Ultrasonic Thermal-Assisted Extraction of Phosvitin from Egg Yolk and Evaluation of Its Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
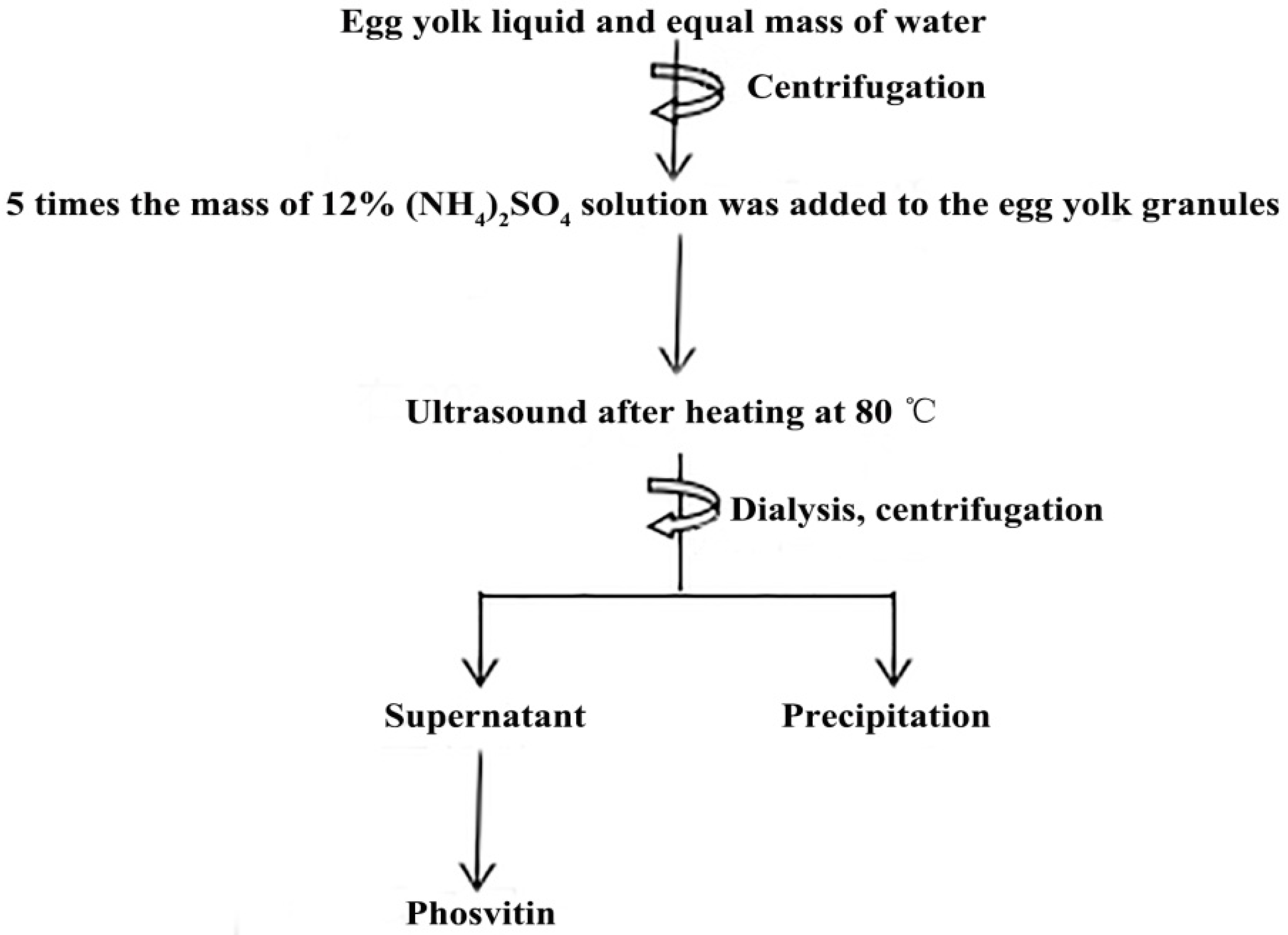
2.2. Determination of the Process Parameters for Pv Isolation
2.3. Determination of Purity
2.4. Determination of Activity
2.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
2.6. Liquid Chromatography-Nanoelectrospray Ionization Mass Spectrometry (Nano LC-ESI-MS/MS) Analysis
2.7. Fourier-Transform Infrared (FT-IR)
2.8. Iron-Binding Ability of Phosvitin
2.9. Surface Hydrophobicity Measurements
2.10. Statistical Analysis
3. Results and Discussion
3.1. Single-Factor Variable Analysis
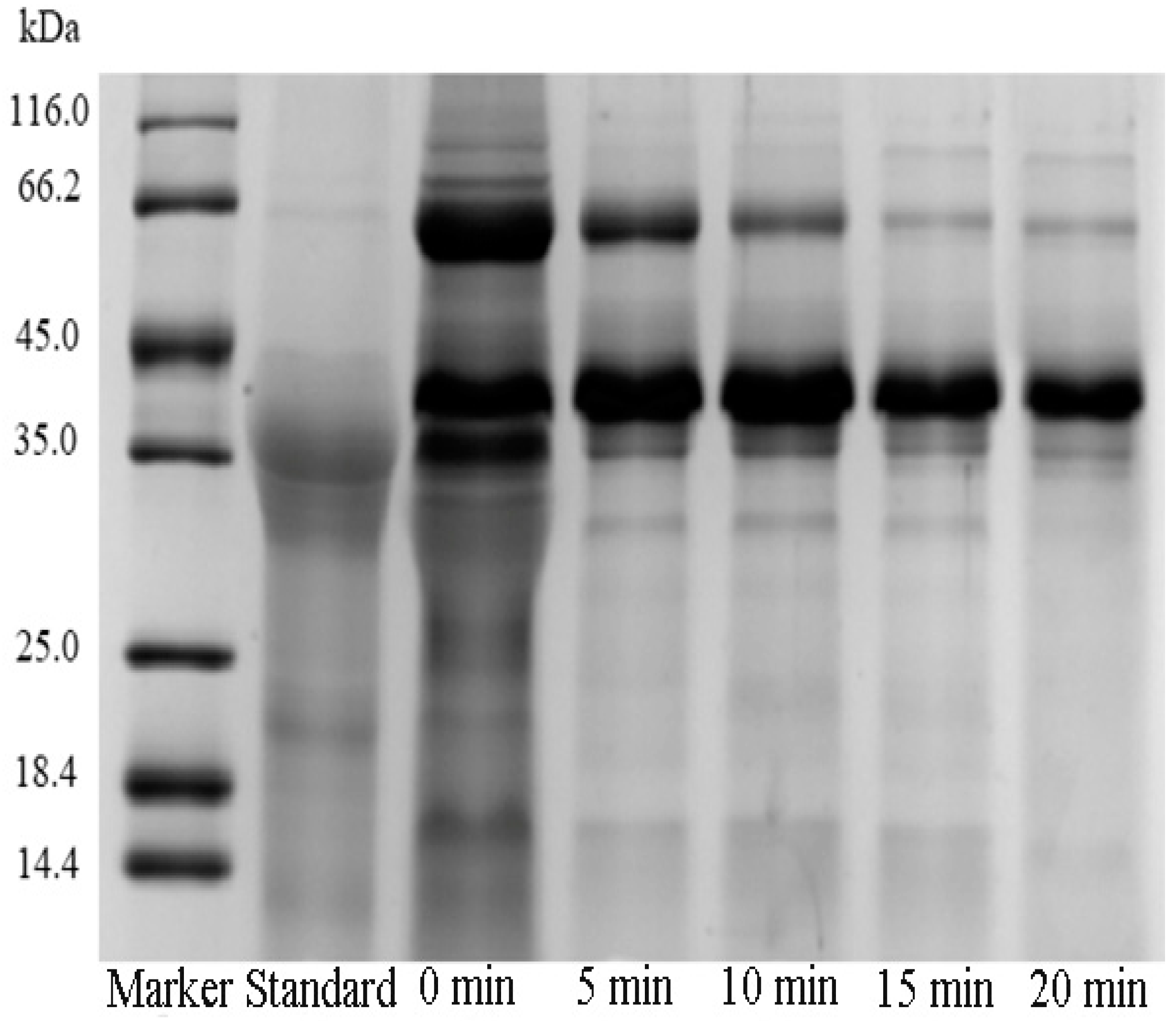
3.1.1. Effect of Heating Time on Pv Extraction
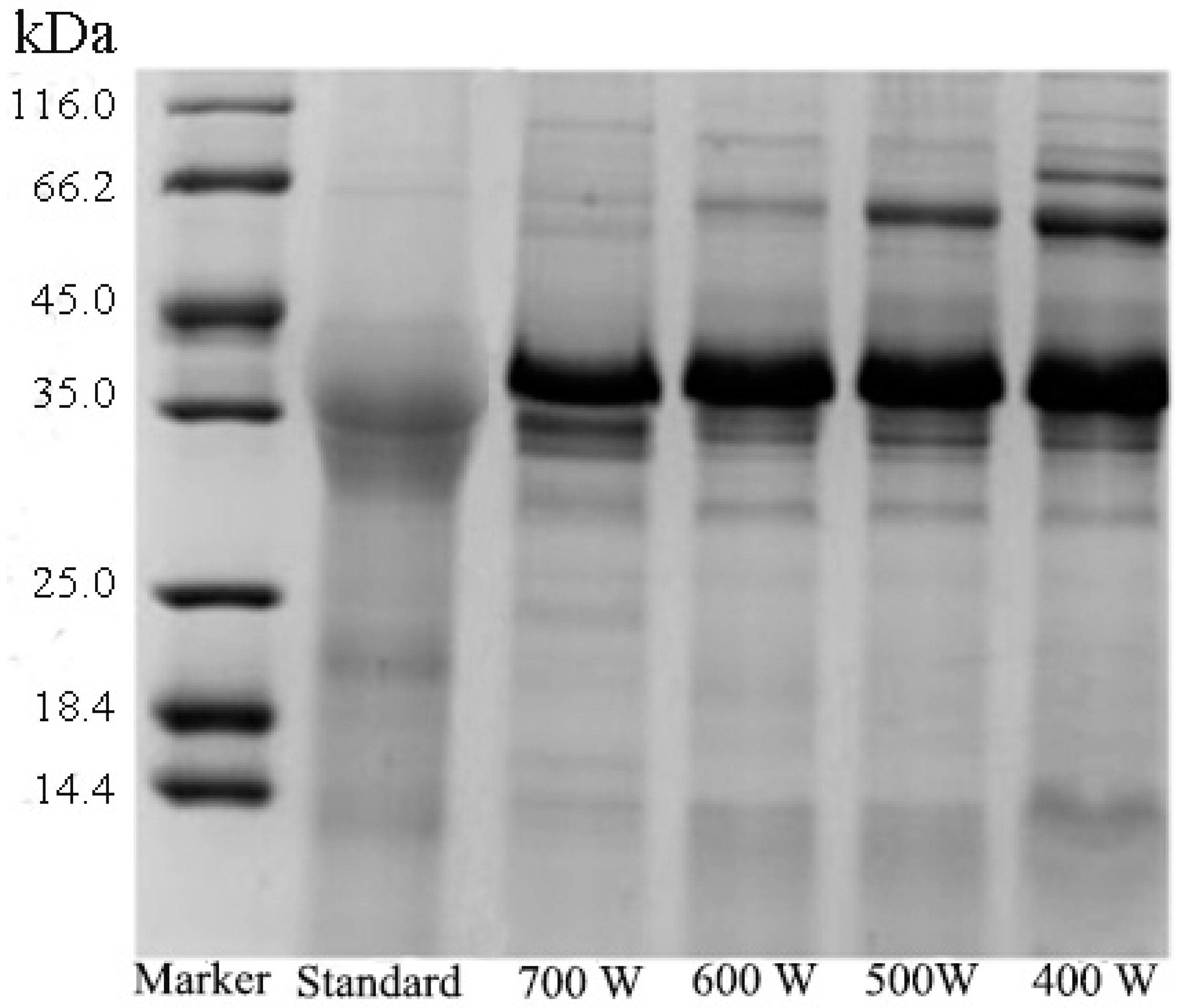
3.1.2. Effect of Ultrasound Power on Pv Extraction
3.1.3. Effect of Ultrasound Time on Pv Extraction
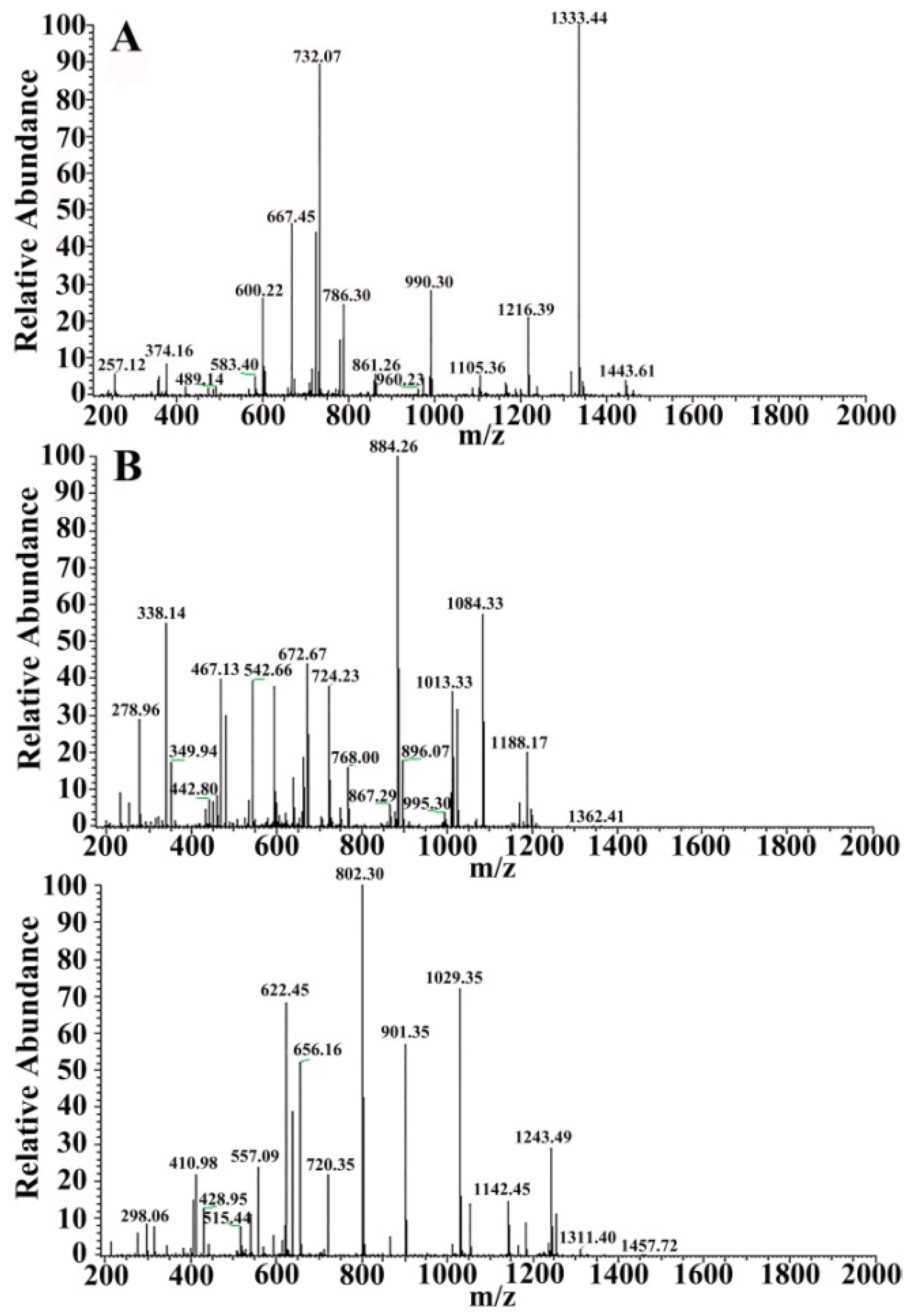
3.2. Identification of Pv by Tandem Mass Spectrometry
3.3. Infrared Spectra Analysis
3.4. Iron Binding Ability of Pv
3.5. Analysis of Surface Hydrophobicity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Finn, R.N.; Kristoffersen, B.A. Vertebrate vitellogenin gene duplication in relation to the “3r hypothesis”: Correlation to the pelagic egg and the oceanic radiation of teleosts. PLoS ONE 2007, 2, e169. [Google Scholar] [CrossRef]
- Burley, R.W.; Cook, W.H. Isolation and composition of avian egg yolk granules and their constituent α- and β-lipovitellins. Can. J. Biochem. Physiol. 1961, 39, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.C. The primary structure of avian phosvitins. Contributions through the edman degradation of methylmercaptovitins prepared from the constituent phosphoproteins. Int. J. Biochem. 1985, 17, 983–988. [Google Scholar] [CrossRef]
- Byrne, B.; Schip, A.; Vandeklundert, J.; Arnberg, A.C.; Gruber, M.; Ab, G. Amino-acid-sequence of phosvitin derived from the nucleotide-sequence of part of the chicken vitellogenin gene. Biochemistry 1984, 23, 4275. [Google Scholar] [CrossRef] [PubMed]
- Mecham, D.K.; Olcott, H.S. Phosvitin, the principal phosphoprotein of egg yolk. J. Am. Chem. Soc. 1949, 71, 3670–3679. [Google Scholar] [CrossRef]
- Chung, S.L.; Ferrier, L.K. Heat denaturation and emulsifying properties of egg yolk phosvitin. J. Food Sci. 1995, 60, 3. [Google Scholar] [CrossRef]
- Greengard, O.; Sentenac, A.; Mendelsohn, N. Phosvitin, the iron carrier of egg yolk. Biochim. Biophys. Acta BBA Gen. Subj. 1964, 90, 406–407. [Google Scholar] [CrossRef]
- Grizzuti, K.; Perlmann, G.E. Binding of magnesium and calcium ions to the phosphoglycoprotein phosvitin. Biochemistry 1973, 12, 4399. [Google Scholar]
- Grizzuti, K.; Perlmann, G.E. Further studies on the binding of divalent cations to the phosphoglycoprotein phosvitin. Biochemistry 1975, 14, 2171. [Google Scholar] [CrossRef]
- Chen, H.; Jin, Y.; Ding, X.; Wu, F.; Bashari, M.; Chen, F.; Cui, Z.; Xu, X. Improved the emulsion stability of phosvitin from hen egg yolk against different ph by the covalent attachment with dextran. Food Hydrocoll. 2014, 39, 104–112. [Google Scholar] [CrossRef]
- Ishikawa, S.; Yano, Y.; Arihara, K.; Itoh, M. Egg yolk phosvitin inhibits hydroxyl radical formation from the fenton reaction. Biosci. Biotechnol. Biochem. 2004, 68, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, M.; Ma, H.; Xu, X.; Jin, Z.; Liu, X. Physicochemical properties and antioxidant potential of phosvitin-resveratrol complexes in emulsion system. Food Chem. 2016, 206, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Miyazaki, S.; Kawamoto, A.; Kobayashi, K. Effects of phosphate residues on the excellent emulsifying properties of phosphoglycoprotein phosvitin. Agric. Biol. Chem. 2016, 51, 2989–2994. [Google Scholar]
- Castellani, O.; Davidbriand, E.; Guerindubiard, C.; Anton, M. Effect of aggregation and sodium salt on emulsifying properties of egg yolk phosvitin. Food Hydrocoll. 2005, 19, 769–776. [Google Scholar] [CrossRef]
- Sattar Khan, M.A.; Babiker, E.E.; Azakami, H.; Kato, A. Effect of protease digestion and dephosphorylation on high emulsifying properties of hen egg yolk phosvitin. J. Agric. Food Chem. 1998, 46, 87–90. [Google Scholar] [CrossRef]
- Castellani, O.; Martinet, V.; David-Briand, E.; Guérin-Dubiard, C.; Anton, M. Egg yolk phosvitin: Preparation of metal-free purified protein by fast protein liquid chromatography using aqueous solvents. J. Chromatogr. B 2003, 791, 273–284. [Google Scholar] [CrossRef]
- Lei, B.; Wu, J. Purification of egg yolk phosvitin by anion exchange chromatography. J. Chromatogr. A 2012, 1223, 41–46. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, N.; Geng, F.; Ma, M. Simply and effectively preparing high-purity phosvitin using polyethylene glycol and anion-exchange chromatography. J. Sep. Sci. 2011, 34, 3295–3301. [Google Scholar] [CrossRef]
- Ren, J.; Jianping, W. Thermal-aided phosvitin extraction from egg yolk. J. Sci. Food Agric. 2015, 95, 2595–2600. [Google Scholar] [CrossRef]
- Zou, T.; Kang, L.; Yang, C.; Song, H.; Liu, Y. Flavour precursor peptide from an enzymatic beef hydrolysate maillard reaction-ii: Mechanism of the synthesis of flavour compounds from a sulphur-containing peptide through a maillard reaction. LWT 2019, 110, 8–18. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Du, L.; Zhang, X.; Yang, W.; Zhang, H. Effect of ultrasound assisted heating on structure and antioxidant activity of whey protein peptide grafted with galactose. LWT 2019, 109, 130–136. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Lee, S.Y.; Zhu, L.; Show, P.L. Enhanced microalgal protein extraction and purification using sustainable microwave-assisted multiphase partitioning technique. Chem. Eng. J. 2019, 367, 1–8. [Google Scholar] [CrossRef]
- Meza-Jiménez, M.d.L.; Pokhrel, P.-R.; Robles de la Torre, R.R.; Barbosa-Canovas, G.V.; Hernández-Sánchez, H. Effect of pulsed electric fields on the activity of food-grade papain in a continuous system. LWT 2019, 109, 336–341. [Google Scholar] [CrossRef]
- Sakdasri, W.; Ngamprasertsith, S.; Sooksai, S.; Noitang, S.; Sukaead, W.; Sawangkeaw, R. Defatted fiber produced from lemon basil (ocimum citriodorum vis.) seed with supercritical co2: Economic analysis. Ind. Crops Prod. 2019, 135, 188–195. [Google Scholar] [CrossRef]
- Gorguc, A.; Bircan, C.; Yilmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.A.; Clevenstine, S.M.; Piazza, G.J. Ultrasonic processing for recovery of chicken erythrocyte hemoglobin. Food Bioprod. Proc. 2015, 94, 1–9. [Google Scholar] [CrossRef]
- Chen, G.; Fang, C.; Chen, X.; Wang, Z.; Liu, M.; Kan, J. High-pressure ultrasonic-assisted extraction of polysaccharides from mentha haplocalyx: Structure, functional and biological activities. Ind. Crops Prod. 2019, 130, 273–284. [Google Scholar] [CrossRef]
- Vinatoru, M.; Toma, M.; Radu, O.; Filip, P.I.; Lazurca, D.; Mason, T.J. The use of ultrasound for the extraction of bioactive principles from plant materials. Ultrason. Sonochem. 1997, 4, 135–139. [Google Scholar] [CrossRef]
- Tsochatzidis, N.A.; Guiraud, P.; Wilhelm, A.M.; Delmas, H. Determination of velocity, size and concentration of ultrasonic. Chem. Eng. Sci. 2001, 56, 1831–1840. [Google Scholar] [CrossRef]
- Li, K.; Ma, H.; Li, S.; Zhang, C.; Dai, C. Effect of ultrasound on alkali extraction protein from rice dreg flour. J. Food Process Eng. 2017, 40, e12377. [Google Scholar] [CrossRef]
- Vernes, L.; Abert-Vian, M.; El Maataoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of ultrasound for green extraction of proteins from spirulina. Mechanism, optimization, modeling, and industrial prospects. Ultrason. Sonochem. 2019, 54, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Álvarez, C.; Bobo, G.; Aguiló-Aguayo, I. Characterization of functional properties of proteins from ganxet beans (Phaseolus vulgaris L. Var. Ganxet) isolated using an ultrasound-assisted methodology. LWT 2018, 98, 106–112. [Google Scholar] [CrossRef]
- Jung, S.; Ahn, D.U.; Nam, K.C.; Kim, H.J.; Jo, C. Separation of phosvitin from egg yolk without using organic solvents. Asian Australas. J. Anim. Sci. 2013, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Geng, F.; Huang, X.; Ma, M. Calcium binding characteristics and structural changes of phosvitin. J. Inorg. Biochem. 2016, 159, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Abeyrathne, E.D.; Choi, I.; Suh, J.W.; Ahn, D.U. Sequential separation of immunoglobulin y and phosvitin from chicken egg yolk without using organic solvents. Poultry Sci. 2014, 93, 2668–2677. [Google Scholar] [CrossRef]
- Jiang, B.; Yuan, Y.; Zhang, X.; Feng, Z.; Liu, C. Separation and enrichment of lectin from zihua snap-bean (Phaseolus vulgaris) seeds by peg 600-ammonium sulfate aqueous two-phase system. Molecules 2017, 22, 1596. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, L.; Na, J.; Zhang, X.; Yuan, Y.; Liu, C.; Feng, Z. Environmentally-friendly strategy for separation of α-lactalbumin from whey by aqueous two phase flotation. Arab. J. Chem. 2018. [Google Scholar] [CrossRef]
- Qin, J.; Xiaolong, Z. Identifi cation of in vivo protein phosphorylation sites with mass spectrometry. Methods Mol. Biol. 2002, 194, 211–221. [Google Scholar]
- Jiang, B.; Wang, X.; Wang, L.; Lv, X.; Li, D.; Liu, C.; Feng, Z. Two-step isolation, purification, and characterization of lectin from zihua snap bean (Phaseolus vulgaris) seeds. Polymers 2019, 11, 785. [Google Scholar] [CrossRef]
- Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Reutilization of food waste: One-step extration, purification and characterization of ovalbumin from salted egg white by aqueous two-phase flotation. Foods 2019, 8, 286. [Google Scholar] [CrossRef]
- Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Eco-innovation in reusing food by-products: Separation of ovalbumin from salted egg white using aqueous two-phase system of PEG 1000/(NH4)2SO4. Polymers 2019, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, Y.Y.; Jiang, J.Y.; Ji, Q.Y.; Fu, Y.; Zhao, L.X.; Li, C.Y.; Ye, F. Physicochemical properties and fungicidal activity of inclusion complexes of fungicide chlorothalonil with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. J. Mol. Liq. 2019, 293, 111513. [Google Scholar] [CrossRef]
- Ji, X.; Peng, Q.; Yuan, Y.; Liu, F.; Wang, M. Extraction and physicochemical properties of polysaccharides from ziziphus jujuba cv. Muzao by ultrasound-assisted aqueous two-phase extraction. Int. J. Biol. Macromol. 2018, 108, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, D.; Ma, C.; Gao, Y. Conjugation of polyphenols prevents lactoferrin from thermal aggregation at neutral ph. Food Hydrocoll. 2016, 58, 49–59. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Paniwnyk, L.; Herceg, Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 2014, 121, 15–23. [Google Scholar] [CrossRef]
- Xiaowei, Z. Isolation, Purification, Structural Characterization and Functional Properties of Phosvitin; Wuhan Huazhong Agricultural University: Wuhan, China, 2014; pp. 1–50. [Google Scholar]
- Mauerer, A.; Lee, G. Changes in the amide i ft-ir bands of poly-l-lysine on spray-drying from alpha-helix, beta-sheet or random coil conformations. Eur. J. Pharm. Biopharm. 2006, 62, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A fourier transform infrared (ft-ir) spectroscopic study. Amino Acids 2011, 43, 911–921. [Google Scholar] [CrossRef]
- Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Separation and enrichment of antioxidant peptides from whey protein isolate hydrolysate by aqueous two-phase extraction and aqueous two-phase flotation. Foods 2019, 8, 34. [Google Scholar] [CrossRef]
- Taborsky, G. Iron binding by phosvitin and its conformational consequences. J. Biol. Chem. 1980, 255, 2976–2984. [Google Scholar]
- Renugopalakrishnan, V.; Horowitz, P.M.; Glimcher, M.J. Structural studies of phosvitin in solution and in the solid state. J. Biol. Chem. 1985, 260, 11406. [Google Scholar]
- Lu, C.; Baker, R.C. Effect of ph and food ingredients on the stability of egg yolk phospholipids and the metal-chelator antioxidant activity of phosvitin. J. Food Sci. 1987, 52, 613–616. [Google Scholar] [CrossRef]
- Albright, K.J.; Gordon, D.T.; Cotterill, O.J. Release of iron from phosvitin by heat and food additives. J. Food Sci. 1984, 49, 78–81. [Google Scholar] [CrossRef]
- Hegenauer, J.; Saltman, P.; Nace, G. Iron (iii)-phosphoprotein chelates: Stoichiometric equilibrium constant for interaction of iron (iii) and phosphorylserine residues of phosvitin and casein. Biochemistry 1979, 18, 3865–3879. [Google Scholar] [CrossRef] [PubMed]



| Heating Time (min) | Purity (%) | N/P | Activity (%) |
|---|---|---|---|
| 0 | 65 ± 1 a | 3.8 ± 0.0 d | 66 ± 1 a |
| 5 | 74 ± 1 b | 3.6 ± 0.0 c | 76 ± 1 b |
| 10 | 75 ± 1 b | 3.4 ± 0.0 b | 84 ± 1 c |
| 15 | 80 ± 2 c | 3.1 ± 0.0 a | 98 ± 1 d |
| 20 | 80 ± 1 c | 3.2 ± 0.0 a | 96 ± 1 d |
| Ultrasound Power (W) | Purity (%) | N/P | Activity (%) |
|---|---|---|---|
| 400 | 73 ± 1 a | 3.6 ± 0.0 c | 72 ± 1 a |
| 500 | 77 ± 1 b | 3.3 ± 0.0 b | 88 ± 1 b |
| 600 | 80 ± 2 c | 3.1 ± 0.0 a | 98 ± 1 c |
| 700 | 80 ± 1 c | 3.2 ± 0.0 a | 97 ± 1 c |
| Ultrasound Time (min) | Purity (%) | N/P | Activity (%) |
|---|---|---|---|
| 0 | 72 ± 2 a | 3.6 ± 0.0 c | 74 ± 1 a |
| 5 | 77 ± 1 b | 3.4 ± 0.0 b | 89 ± 1 b |
| 10 | 80 ± 2 c | 3.1 ± 0.0 a | 98 ± 1 c |
| 15 | 80 ± 1 c | 3.2 ± 0.0 a | 96 ± 1 c |
| Hits | Protein Molecule Weight (Da) | Number of Peptides | Link | Relative Abundance |
|---|---|---|---|---|
| 1 | 206,730.27 | 101 | P02845 | 52.9% |
| 2 | 212,606.75 | 85 | P87498 | 37.6% |
| 3 | 71,867.75 | 5 | P19121 | 1.1% |
| 4 | 28,276.62 | 4 | P02752 | 7.8% |
| 5 | 43,195.66 | 2 | P01012 | 0.6% |
| Peptide Number | Amino Acid Sequence | m/z |
|---|---|---|
| 5162 | KVDHQSLSR | 1068.57 |
| 6126 | EKHNELLMPNHK | 1488.75 |
| 6134 | SCNVVVAQDCTEHPK | 1742.77 |
| 6147 | HNELLMPNHK | 1231.60 |
| 6315 | VGFHCFPK | 990.47 |
| 6414 | ESVLSDSGVSEYEK | 1527.68 |
| 6454 | TVIVEAPIHGLK | 1275.75 |
| 6462 | VTVASWMR | 948.48 |
| 6469 | ESVLSDSGVSEYEKDNIK | 1997.93 |
| 6504 | NVNFDGEILK | 1147.59 |
| 6548 | NPTIDGEESTCYSVDPVLK | 2122.97 |
| 6694 | ATAVSLLEWQR | 1272.68 |
| 7964 | DCTPIEK | 861.39 |
| Peptide Number | Amino Acid Sequence | m/z |
|---|---|---|
| 5178 | SAGEATNLK | 889.45 |
| 5996 | KKPMDEEENDQVK | 1588.74 |
| 6104 | YDAECEQEYR | 1361.52 |
| 6146 | IKTFNEVK | 977.55 |
| 6165 | MPNGYLAK | 892.45 |
| 6167 | TVQLAGVDSK | 1016.55 |
| 6176 | SFVKLEK | 849.50 |
| 6189 | MPNGYLAK | 892.45 |
| 6247 | CYSTEPVLR | 1123.53 |
| 6264 | IGSHEIDMHPVNGQVK | 1759.86 |
| 6387 | SAGEATNLKAINIK | 1428.79 |
| 6584 | TTPVTVGFHCLPADSANSLTDK | 2330.12 |
| 6606 | SAGEATNLKAINIKIGSHEIDMHPVNGQVK | 3170.65 |
| 6742 | NAVSFGHSWILEEAPCR | 1971.93 |
| 7027 | YDAECEQEYR | 1361.52 |
| 7251 | CYSTEPVLR | 1123.53 |
| 7466 | TVQLAGVDSK | 1016.54 |
| Source | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Unordered (%) |
|---|---|---|---|---|
| Pv standard | 17.4 ± 0.14 a | 26.4 ± 0.12 a | 39.5 ± 0.11 a | 16.7 ± 0.14 a |
| Purified Pv | 17.1 ± 0.11 a | 27.3 ± 0.13 a | 39.0 ± 0.14 a | 16.6 ± 0.15 a |
| Source | Bind with Fe2+ | Bind with Fe3+ |
|---|---|---|
| Pv standard | 28.1 ± 0.72 a | 53.8 ± 1.11 a |
| purified Pv | 27.2 ± 0.65 a | 53.6 ± 1.1 a |
| Source | Surface Hydrophobicity |
|---|---|
| Pv standard | 488.6 ± 2.14 a |
| purified Pv | 493.48 ± 2.31 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Wang, L.; Wang, X.; Wu, S.; Li, D.; Liu, C.; Feng, Z. Ultrasonic Thermal-Assisted Extraction of Phosvitin from Egg Yolk and Evaluation of Its Properties. Polymers 2019, 11, 1353. https://doi.org/10.3390/polym11081353
Jiang B, Wang L, Wang X, Wu S, Li D, Liu C, Feng Z. Ultrasonic Thermal-Assisted Extraction of Phosvitin from Egg Yolk and Evaluation of Its Properties. Polymers. 2019; 11(8):1353. https://doi.org/10.3390/polym11081353
Chicago/Turabian StyleJiang, Bin, Linlin Wang, Xiaojing Wang, Shuang Wu, Dongmei Li, Chunhong Liu, and Zhibiao Feng. 2019. "Ultrasonic Thermal-Assisted Extraction of Phosvitin from Egg Yolk and Evaluation of Its Properties" Polymers 11, no. 8: 1353. https://doi.org/10.3390/polym11081353
APA StyleJiang, B., Wang, L., Wang, X., Wu, S., Li, D., Liu, C., & Feng, Z. (2019). Ultrasonic Thermal-Assisted Extraction of Phosvitin from Egg Yolk and Evaluation of Its Properties. Polymers, 11(8), 1353. https://doi.org/10.3390/polym11081353