A Novel Hierarchically Porous Polypyrrole Sphere Modified Separator for Lithium-Sulfur Batteries
Abstract
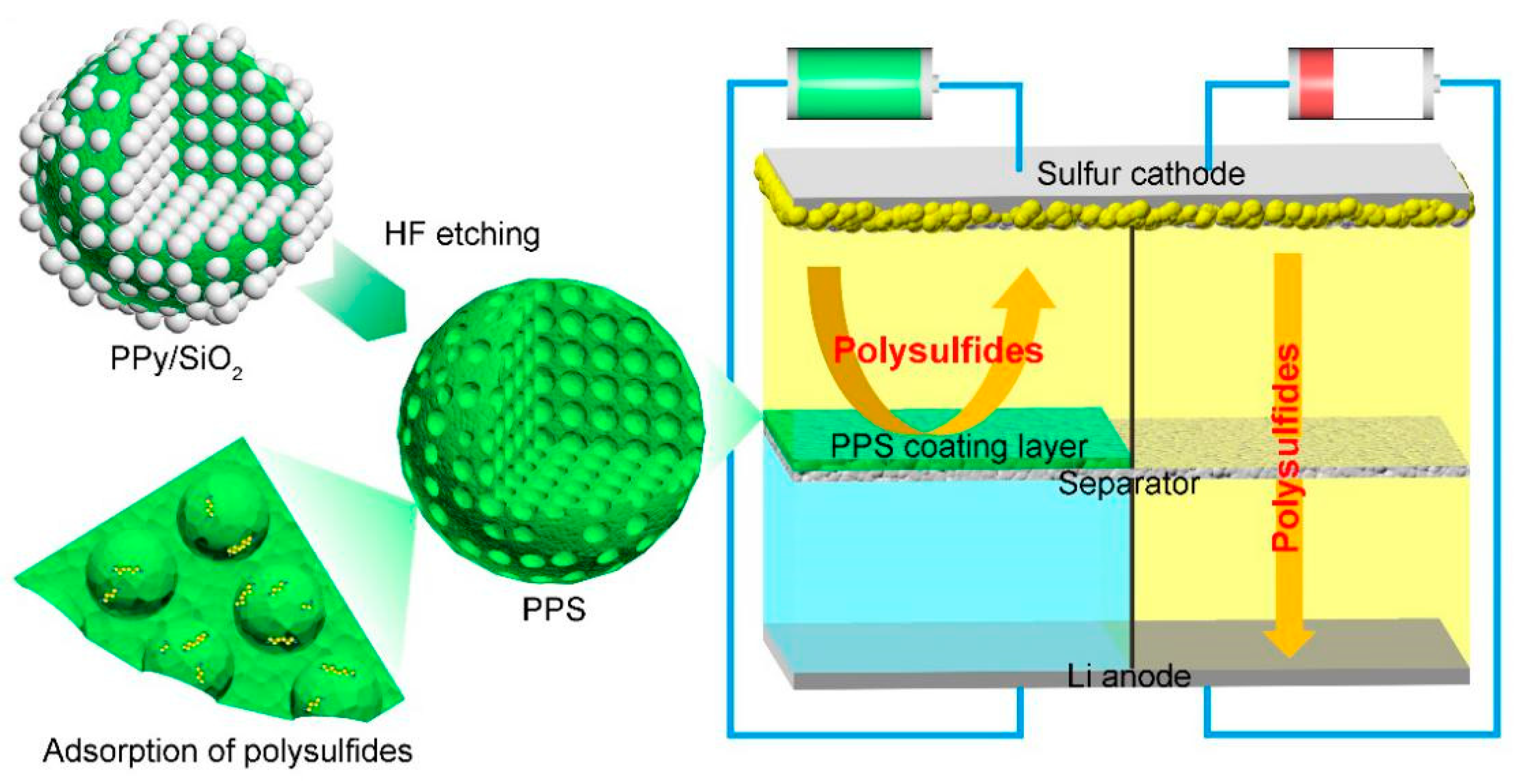
1. Introduction
2. Materials and Methods
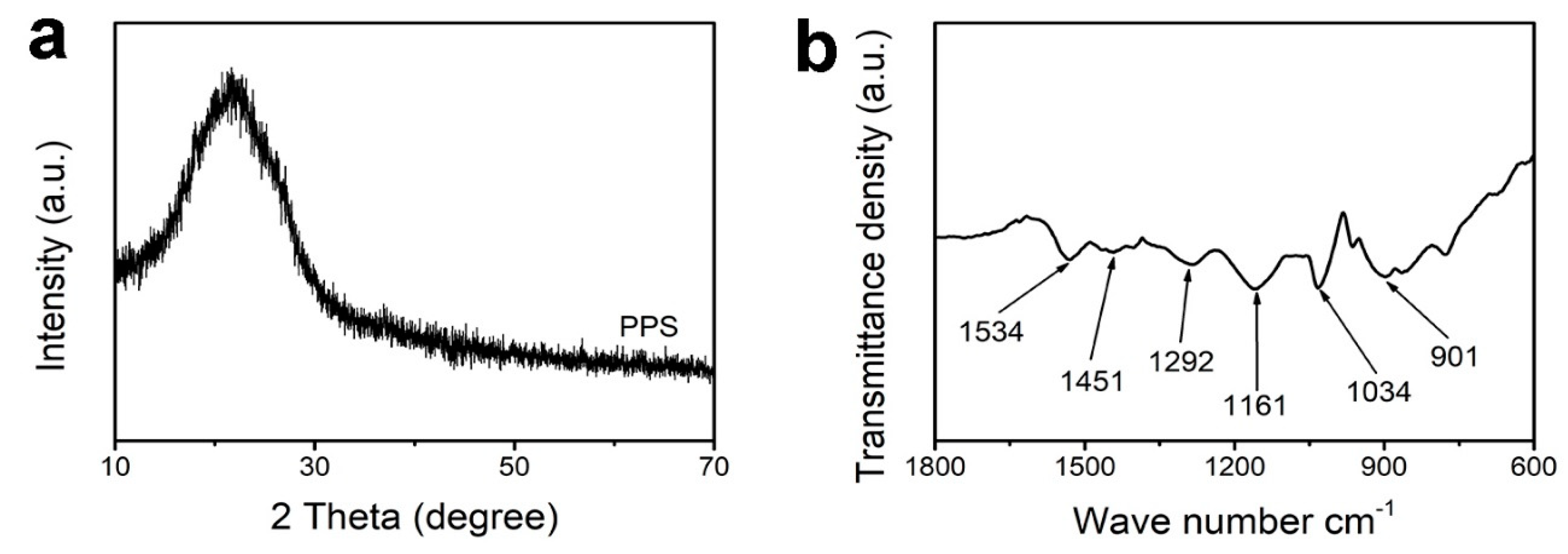
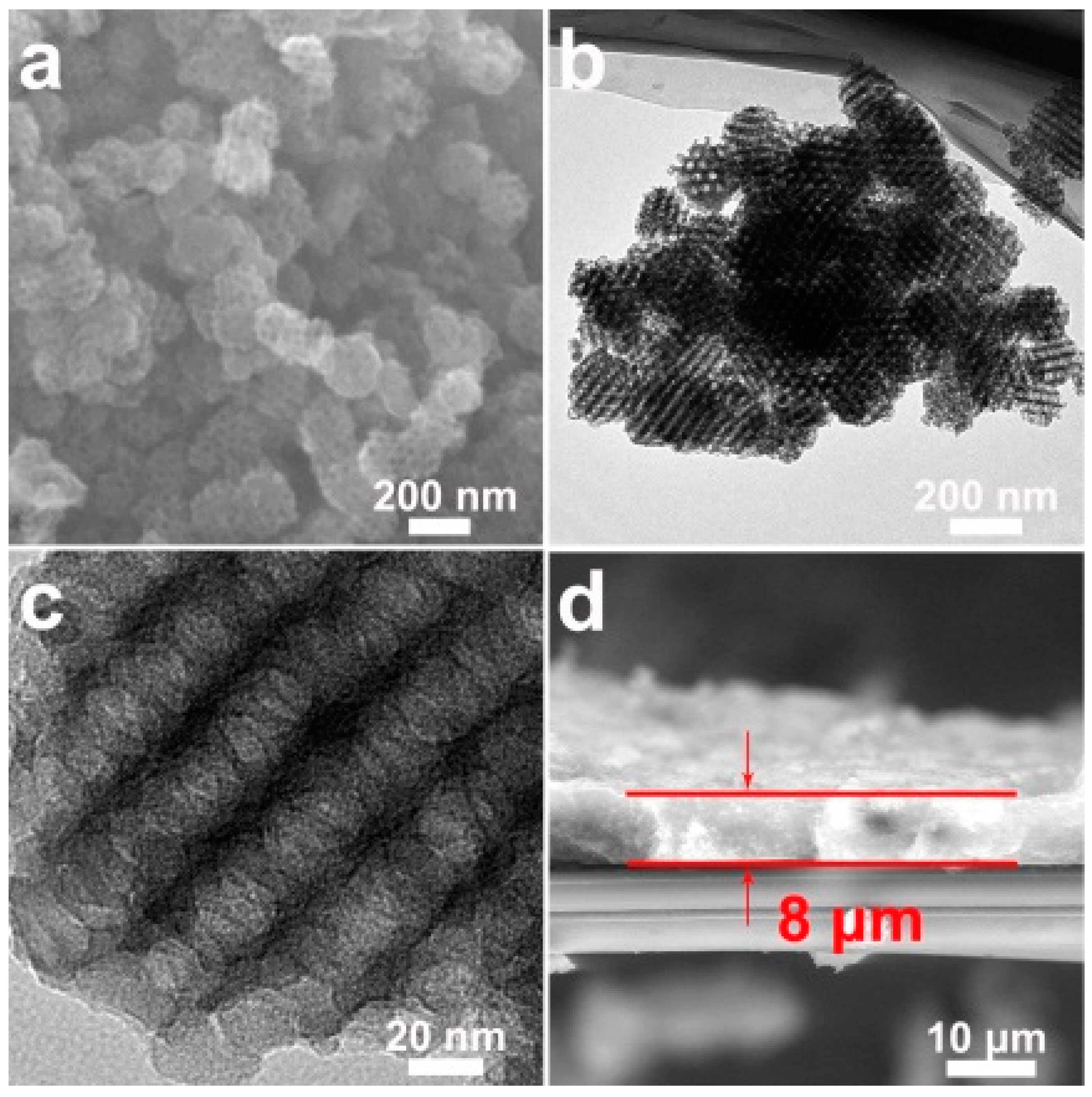
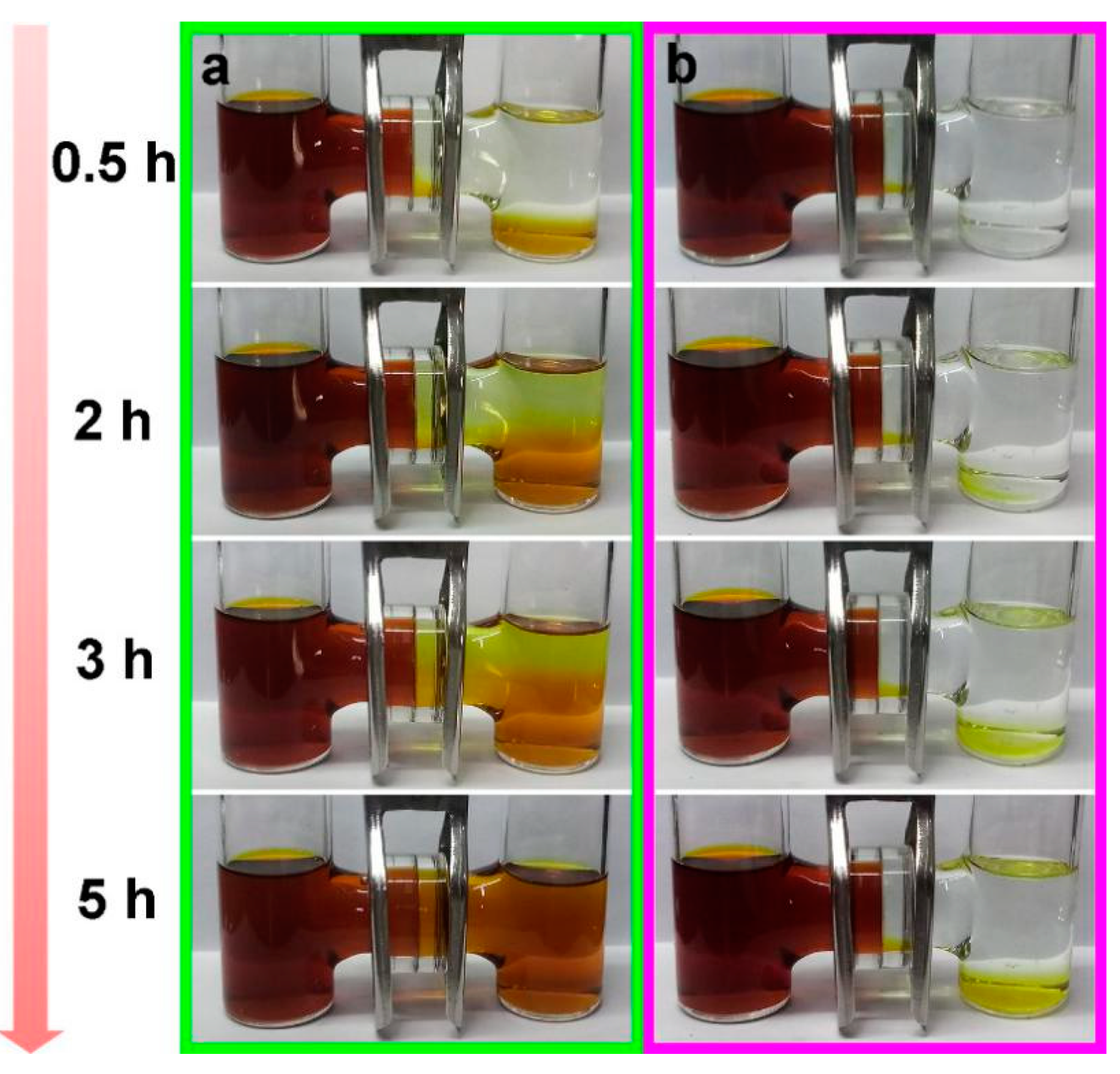
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chu, S.; Cui, Y.; Liu, N. The Path Towards Sustainable Energy. Nat. Mater. 2016, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Review-The Importance of Chemical Interactions between Sulfur Host Materials and Lithium Polysulfides for Advanced Lithium-Sulfur Batteries. J. Electrochem. Soc. 2015, 162, A2567–A2576. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lee, H.; Seh, Z.W.; Zheng, G.; Sun, J.; Li, Y.; Cui, Y. Lithium Sulfide/Metal Nanocomposite as a High-Capacity Cathode Prelithiation Material. Adv. Energy Mater. 2016, 6, 1600154. [Google Scholar] [CrossRef]
- Gao, X.; Yang, H. Multi-electron reaction materials for high energy density batteries. Energy Environ. Sci. 2010, 3, 174–189. [Google Scholar] [CrossRef]
- Li, Z.; Guan, B.Y.; Zhang, J.; Lou, X.W. A Compact Nanoconfined Sulfur Cathode for High-Performance Lithium-Sulfur Batteries. Joule 2017, 1, 576–587. [Google Scholar] [CrossRef]
- Sun, Y.; Lee, H.; Seh, Z.W.; Liu, N.; Sun, J.; Li, Y.; Cui, Y. High-capacity battery cathode prelithiation to offset initial lithium loss. Nat. Energy 2016, 1, 15008. [Google Scholar] [CrossRef]
- Park, J.; Yu, S.H.; Sung, Y.E. Design of Structural and Functional Nanomaterials for Lithium-Sulfur Batteries. Nano Today 2018, 18, 35–64. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhao, Y.; Yermukhambetova, A.; Bakenov, Z.; Chen, P. Ternary sulfur/polyacrylonitrile/Mg0.6Ni0.4O composite cathodes for high performance lithium/sulfur batteries. J. Mater. Chem. A 2013, 1, 295–301. [Google Scholar] [CrossRef]
- Sun, Y.; Lee, H.; Zheng, G.; Seh, Z.W.; Sun, J.; Li, Y.; Cui, Y. In Situ Chemical Synthesis of Lithium Fluoride/Metal Nanocomposite for High Capacity Prelithiation of Cathodes. Nano Lett. 2016, 16, 1497–1501. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, Y.; Zhang, Y.; Ricardezsandoval, L.A.; Wang, X.; Li, J. Construction of Oxygen-deficient La(OH)3 Nanorods Wrapped by Reduced Graphene Oxide for Polysulfide Trapping toward High-Performance Lithium/Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 23271–23279. [Google Scholar] [CrossRef]
- Zheng, C.; Niu, S.; Lv, W.; Zhou, G.; Li, J.; Fan, S.; Deng, Y.; Pan, Z.; Li, B.; Kang, F.; et al. Propelling polysulfides transformation for high-rate and long-life lithium–sulfur batteries. Nano Energy 2017, 33, 306–312. [Google Scholar] [CrossRef]
- Gueon, D.; Hwang, J.T.; Yang, S.B.; Cho, E.; Sohn, K.; Yang, D.; Moon, J.H. Spherical Macroporous Carbon Nanotube Particles with Ultrahigh Sulfur Loading for Lithium–Sulfur Battery Cathodes. ACS Nano 2018, 1, 226–233. [Google Scholar] [CrossRef]
- Agostini, M.; Scrosati, B.; Hassoun, J. An Advanced Lithium-Ion Sulfur Battery for High Energy Storage. Adv. Energy Mater. 2015, 5, 1500481. [Google Scholar] [CrossRef]
- Lin, H.; Yang, L.; Jiang, X.; Li, G.; Zhang, T.; Yao, Q.; Zheng, J.W.; Lee, J.Y. Electrocatalysis of polysulfide conversion by sulfur-deficient MoS2 nanoflakes for lithium–sulfur batteries. Energy Environ. Sci. 2017, 10, 1476–1486. [Google Scholar] [CrossRef]
- Xu, G.; Yan, Q.; Wang, S.; Kushima, A.; Bai, P.; Liu, K.; Zhang, X.; Tang, Z.; Li, J. A Thin Multifunctional Coating on a Separator Improves the Cyclability and Safety of Lithium Sulfur Batteries. Chem. Sci. 2017, 8, 6619–6625. [Google Scholar] [CrossRef]
- Song, X.; Chen, G.; Wang, S.; Huang, Y.; Jiang, Z.; Ding, L.X.; Wang, H. Self-Assembled Close-Packed MnO2 Nanoparticles Anchored on a Polyethylene Separator for Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 26274–26282. [Google Scholar] [CrossRef]
- Zhou, G.; Pei, S.; Li, L.; Wang, D.W.; Wang, S.; Huang, K.; Yin, L.C.; Li, F.; Cheng, H.M. A Graphene-Pure-Sulfur Sandwich Structure for Ultrafast, Long-Life Lithium-Sulfur Batteries. Adv. Mater. 2014, 26, 625–631. [Google Scholar] [CrossRef]
- Sun, Y.; Seh, Z.W.; Li, W.; Yao, H.; Zheng, G.; Cui, Y. In-operando optical imaging of temporal and spatial distribution of polysulfides in lithium-sulfur batteries. Nano Energy 2015, 11, 579–586. [Google Scholar] [CrossRef]
- Liu, X.; Qian, T.; Liu, J.; Tian, J.; Zhang, L.; Yan, C. Greatly Improved Conductivity of Double-Chain Polymer Network Binder for High Sulfur Loading Lithium-Sulfur Batteries with a Low Electrolyte/Sulfur Ratio. Small 2018, 14, 1801536. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, J.; Yin, L.; Hu, G.; Fang, R.; Cheng, H.M.; Li, F. Conductive porous vanadium nitride/graphene composite as chemical anchor of polysulfides for lithium-sulfur batteries. Nat. Commun. 2017, 8, 14627. [Google Scholar] [CrossRef]
- Dong, Y.F.; Zheng, S.H.; Qin, J.Q.; Zhao, X.J.; Shi, H.D.; Wang, X.H.; Chen, J.; Wu, Z.S. All-Mxene-based integrated electrode constructed by Ti3C2 nanoribbon framework host and nanosheet interlayer for high-energy-density Li-S batteries. ACS Nano 2018, 12, 2381–2388. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Qiu, W.; An, C.; Yan, Y.; Xu, J.; Zhang, Z.; Guo, W.; Wang, Z.; Zheng, Z.; Wang, Z.; Deng, Q.; et al. Suppressed polysulfide shuttling and improved Li+ transport in Li-S batteries enabled by NbN modified PP separator. J. Power Sources 2019, 423, 98–105. [Google Scholar] [CrossRef]
- Li, N.; Chen, Z.; Chen, F.; Hu, G.; Wang, S.; Sun, Z.; Sun, X.; Li, F. From interlayer to lightweight capping layer: Rational design of mesoporous TiO2 threaded with CNTs for advanced Li-S batteries. Carbon 2019, 143, 523–530. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Zhou, G.; Lv, W.; Ling, G.; Zhi, L.; Yang, Q. Catalytic effects in lithium-sulfur batteries: Promoted sulfur transformation and reduced shuttle effect. Adv. Sci. 2018, 5, 1700270. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Z.; Cheng, B.; Chen, R.; Hu, Y.; Ma, L.; Zhu, J.; Liu, J.; Jin, Z. Self-Templated Formation of Interlaced Carbon Nanotubes Threaded Hollow Co3S4 Nanoboxes for High-Rate and Heat-Resistant Lithium–Sulfur Batteries. J. Am. Chem. Soc. 2017, 139, 12710–12715. [Google Scholar] [CrossRef]
- Li, Y.; Shi, B.; Liu, W.; Guo, R.; Pei, H.; Ye, D.; Xie, J.; Kong, J. Hollow polypyrrole@MnO2 spheres as nano-sulfur hosts for improved lithium-sulfur batteries. Electrochim. Acta 2018, 260, 912–920. [Google Scholar] [CrossRef]
- Peng, Y.; Wen, Z.; Liu, C.; Zeng, J.; Wang, Y.; Zhao, J. Refining Interfaces between Electrolyte and Both Electrodes with Carbon Nanotube Paper for High-Loading Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 6986–6994. [Google Scholar] [CrossRef]
- Wu, X.; Fan, L.; Qiu, Y.; Wang, M.; Cheng, J.; Guan, B.; Guo, Z.; Zhang, N.; Sun, K. Ion-Selective Prussian-Blue-Modified Celgard Separator for High-Performance Lithium-Sulfur Battery. ChemSusChem 2018, 11, 3345–3351. [Google Scholar] [CrossRef]
- Li, F.; Kaiser, M.R.; Ma, J.; Guo, Z.; Liu, H.; Wang, J. Free-standing sulfur-polypyrrole cathode in conjunction with polypyrrole-coated separator for flexible Li-S batteries. Energy Storage Mater. 2018, 13, 312–322. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Wang, Q.; Shen, C.; Peng, P.; Jin, J.; Wu, X. Enhanced performance of lithium sulfur battery with self-assembly polypyrrole nanotube film as the functional interlayer. J. Power Sources 2015, 273, 511–516. [Google Scholar] [CrossRef]
- Yin, F.; Ren, J.; Zhang, Y.; Tan, T.; Chen, Z. A PPy/ZnO functional interlayer to enhance electrochemical performance of lithium/sulfur batteries. Nanoscale Res. Lett. 2018, 1, 307. [Google Scholar] [CrossRef]
- Li, H.; Sun, L.; Zhao, Y.; Tan, T.; Zhang, Y. Blackberry-like hollow graphene spheres synthesized by spray drying for high-performance lithium-sulfur batteries. Electrochim. Acta 2019, 295, 822–828. [Google Scholar] [CrossRef]
- Yin, F.; Ren, J.; Wu, G.; Zhang, C.; Zhang, Y. Polypyrrole Nanowires with Ordered Large Mesopores: Synthesis, Characterization and Applications in Supercapacitor and Lithium/Sulfur Batteries. Polymers 2019, 2, 277. [Google Scholar] [CrossRef]
- Rao, J.; Xu, R.; Zhou, T.; Zhang, D.; Zhang, C. Rational design of self-supporting graphene-Polypyrrole/sulfur-Graphene sandwich as structural paper electrode for lithium sulfur batteries. J. Alloys Compd. 2017, 728, 376–382. [Google Scholar] [CrossRef]
- Yin, F.; Liu, X.; Zhang, Y.; Zhao, Y.; Menbayeva, A.; Bakenov, Z.; Wang, X. Well-dispersed sulfur anchored on interconnected polypyrrole nanofiber network as high performance cathode for lithium-sulfur batteries. Solid State Sci. 2017, 66, 44–49. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Chen, J.; Li, X.; Walsh, F.; Ouyang, J.; Jia, D.; Zhou, Y. Three-dimensional graphene oxide/polypyrrole composite electrodes fabricated by one-step electrodeposition for high performance supercapacitors. J. Mater. Chem. A 2015, 3, 14445–14457. [Google Scholar] [CrossRef]
- Pei, F.; Lin, L.; Fu, A.; Mo, S.; Ou, D.; Fang, X.; Zheng, N. A Two-Dimensional Porous Carbon-Modified Separator for High-Energy-Density Li-S Batteries. Joule 2017, 2, 323–336. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, W.; Kong, L.; Zhang, L.; Zhu, X.; Shao, Y.; Ding, F.; Zhang, Q.; Sun, J.; Liu, Z.; et al. Synchronous Immobilization and Conversion of Polysulfides on VO2-VN Binary Host Targeting High Sulfur Loading Li−S Batteries. Energy Environ. Sci. 2018, 11, 2620–2630. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Yan, X.; Su, Q.; Lu, Y.; Qiu, J.; Wang, Z.; Lin, X.; Huang, J.; Liu, R.; et al. Superhierarchical Cobalt-Embedded Nitrogen-Doped Porous Carbon Nanosheets as Two-in-One Hosts for High-Performance Lithium-Sulfur Batteries. Adv. Mater. 2018, 30, 1706895. [Google Scholar] [CrossRef]
- Kong, W.; Yan, L.; Luo, Y.; Wang, D.; Jiang, K.; Li, Q.; Fan, S.; Wang, J. Ultrathin MnO2/Graphene Oxide/Carbon Nanotube Interlayer as Efficient Polysulfide-Trapping Shield for High-Performance Li−S Batteries. Adv. Funct. Mater. 2017, 27, 1606663. [Google Scholar] [CrossRef]
- He, Y.; Shan, Z.; Tan, T.; Chen, Z.; Zhang, Y. Ternary Sulfur/Polyacrylonitrile/SiO2 Composite Cathodes for High-Performance Sulfur/Lithium Ion Full Batteries. Polymers 2018, 8, 930. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, H.; Li, X.; Xiang, X.; Liao, Y.; Xue, Z.; Ye, Y.; Xie, X. Ultralight Layer-by-Layer Self-Assembled MoS2-Polymer Modified Separator for Simultaneously Trapping Polysulfides and Suppressing Lithium Dendrites. Adv. Energy Mater. 2018, 8, 1802430. [Google Scholar] [CrossRef]
- Son, B.D.; Cho, S.H.; Bae, K.Y.; Kim, B.H.; Yoon, W.Y. Dual functional effect of the ferroelectricity embedded interlayer in lithium sulfur battery. J. Power Sources 2019, 419, 35–41. [Google Scholar] [CrossRef]
- Yao, H.; Yan, K.; Li, W.; Zheng, G.; Kong, D.; Seh, Z.W.; Narasimhan, V.K.; Liang, Z.; Cui, Y. Improved lithium-sulfur batteries with a conductive coating on the separator to prevent the accumulation of inactive S-related species at the cathode-separator interface. Energy Environ. Sci. 2014, 7, 3381–3390. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Liu, X.; Mao, E.; Wang, M.; Li, G.; Fu, L.; Li, Z.; Eng, Y.S.; Seh, Z.W.; et al. Engineering stable electrode-separator interfaces with ultrathin conductive polymer layer for high-energy-density Li-S batteries. Energy. Storage Mater. 2019. [Google Scholar] [CrossRef]
- Li, H.; Sun, L.; Zhang, Y.; Tan, T.; Wang, G.; Bakenov, Z. Enhanced cycle performance of Li/S battery with the reduced graphene oxide/activated carbon functional interlayer. J. Energy Chem. 2017, 26, 1276–1281. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, Q.; Tang, J.; Bai, T.; Chen, F.; Yang, J. A high-level N-doped porous carbon nanowire modified separator for long-life lithium–sulfur batteries. J. Electroanal. Chem. 2016, 768, 55–61. [Google Scholar] [CrossRef]
- Kong, L.; Chen, X.; Li, B.; Peng, H.; Huang, J.; Xie, J.; Zhang, Q. A Bifunctional Perovskite Promoter for Polysulfide Regulation toward Stable Lithium–Sulfur Batteries. Adv. Mater. 2018, 30, 1705219. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, Y.; Li, B.; Tan, T.; Zhao, Y. ZnO/carbon nanotube/reduced graphene oxide composite film as an effective interlayer for lithium/sulfur batteries. Solid State Sci. 2019, 95, 105924. [Google Scholar] [CrossRef]



| Sample | Sulfur Loading of Cathode (Content or Areal Loading) | Initial Capacity (mAh·g−1, at n C) | Final Capacity (mAh·g−1, after n Cycles) | High Rate Performance (mAh·g−1, at n C) | Ref. |
|---|---|---|---|---|---|
| Super P coated separator | 1.5–2.0 mg·cm−2 | ~1000 mAh·g−1 (0.1 C) | 610 mAh·g−1 (200 th) | ~390 mAh·g−1 (1 C) | [46] |
| PPy modified separator | 70%, 1.2 mg·cm−2 | 985 mAh·g−1 (0.5 C) | 805 mAh·g−1 (250 th) | 682 mAh·g−1 (2 C) | [47] |
| RGO/AC interlayer | 58.2% | 1078 mAh·g−1 (0.1 C) | 655 mAh·g−1 (100 th) | 348 mAh·g−1 (1.5 C) | [48] |
| N-PCNW- modified separator | 1.5–1.7 mg·cm−2 | 1430 mAh·g−1 (0.2 C) | 881.5 mAh·g−1 (200 th) | 618 mAh·g−1 (2 C) | [49] |
| PrNPs | 70%, 1.1 mg·cm−2 | 986 mAh·g−1 (0.2 C) | 695 mAh·g−1 (200 th) | 753 mAh·g−1 (2 C) | [50] |
| ZnO/CNT/RGO interlayer | 68.3%, 1.7 mg·cm−2 | 1061 mAh·g−1 (0.2 C) | 768 mAh·g−1 (200 th) | 597 mAh·g−1 (2 C) | [51] |
| PPS-modified separator | 2.1 mg·cm−2 | 1274 mAh·g−1 (0.2 C) | 855 mAh·g−1 (100th) | 507 mAh·g−1 (3 C) | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Sun, Z.; Zhao, Y.; Bakenov, Z. A Novel Hierarchically Porous Polypyrrole Sphere Modified Separator for Lithium-Sulfur Batteries. Polymers 2019, 11, 1344. https://doi.org/10.3390/polym11081344
Li B, Sun Z, Zhao Y, Bakenov Z. A Novel Hierarchically Porous Polypyrrole Sphere Modified Separator for Lithium-Sulfur Batteries. Polymers. 2019; 11(8):1344. https://doi.org/10.3390/polym11081344
Chicago/Turabian StyleLi, Baoe, Zhenghao Sun, Yan Zhao, and Zhumabay Bakenov. 2019. "A Novel Hierarchically Porous Polypyrrole Sphere Modified Separator for Lithium-Sulfur Batteries" Polymers 11, no. 8: 1344. https://doi.org/10.3390/polym11081344
APA StyleLi, B., Sun, Z., Zhao, Y., & Bakenov, Z. (2019). A Novel Hierarchically Porous Polypyrrole Sphere Modified Separator for Lithium-Sulfur Batteries. Polymers, 11(8), 1344. https://doi.org/10.3390/polym11081344






