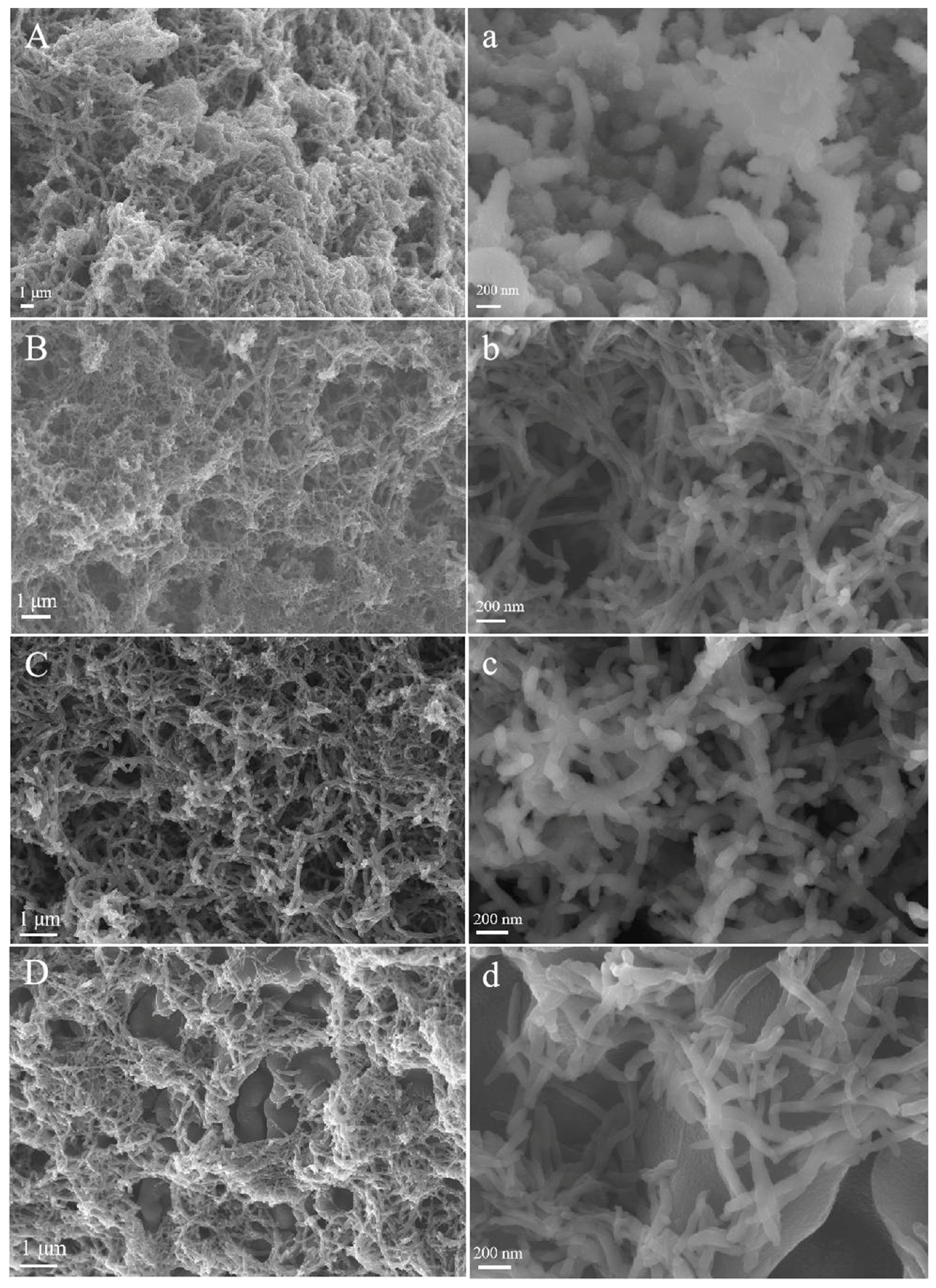
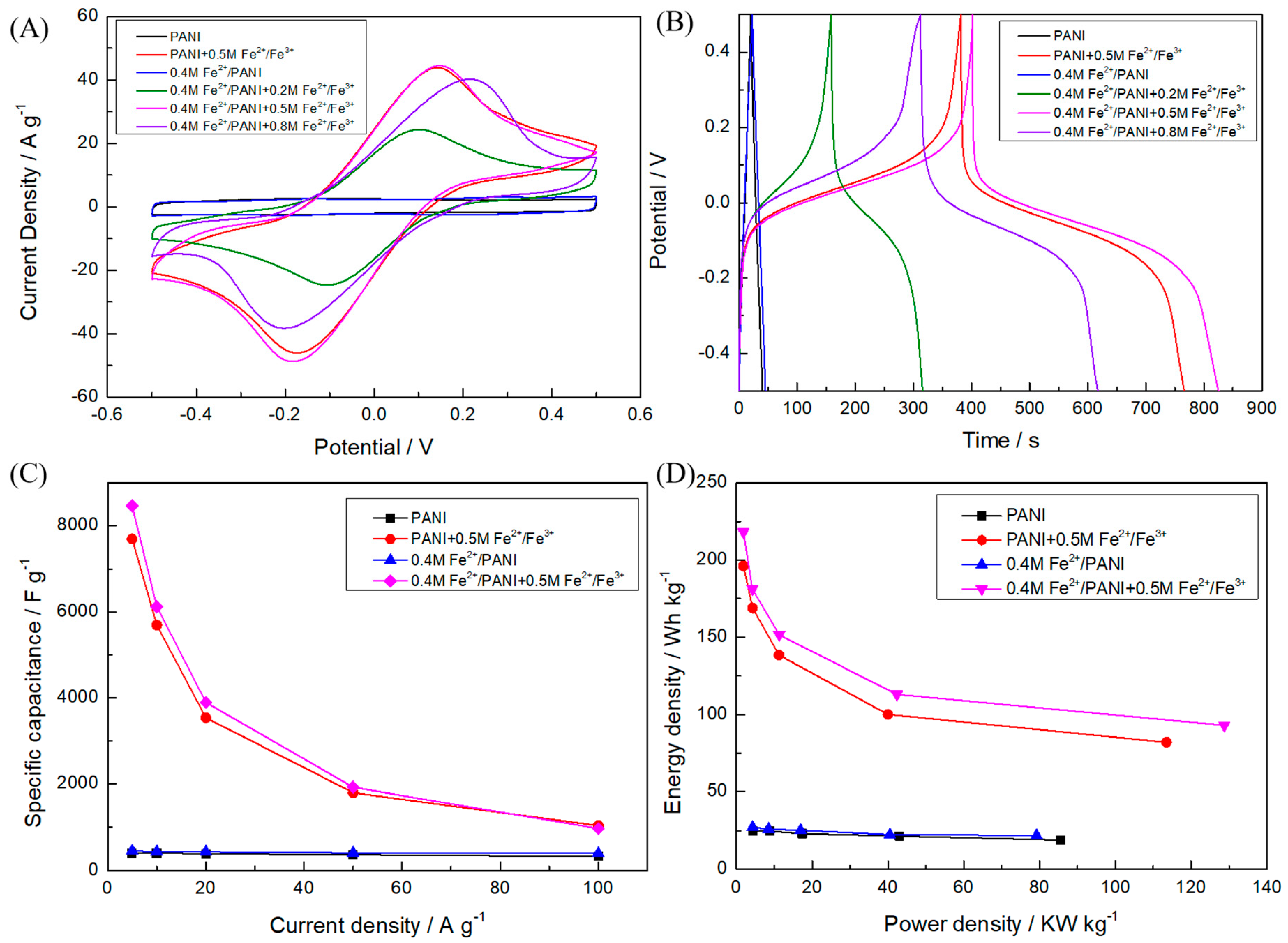
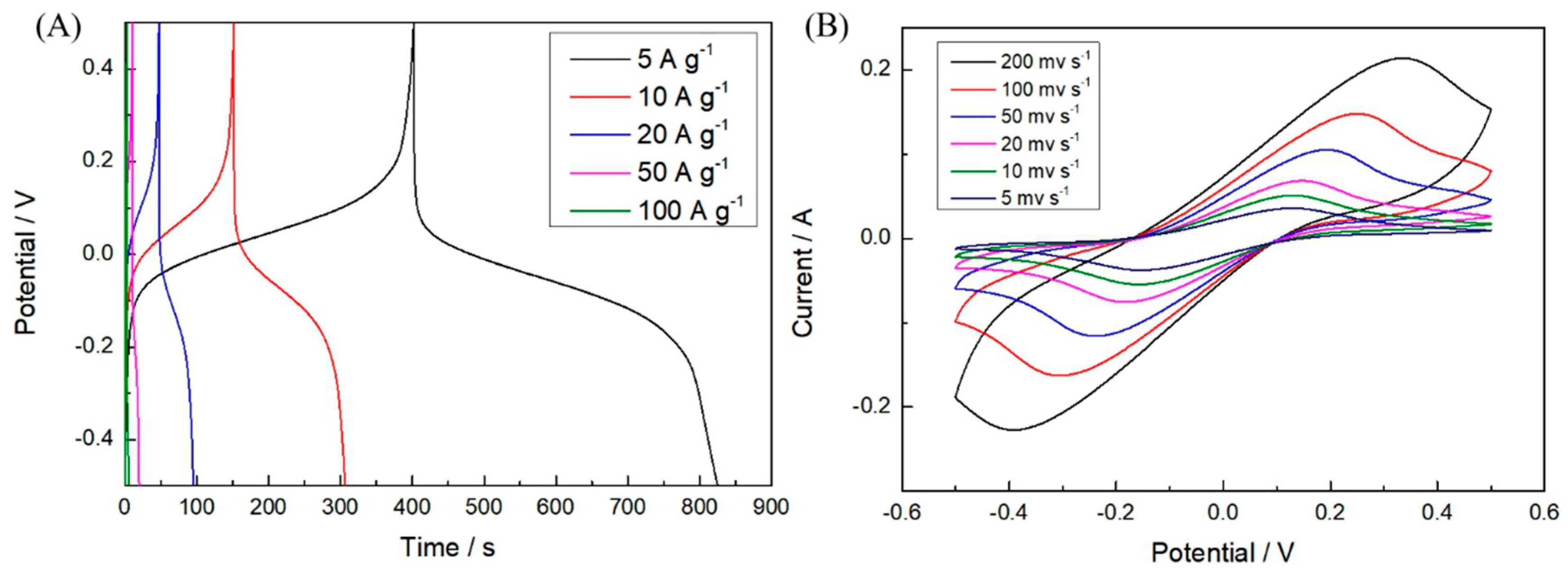
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Senthilkumar, S.T.; Selvan, R.K.; Melo, J.S. Redox additive/active electrolytes: A novel approach to enhance the performance of supercapacitors. J. Mater. Chem. A 2013, 1, 12386–12394. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Jing, H.; Wang, Z.; Li, J.; Hu, M.; Lv, R.; Zhang, R.; Chen, D. Coupled ultrasonication-milling synthesis of hierarchically porous carbon for high-performance supercapacitor. J. Colloid Interface Sci. 2018, 528, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Long, J.Y.; Yan, Z.S.; Gong, Y.; Lin, J.H. MOF-derived Cl/O-doped C/CoO and C nanoparticles for high performance supercapacitor. Appl. Surf. Sci. 2018, 448, 50–63. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Sun, F.-F.; Batmunkh, M.; Li, W.-H.; Li, H.; Sun, Y.; Zhao, Q.; Liu, X.; Ma, T.-Y. Zinc–nickel–cobalt ternary hydroxide nanoarrays for high-performance supercapacitors. J. Mater. Chem. A 2019, 7, 11826–11835. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, K.; Zhang, Y.; Wei, Z. Hierarchical Porous Graphene/Polyaniline Composite Film with Superior Rate Performance for Flexible Supercapacitors. Adv. Mater. 2013, 25, 6985–6990. [Google Scholar] [CrossRef]
- Ma, L.; Su, L.; Zhang, J.; Zhao, D.; Qin, C.; Jin, Z.; Zhao, K. A controllable morphology GO/PANI/metal hydroxide composite for supercapacitor. J. Electroanal. Chem. 2016, 777, 75–84. [Google Scholar] [CrossRef]
- He, H.; Ma, L.; Fu, S.; Gan, M.; Hu, L.; Zhang, H.; Xie, F.; Jiang, M. Fabrication of 3D ordered honeycomb-like nitrogen-doped carbon/PANI composite for high-performance supercapacitors. Appl. Surf. Sci. 2019, 484, 1288–1296. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Wang, Q.; Du, P.; Wei, W.; Liu, P. PANI coated microporous graphene fiber capable of subjecting to external mechanical deformation for high performance flexible supercapacitors. Carbon 2019, 143, 147–153. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, J.; Araby, S.; Shi, D.; Dong, W.; Tang, T.; Chen, M. High-mass loading electrodes with exceptional areal capacitance and cycling performance through a hierarchical network of MnO2 nanoflakes and conducting polymer gel. J. Power Sources 2019, 412, 655–663. [Google Scholar] [CrossRef]
- Zhu, C.; He, Y.; Liu, Y.; Kazantseva, N.; Saha, P.; Cheng, Q. ZnO@MOF@PANI core-shell nanoarrays on carbon cloth for high-performance supercapacitor electrodes. J. Energy Chem. 2019, 35, 124–131. [Google Scholar] [CrossRef]
- Xia, X.; Hao, Q.; Lei, W.; Wang, W.; Sun, D.; Wang, X. Nanostructured ternary composites of graphene/Fe2O3/polyaniline for high-performance supercapacitors. J. Mater. Chem. 2012, 22, 16844–16850. [Google Scholar] [CrossRef]
- Geethalakshmi, D.; Muthukumarasamy, N.; Balasundaraprabhu, R. Effect of dopant concentration on the properties of HCl-doped PANI thin films prepared at different temperatures. Optik 2014, 125, 1307–1310. [Google Scholar] [CrossRef]
- Gizdavic-Nikolaidis, M.R.; Jevremovic, M.M.; Milenkovic, M.; Allison, M.C.; Stanisavljev, D.R.; Bowmaker, G.A.; Zujovic, Z.D. High yield and facile microwave-assisted synthesis of conductive H2SO4 doped polyanilines. Mater. Chem. Phys. 2016, 173, 255–261. [Google Scholar] [CrossRef]
- Murugesan, R.; Subramanian, E. Effect of organic dopants on electrodeposition and characteristics of polyaniline under the varying influence of H2SO4 and HClO4 electrolyte media. Mater. Chem. Phys. 2003, 80, 731–739. [Google Scholar] [CrossRef]
- Xu, W.; Mu, B.; Wang, A. Morphology control of polyaniline by dopant grown on hollow carbon fibers as high-performance supercapacitor electrodes. Cellulose 2017, 24, 5579–5592. [Google Scholar] [CrossRef]
- Dominic, J.; David, T.; Vanaja, A.; Muralidharan, G.; Maheswari, N.; Kumar, K.S. Supercapacitor performance study of lithium chloride doped polyaniline. Appl. Surf. Sci. 2018, 460, 40–47. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Peng, Z.; Zhuang, J.; Zhang, J. Investigation of polyaniline films doped with Ni2+ as the electrode material for electrochemical supercapacitors. Electrochim. Acta 2013, 90, 393–399. [Google Scholar] [CrossRef]
- Xu, H.; Wu, J.-X.; Li, C.-X.; Zhang, J.-X.; Wang, X.-X. Investigation of polyaniline films doped with Co2+ as the electrode material for electrochemical supercapacitors. Ionics 2015, 21, 1163–1170. [Google Scholar] [CrossRef]
- Xu, H.; Wu, J.; Li, C.; Zhang, J.; Liu, J. Investigation of polyaniline films doped with Fe3+ as the electrode material for electrochemical supercapacitors. Electrochim. Acta 2015, 165, 14–21. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Chen, Y.; Lu, H.; Zhuang, J. Electrochemical polymerization of polyaniline doped with Zn2+ as the electrode material for electrochemical supercapacitors. J. Solid State Electrochem. 2014, 18, 813–819. [Google Scholar] [CrossRef]
- Li, J.; Cui, M.; Lai, Y.; Zhang, Z.; Lu, H.; Fang, J.; Liu, Y. Investigation of polyaniline co-doped with Zn2+ and H+ as the electrode material for electrochemical supercapacitors. Synth. Met. 2010, 160, 1228–1233. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Chen, Y.; Lu, H.; Zhuang, J. Electrochemical polymerization of polyaniline doped with Cu2+ as the electrode material for electrochemical supercapacitors. RSC Adv. 2014, 4, 5547–5552. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Chen, Y.; Zhuang, J.; Lu, H. Electrochemical polymerization of polyaniline doped with Mn2+ as electrode material for electrochemical supercapacitors. Polym. Mater. Sci. Eng. 2014, 30, 23–27. [Google Scholar]
- Wang, X.; Xu, M.; Fu, Y.; Wang, S.; Yang, T.; Jiao, K. A Highly Conductive and Hierarchical PANI Micro/nanostructure and Its Supercapacitor Application. Electrochim. Acta 2016, 222, 701–708. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Parvez, K.; Li, S.; Yang, S.; Liu, Z.; Liu, S.; Feng, X.; Müllen, K. Alternating Stacked Graphene-Conducting Polymer Compact Films with Ultrahigh Areal and Volumetric Capacitances for High-Energy Micro-Supercapacitors. Adv. Mater. 2015, 27, 4054–4061. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.C.; Patil, U.M.; Lee, S.C.; Kulkarni, S.B.; Sohn, J.S.; Nam, M.S.; Han, S. Nanostructured pseudocapacitive materials decorated 3D graphene foam electrodes for next generation supercapacitors. Nanoscale 2015, 7, 6999–7021. [Google Scholar]
- Palaniappan, S.; John, A.; Amarnath, C.A.; Rao, V.J. Mannich-type reaction in solvent free condition using reusable polyaniline catalyst. J. Mol. Catal. A Chem. 2004, 218, 47–53. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Jeyaprabha, C.; Venkatachari, G. Influence of metal cations on the inhibitive effect of polyaniline for iron in 0.5M H2SO4. Mater. Chem. Phys. 2008, 107, 350–355. [Google Scholar] [CrossRef]
- Tao, S.; Hong, B.; Kerong, Z. An infrared and Raman spectroscopic study of polyanilines co-doped with metal ions and H+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 66, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Chen, J.G.; Xiao, J.Q. Nanostructured Electrodes for High-Performance Pseudocapacitors. Angew. Chem. Int. Ed. 2013, 52, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Krüner, B.; Tolosa, A.; Kim, D.; Choudhury, S.; Presser, V.; Lee, J.; Sathyamoorthi, S.; Seo, K.-H. Tin/vanadium redox electrolyte for battery-like energy storage capacity combined with supercapacitor-like power handling. Energy Environ. Sci. 2016, 9, 3392–3398. [Google Scholar]
- Zheng, C.; Yoshio, M.; Qi, L.; Wang, H. A 4 V-electrochemical capacitor using electrode and electrolyte materials free of metals. J. Power Sources 2014, 260, 19–26. [Google Scholar] [CrossRef]
- Fic, K.; Frackowiak, E.; Béguin, F. Unusual energy enhancement in carbon-based electrochemical capacitors. J. Mater. Chem. 2012, 22, 24213–24223. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Z.; Qian, M.; Zhang, Z.; Lin, J.; Huang, F. Enhanced specific capacitance by a new dual redox-active electrolyte in activated carbon-based supercapacitors. Carbon 2019, 143, 300–308. [Google Scholar] [CrossRef]
- Wang, A.-Y.; Chaudhary, M.; Lin, T.-W. Enhancing the stability and capacitance of vanadium oxide nanoribbons/3D-graphene binder-free electrode by using VOSO4 as redox-active electrolyte. Chem. Eng. J. 2019, 355, 830–839. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K.; Lee, Y.S.; Melo, J.S. Electric double layer capacitor and its improved specific capacitance using redox additive electrolyte. J. Mater. Chem. A 2013, 1, 1086–1095. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K.; Ponpandian, N.; Melo, J.S.; Lee, Y.S. Improved performance of electric double layer capacitor using redox additive (VO2+/VO2+) aqueous electrolyte. J. Mater. Chem. A 2013, 1, 7913–7919. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, M.; Xu, H.; Lu, L.; Xiao, Z.; Liu, S. Synergistic interaction between redox-active electrolytes and functionalized carbon in increasing the performance of electric double-layer capacitors. J. Energy Chem. 2018, 27, 1219–1224. [Google Scholar] [CrossRef]
- Chen, W.; Xia, C.; Rakhi, R.; Alshareef, H.N. A general approach toward enhancement of pseudocapacitive performance of conducting polymers by redox-active electrolytes. J. Power Sources 2014, 267, 521–526. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, G.; Yan, Z.; Kang, L.; Xu, H.; Shi, F.; Lei, Z.; Liu, Z.-H. High capacitive property for supercapacitor using Fe3+/Fe2+ redox couple additive electrolyte. Electrochim. Acta 2017, 231, 705–712. [Google Scholar] [CrossRef]
- Xia, C.; Leng, M.; Tao, W.; Wang, Q.; Gao, Y.; Zhang, Q. Polyaniline/carbon nanotube core–shell hybrid and redox active electrolyte for high-performance flexible supercapacitor. J. Mater. Sci. Mater. Electron. 2019, 30, 4427–4436. [Google Scholar] [CrossRef]
- Liu, T.T.; Zhu, Y.H.; Liu, E.H.; Luo, Z.Y.; Hu, T.T.; Li, Z.P.; Ding, R. Fe3+/Fe2+ redox electrolyte for high-performance polyaniline/SnO2 supercapacitors. Trans. Nonferrous Met. Soc. China 2015, 25, 2661–2665. [Google Scholar] [CrossRef]
- Bu, Y.; Cao, M.; Jiang, Y.; Gao, L.; Shi, Z.; Xiao, X.; Wang, M.; Yang, G.; Zhou, Y.; Shen, Y. Ultra-thin bacterial cellulose/poly(ethylenedioxythiophene) nanofibers paper electrodes for all-solid-state flexible supercapacitors. Electrochim. Acta 2018, 271, 624–631. [Google Scholar] [CrossRef]
- Mai, L.-Q.; Minhas-Khan, A.; Tian, X.; Hercule, K.M.; Zhao, Y.-L.; Lin, X.; Xu, X. Synergistic interaction between redox-active electrolyte and binder-free functionalized carbon for ultrahigh supercapacitor performance. Nat. Commun. 2013, 4, 2923. [Google Scholar] [CrossRef]
- Asen, P.; Shahrokhian, S.; Zad, A.I. Transition metal ions-doped polyaniline/graphene oxide nanostructure as high performance electrode for supercapacitor applications. J. Solid State Electrochem. 2018, 22, 983–996. [Google Scholar] [CrossRef]
- Chen, W.; Rakhi, R.; Alshareef, H.N. Facile synthesis of polyaniline nanotubes using reactive oxide templates for high energy density pseudocapacitors. J. Mater. Chem. A 2013, 1, 3315–3324. [Google Scholar] [CrossRef]
- Kim, Y.; Foster, C.; Chiang, J.; Heeger, A. Localized charged excitations in polyaniline: Infrared photoexcitation and protonation studies. Synth. Met. 1989, 29, 285–290. [Google Scholar] [CrossRef]
- Chiang, J.-C.; MacDiarmid, A.G. ‘Polyaniline’: Protonic acid doping of the emeraldine form to the metallic regime. Synth. Met. 1986, 13, 193–205. [Google Scholar] [CrossRef]
- Du, X.-S.; Zhou, C.-F.; Wang, G.-T.; Mai, Y.-W. Novel Solid-State and Template-Free Synthesis of Branched Polyaniline Nanofibers. Chem. Mater. 2008, 20, 3806–3808. [Google Scholar] [CrossRef]
- Du, X.-S.; Zhou, C.-F.; Mai, Y.-W. Facile Synthesis of Hierarchical Polyaniline Nanostructures with Dendritic Nanofibers as Scaffolds. J. Phys. Chem. C 2008, 112, 19836–19840. [Google Scholar] [CrossRef]
- Arulmani, S.; Wu, J.J.; Anandan, S. Ultrasound promoted transition metal doped polyaniline nanofibers: Enhanced electrode material for electrochemical energy storage applications. Ultrason. Sonochem. 2019, 51, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, Y.; Yuan, Z.-Y.; Zhu, Y.-P.; Ma, T.-Y. Titanium Phosphonate Based Metal-Organic Frameworks with Hierarchical Porosity for Enhanced Photocatalytic Hydrogen Evolution. Angew. Chem. 2018, 57, 3222–3227. [Google Scholar] [CrossRef] [PubMed]
- Alamin, A.A.; Elhamid, A.E.M.A.; Anis, W.R.; Attiya, A.M. Fabrication of symmetric supercapacitor based on relatively long lifetime polyaniline grown on reduced graphene oxide via Fe2+ oxidation sites. Diam. Relat. Mater. 2019, 96, 182–194. [Google Scholar] [CrossRef]
- Chen, W.; Rakhi, R.; Alshareef, H.N. Capacitance enhancement of polyaniline coated curved-graphene supercapacitors in a redox-active electrolyte. Nanoscale 2013, 5, 4134–4138. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Deshmukh, A.B.; Choudhary, R.B.; Karbhal, I.; Majumder, M.; Shelke, M.V. Facile synthesis and electrochemical evaluation of PANI/CNT/MoS 2 ternary composite as an electrode material for high performance supercapacitor. Mater. Sci. Eng. B 2017, 223, 24–34. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Deng, J.; Han, Y.; Li, X. Fabrication of porous Co3O4 with different nanostructures by solid-state thermolysis of metal-organic framework for supercapacitors. J. Mater. Sci. 2018, 53, 8474–8482. [Google Scholar] [CrossRef]
- Dai, Y.; Zhu, S.; Wang, C.; Cong, Y.; Zeng, Y.; Jiang, T.; Huang, H.; Huang, S.; Meng, X. In-situ fabrication of Co foam@Co3O4 porous nanosheet arrays for high performance supercapacitors. J. Alloy. Compd. 2018, 748, 291–297. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Hou, L.; Yuan, C.; Yang, L.; Zhang, X. Uniform urchin-like nickel cobaltite microspherical superstructures constructed by one-dimension nanowires and their application for electrochemical capacitors. Electrochim. Acta 2012, 81, 172–178. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, D.; Li, Y.; Wu, Z.; Zhuo, R.; Li, S.; Feng, J.; Wang, J.; Yan, P.; Geng, Z. Manganese dioxide nanosheet arrays grown on graphene oxide as an advanced electrode material for supercapacitors. Electrochim. Acta 2014, 117, 528–533. [Google Scholar] [CrossRef]
- Zhou, H.; Zhi, X.; Zhai, H.-J. A facile approach to improve the electrochemical properties of polyaniline-carbon nanotube composite electrodes for highly flexible solid-state supercapacitors. Int. J. Hydrog. Energy 2018, 43, 18339–18348. [Google Scholar] [CrossRef]
- Malik, R.; Zhang, L.; McConnell, C.; Schott, M.; Hsieh, Y.-Y.; Noga, R.; Alvarez, N.T.; Shanov, V. Three-dimensional, free-standing polyaniline/carbon nanotube composite-based electrode for high-performance supercapacitors. Carbon 2017, 116, 579–590. [Google Scholar] [CrossRef]


| Material Fabrication Method | Cs/F·g−1 | Electrolyte | Citation | |
|---|---|---|---|---|
| PANI | Chemical oxidative polymerization | 1062/2 A g−1 | 1 M H2SO4 + 0.8 M Fe3+/2+ | [42] |
| PANI/SnO2 | Chemical oxidative polymerization | 1172/1 A g−1 | 1 M H2SO4 + 0.4 M Fe3+/2+ | [44] |
| PANI/CNT | Electrodeposition | 1128/5 A g−1 | 1 M H2SO4 + 0.02 M Fe3+/2+ | [43] |
| Fe3+-Zn2+-PANI/GO | Electrodeposition | 1140/10 A g−1 | 0.5 M Na2SO4 | [47] |
| PANI/RGO | Chemical oxidative polymerization | 565/0.1 A g−1 | 6 M KOH | [55] |
| Fe3+/PANI | Electrodeposition | 602/3 mA cm−2 | 0.5 M H2SO4 | [21] |
| Fe2+/PANI | Electrodeposition | 8468/5 A g−1 | 1 M H2SO4 + 0.5 M Fe3+/2+ | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, Y.; Meng, W.; Xia, Y.; Du, X. Redox-Active Gel Electrolyte Combined with Branched Polyaniline Nanofibers Doped with Ferrous Ions for Ultra-High-Performance Flexible Supercapacitors. Polymers 2019, 11, 1357. https://doi.org/10.3390/polym11081357
Mo Y, Meng W, Xia Y, Du X. Redox-Active Gel Electrolyte Combined with Branched Polyaniline Nanofibers Doped with Ferrous Ions for Ultra-High-Performance Flexible Supercapacitors. Polymers. 2019; 11(8):1357. https://doi.org/10.3390/polym11081357
Chicago/Turabian StyleMo, Youtian, Wei Meng, Yanlin Xia, and Xusheng Du. 2019. "Redox-Active Gel Electrolyte Combined with Branched Polyaniline Nanofibers Doped with Ferrous Ions for Ultra-High-Performance Flexible Supercapacitors" Polymers 11, no. 8: 1357. https://doi.org/10.3390/polym11081357
APA StyleMo, Y., Meng, W., Xia, Y., & Du, X. (2019). Redox-Active Gel Electrolyte Combined with Branched Polyaniline Nanofibers Doped with Ferrous Ions for Ultra-High-Performance Flexible Supercapacitors. Polymers, 11(8), 1357. https://doi.org/10.3390/polym11081357






