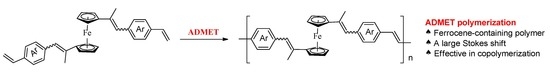
Ferrocene-Containing Conjugated Oligomers Synthesized by Acyclic Diene Metathesis Polymerization
Abstract
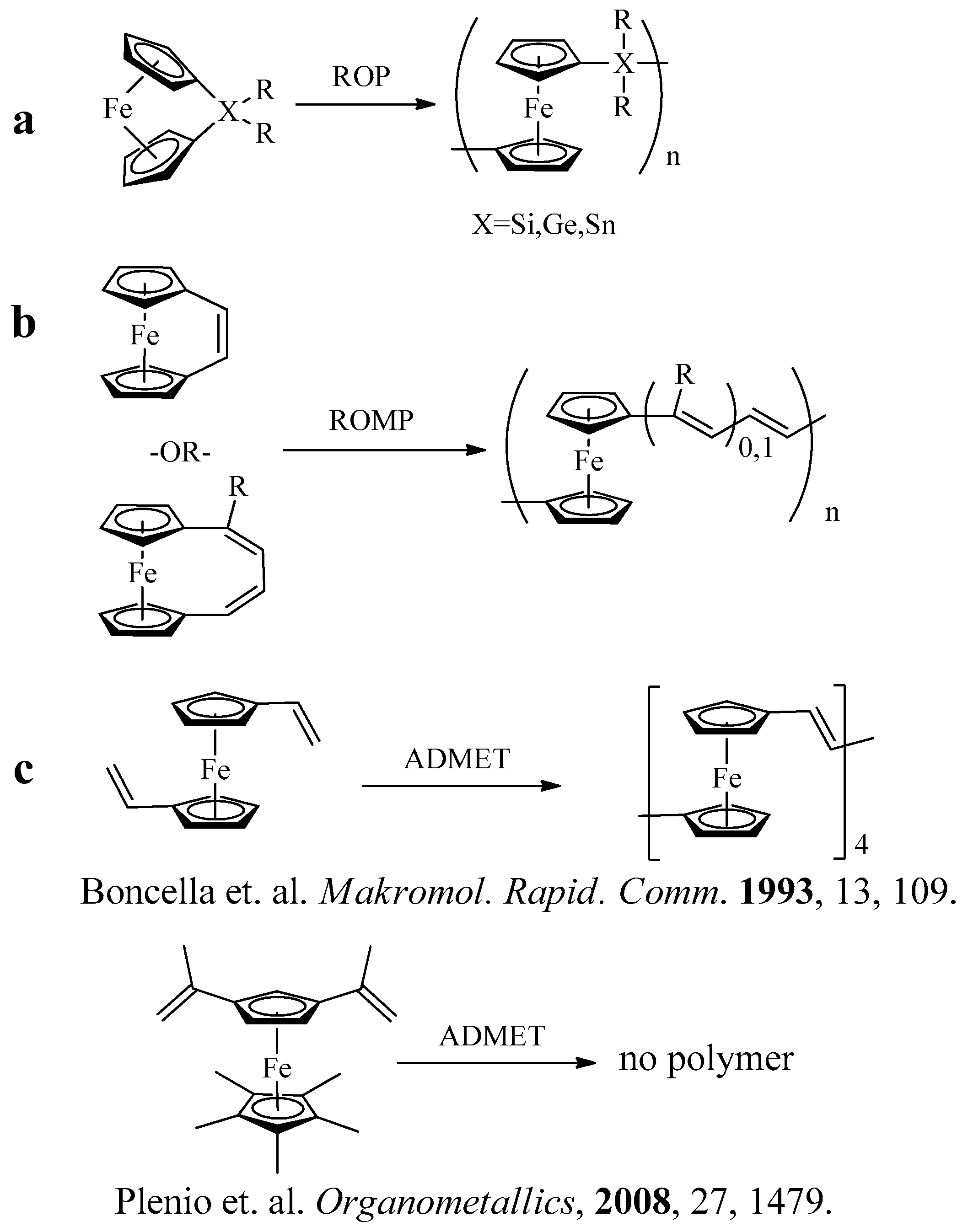
1. Introduction
2. Materials and Methods
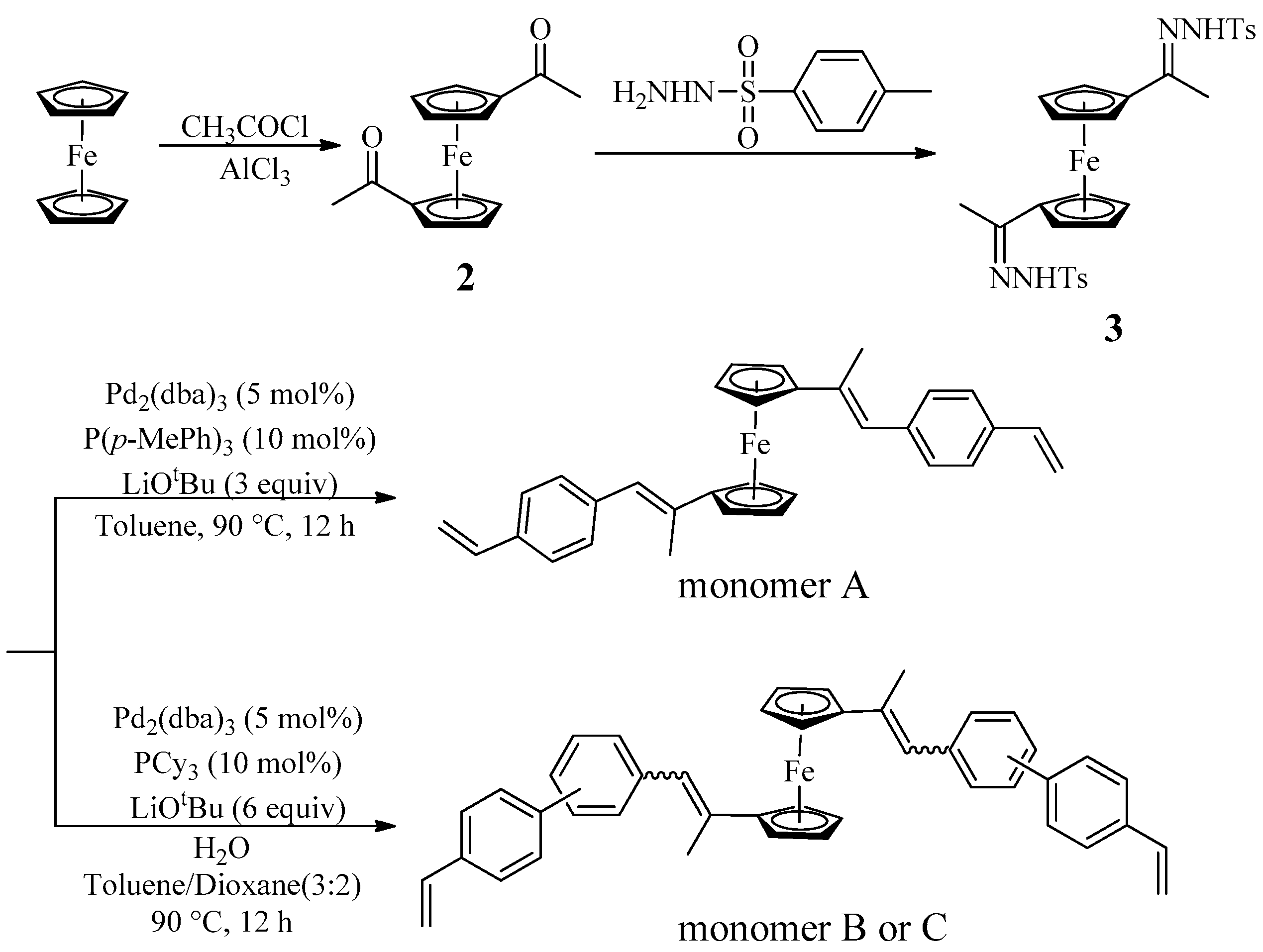
2.1. Synthesis of 1,1’-Diacetylferrocene (2)
2.2. Synthesis of N’,N’’-(1,1’-ferrocenylbis(ethan-1-yl-1-ylidene))bis(4-methylbenzenesulfonohydrazide) (3)
2.3. Synthesis of Monomer A
2.4. Synthesis of Monomer B
2.5. Synthesis of Monomer C
2.6. Synthesis of 9,9-dioctyl-2,7-divinylfluorene (Monomer D)
2.7. Synthesis of Oligomer A
2.8. Synthesis of Copolymer 1
3. Results
3.1. Monomer Synthesis and Characterisation
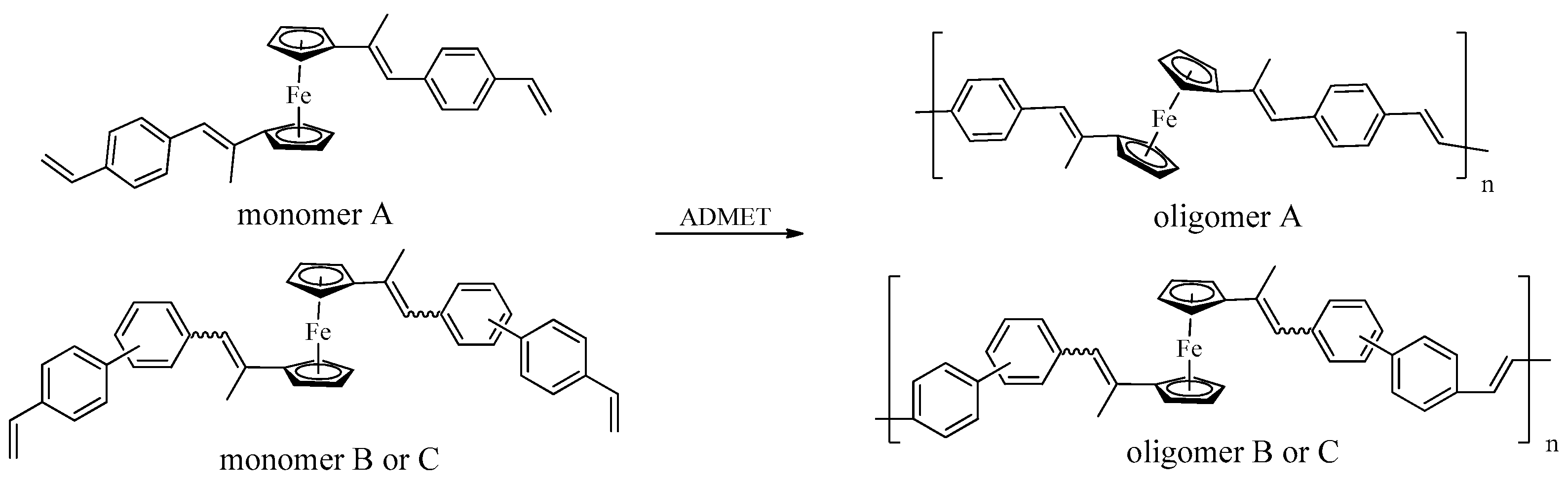
3.2. Acyclic Diene Metathesis (ADMET) Polymerization of Monomers
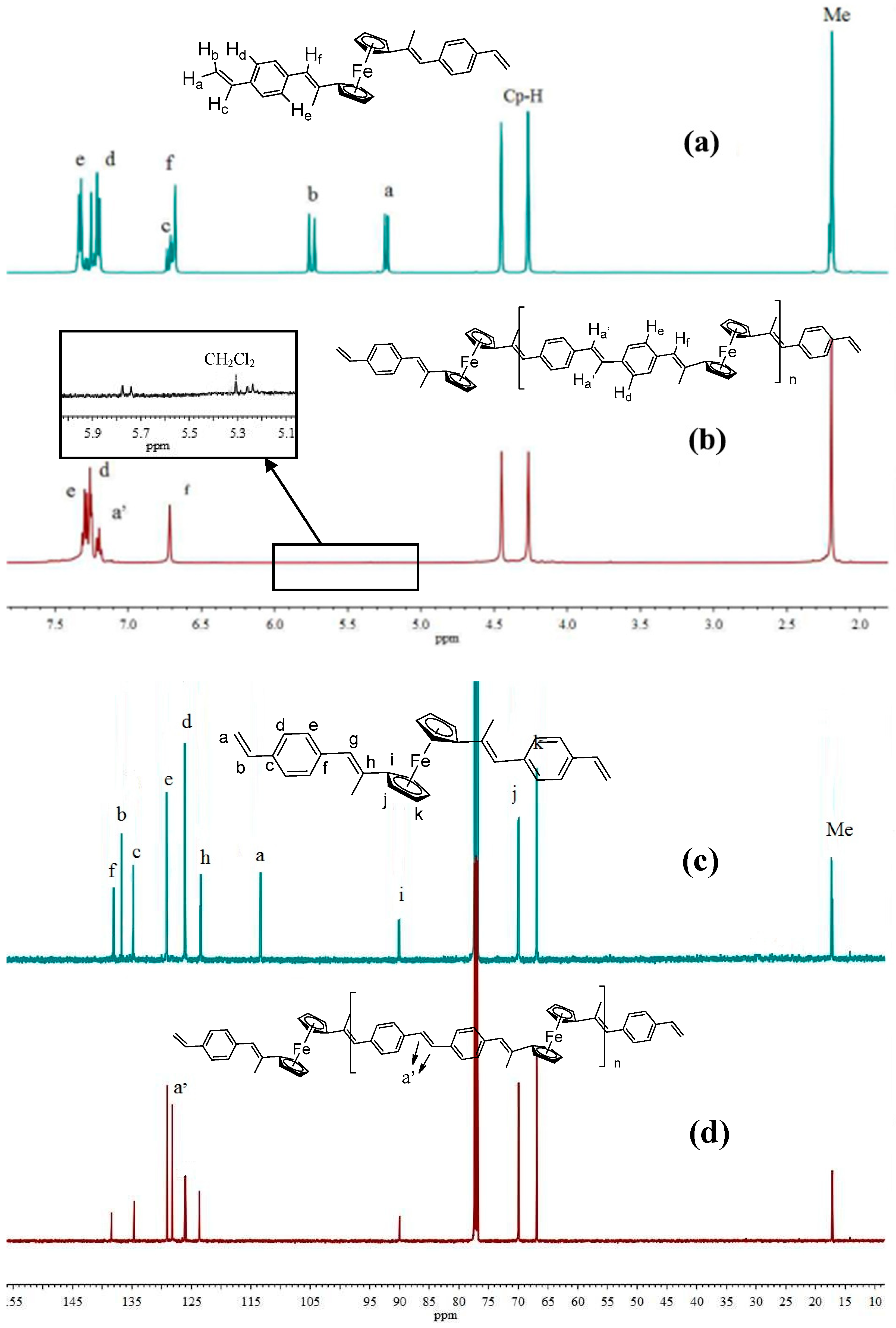
3.3. Microstructure via NMR and FT-IR
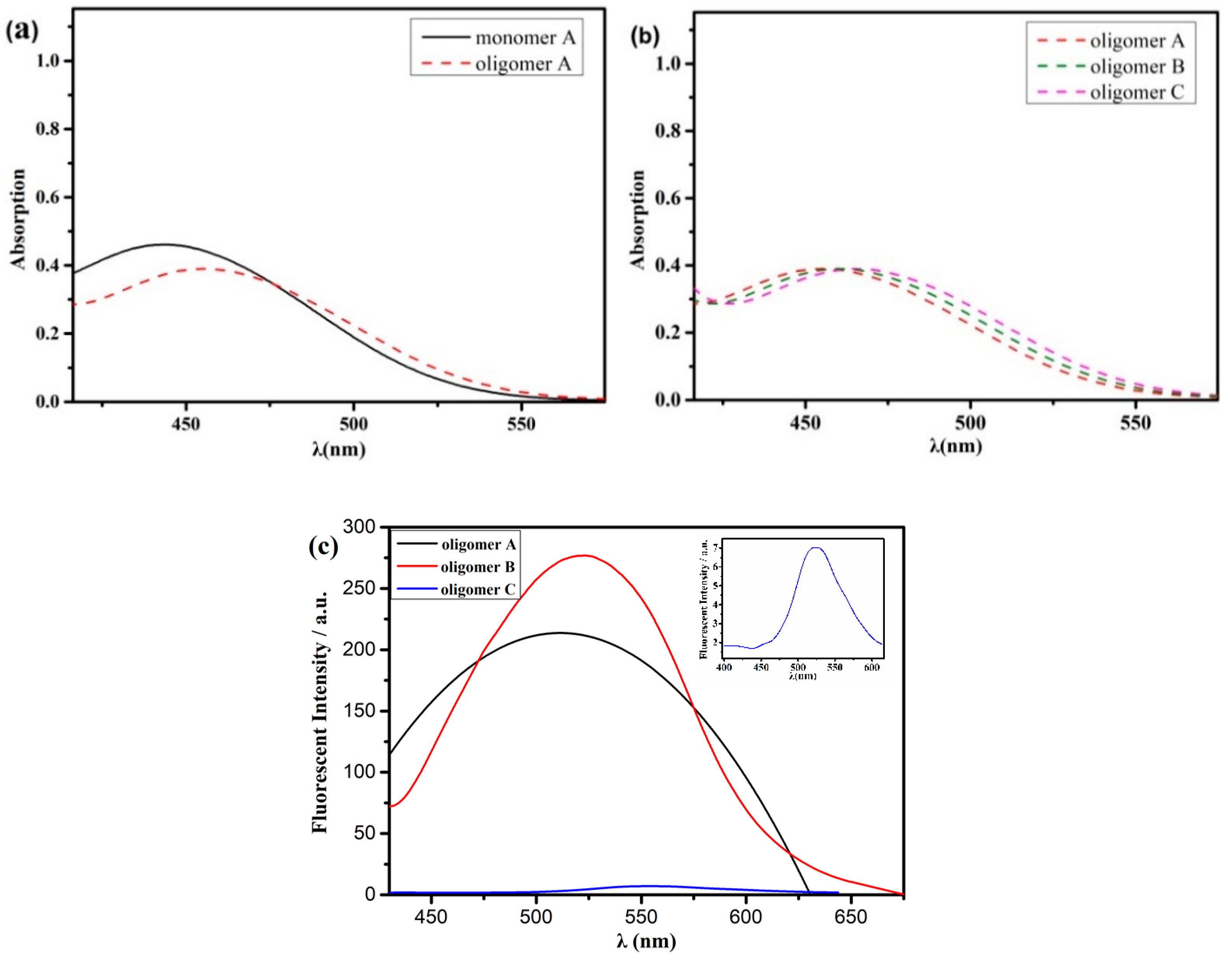
3.4. Optical Property
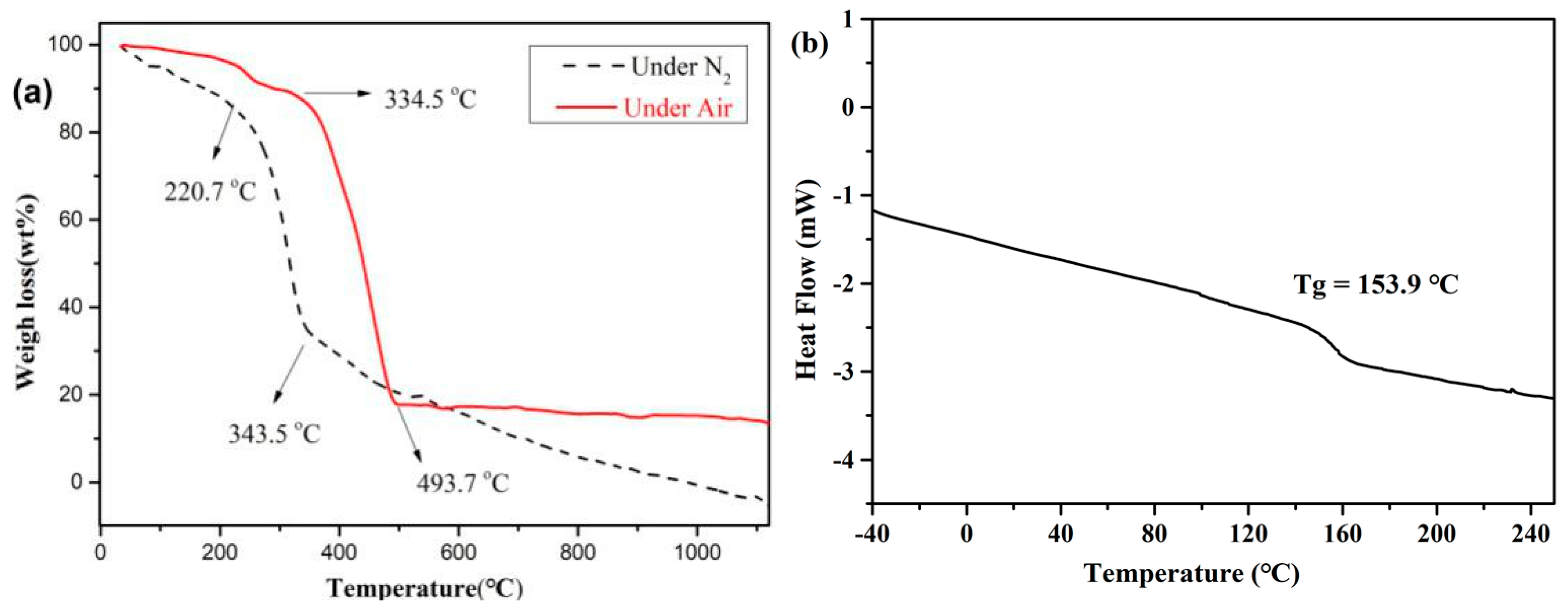
3.5. Thermal Stability Studies
3.6. Electrochemical Properties
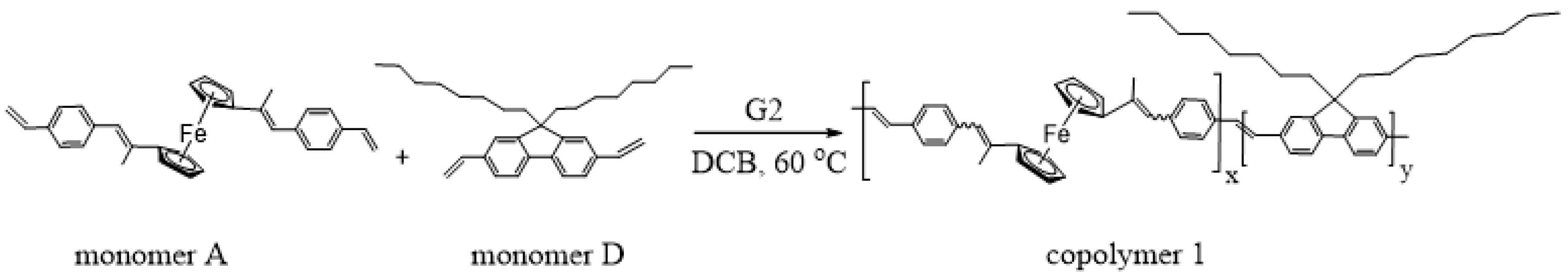
3.7. Copolymerization of Monomer A and 9,9-Dioctyl-2,7-Divinylfluorene (Monomer D)
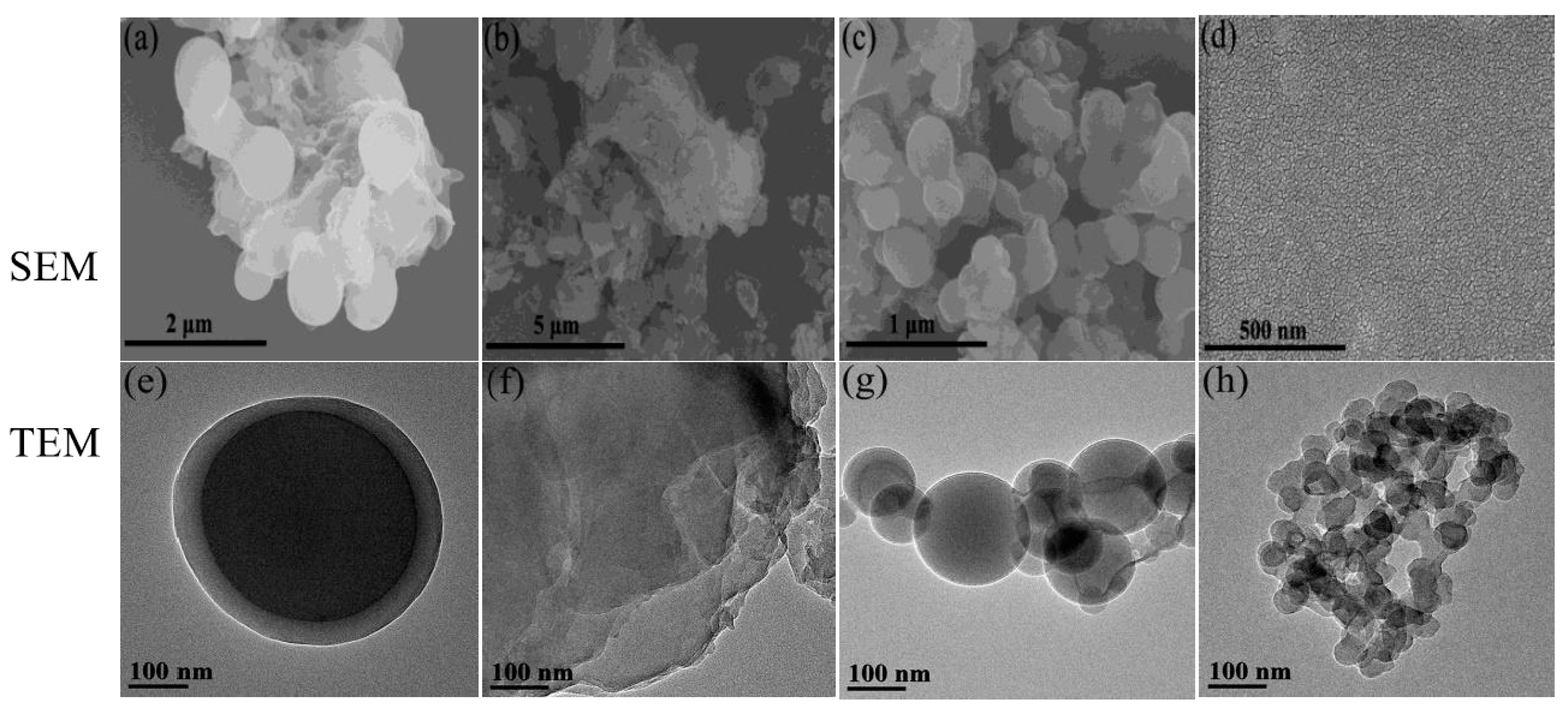
3.8. SEM and TEM Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonçalves, C.S.; Serbena, J.P.; Hümmelgen, I.A.; Gruber, J. A novel ferrocene-DOPPV conjugated copolymer. Macromol. Symp. 2006, 245, 22–26. [Google Scholar] [CrossRef]
- Arimoto, F.S.; Haven, A.C., Jr. Derivatives of dicyclopentadienyliron. J. Am. Chem. Soc. 1955, 77, 6295–6297. [Google Scholar] [CrossRef]
- Hudson, Z.M.; Manners, I. Assembly and disassembly of ferrocene-based nanotubes. Science 2014, 344, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Pittman, C.U., Jr. The discovery of metallocene- and metallocene-like addition polymers. J. Inorg. Organomet. Polym. 2005, 15, 33–55. [Google Scholar] [CrossRef]
- Bellas, V.; Rehahn, M. Polyferrocenylsilane-based polymer systems. Angew. Chem. Int. Ed. 2007, 46, 5082–5104. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, A.S.; Manners, I. Neutral and cationic macromolecules based on iron sandwich complexes. J. Inorg. Organomet. Polym. 2005, 15, 157–195. [Google Scholar] [CrossRef]
- Whittell, G.R.; Manners, I. Metallopolymers: New multifunctional materials. Adv. Mater. 2007, 19, 3439–3468. [Google Scholar] [CrossRef]
- Astruc, D.; Ornelas, C.; Ruiz, J. Metallocenyl dendrimers and their applications in molecular electronics, sensing, and catalysis. Acc. Chem. Res. 2008, 41, 841–856. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Manners, I. Frontiers in Transition Metal-Containing Polymers; John Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Zamora, M.; Bruña, S.; Alonso, B.; Cuadrado, I. Polysiloxanes bearing pendant redox-active dendritic wedges containing ferrocenyl and (η6−aryl)tricarbonylchromium moieties. Macromolecules 2011, 44, 7994–8007. [Google Scholar] [CrossRef]
- Wang, X.; McHale, R. Metal-containing polymers: Building blocks for functional (nano)materials. Macromol. Rapid Commun. 2010, 31, 331–350. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Strohm, E.A. Transition metal-containing macromolecules: En route to new functional materials. Polymer 2012, 53, 4879–4921. [Google Scholar] [CrossRef]
- Choueiri, R.M.; Klinkova, A.; Pearce, S.; Manners, I.; Kumacheva, E. Self-assembly and surface patterning of polyferrocenylsilane-functionalized gold nanoparticles. Macromol. Rapid. Commun. 2018, 39, 1700554. [Google Scholar] [CrossRef]
- Amer, W.A.; Yu, H.; Wang, L.; Vatsadze, S.; Tong, R. Synthesis, characterization and properties of some main-chain ferrocene-based polymers containing aromatic units. J. Inorg. Organomet. Polym. 2013, 23, 1431–1444. [Google Scholar] [CrossRef]
- Bruña, S.; González-Vadillo, A.M.; Nieto, D.; Pastor, J.C.; Cuadrado, I. Redox-active macrocyclic and linear oligo-carbosiloxanes prepared via hydrosilylation from 1,3-divinyl-1,3-dimethyl-1,3-diferrocenyldisiloxane. Macromolecules 2012, 45, 781–793. [Google Scholar] [CrossRef]
- Eloi, J.C.; Chabanne, L.; Whittell, G.R.; Manners, I. Metallopolymers with emerging applications. Mater. Today 2008, 11, 28–36. [Google Scholar] [CrossRef]
- Chai, H.; Hu, J.; Shi, Q.; Zhang, H. Synthesis and chiroptical properties of helical polyallenylferrocenes. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2663–2669. [Google Scholar] [CrossRef]
- Hailes, R.L.N.; Musgrave, R.A.; Kilpatrick, A.F.R.; Russell, A.D.; Whittell, G.R.; O’Hare, D.; Manners, I. Ring-opening polymerisation of low-strain nickelocenophanes: Synthesis and magnetic properties of polynickelocenes with carbon and silicon main chain spacers. Chem. Eur. J. 2019, 25, 1044–1054. [Google Scholar] [CrossRef]
- Williams, K.A.; Boydston, A.J.; Bielawski, C.W. Main-chain organometallic polymers: Synthetic strategies, applications, and perspectives. Chem. Soc. Rev. 2007, 36, 729–744. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, L.; Yan, Y.; Tang, C. Metal-containing and related polymers for biomedical applications. Chem. Soc. Rev. 2016, 45, 5232–5263. [Google Scholar]
- Hailes, R.L.N.; Oliver, A.M.; Gwyther, J.; Whittell, G.R.; Manners, I. Polyferrocenylsilanes: Synthesis, properties, and applications. Chem. Soc. Rev. 2016, 45, 5358–5407. [Google Scholar] [CrossRef]
- Musgrave, R.A.; Russell, A.D.; Hayward, D.W.; Whittell, G.R.; Lawrence, P.G.; Gates, P.J.; Green, J.C.; Manners, I. Main-chain metallopolymers at the static-dynamic boundary based on nickelocene. Nature Chem. 2017, 9, 743–750. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Honjo, K.; Kitagawa, S.; Gwyther, J.; Manners, I.; Uemura, T. Thermal ring-opening polymerization of an unsymmetrical silicon-bridged [1]ferrocenophane in coordination nanochannels. Chem. Comm. 2017, 53, 6945–6948. [Google Scholar] [CrossRef]
- Sharma, H.K.; Lee, F.C.; Mahmoud, J.S.; Pannell, K.H. (Bis{2,4,6-triisopropylphenyl}stannylene)ferrocenophane and related ring-opened products. Organometallics 1999, 18, 399–403. [Google Scholar] [CrossRef]
- Sha, Y.; Zhang, Y.; Zhu, T.; Tan, S.; Cha, Y.; Craig, S.L.; Tang, C. Ring-closing metathesis and ring-opening metathesis polymerization toward main-chain ferrocene-containing polymers. Macromolecules 2018, 51, 9131–9139. [Google Scholar] [CrossRef]
- Buretea, M.A.; Tilley, T.D. Poly(ferrocenylenevinylene) from ring-opening metathesis polymerization of ansa-(vinylene)ferrocene. Organometallics 1997, 16, 1507–1510. [Google Scholar] [CrossRef]
- Stanton, C.E.; Lee, T.R.; Grubbs, R.H.; Lewis, N.S. Routes to conjugated polymers with ferrocenes in their backbones: Synthesis and characterization of poly(ferrocenylenedivinylene) and poly(ferrocenylenebutenylene). Macromolecules 1995, 28, 8713–8721. [Google Scholar] [CrossRef]
- Heo, R.W.; Somoza, F.B.; Lee, T.R. Soluble conjugated polymers that contain ferrocenylene units in the backbone. J. Am. Chem. Soc. 1998, 120, 1621–1622. [Google Scholar] [CrossRef]
- Masson, G.; Lough, A.J.; Manners, I. Soluble poly(ferrocenylenevinylene) with t-butyl substituents on the cyclopentadienyl ligands via ring-opening metathesis polymerization. Macromolecules 2008, 41, 539–547. [Google Scholar] [CrossRef]
- Caire da Silva, L.; Rojas, G.; Schulz, M.D.; Wagener, K.B. Acyclic diene metathesis polymerization: History, methods and applications. Prog. Polym. Sci. 2017, 69, 79–107. [Google Scholar] [CrossRef]
- Liu, F.; Cao, J.; Sun, R.; Zhang, H. Ferrocene-containing polymers synthesized by acyclic diene metathesis (ADMET) polymerization. Chinese J. Polym. Sci. 2016, 34, 242–252. [Google Scholar]
- Weychardt, H.; Plenio, H. Acyclic diene metathesis polymerization of divinylarenes and divinylferrocenes with grubbs-type olefin metathesis catalysts. Organometallics 2008, 27, 1479–1485. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, L.; Zhao, Y.; Lin, J.; Chen, M. Palladium-catalyzed cross-coupling polymerization: A new access to cross-conjugated polymers with modifiable structure and tunable optical/conductive properties. Macromolecules 2018, 51, 9662–9668. [Google Scholar] [CrossRef]
- Holliday, B.J.; Swager, T.M. Conducting metallopolymers: The roles of molecular architecture and redox matching. Chem. Commun. 2005, 1, 23–26. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, X. Oligomer molecules for efficient organic photovoltaics. Acc. Chem. Res. 2016, 49, 175–183. [Google Scholar] [CrossRef]
- Kagota, D.; Haque, T.; Koinuma, M.; Inagaki, A.; Asano, M.S.; Nomur, K. Time-resolved fluorescence spectra in the end-functionalized conjugated triblock copolymers consisting of poly(fluorene vinylene) and oligo(phenylene vinylene): Proposal of dynamical distortion in the excited state. Macromolecules 2015, 48, 6233–6240. [Google Scholar]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Kraft, A.; Grimsdale, A.C.; Holmes, A.B. Electroluminescent conjugated polymers-seeing polymers in a new light. Angew. Chem. Int. Ed. 1998, 37, 402–428. [Google Scholar] [CrossRef]
- Barluenga, J.; Tomás-Gamasa, M.; Aznar, F.; Valdés, C. Metal-free carbon–carbon bond-forming reductive coupling between boronic acids and tosylhydrazones. Nat. Chem. 2009, 1, 494–499. [Google Scholar] [CrossRef]
- Scholl, M.; Ding, S.; Lee, C.W.; Grubbs, R.H. Synthesis and activity of a new generation of ruthenium-based olefin metathesis catalysts coordinated with 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene Ligands. Org. Lett. 1999, 1, 953–956. [Google Scholar] [CrossRef]
- Garber, S.B.; Kingsbury, J.S.; Gray, B.L.; Hoveyda, A.H. Efficient and recyclable monomeric and dendritic Ru-based metathesis catalysts. J. Am. Chem. Soc. 2000, 122, 8168–8179. [Google Scholar] [CrossRef]
- Heo, R.W.; Park, J.-S.; Goodson, J.T.; Claudio, G.C.; Takenaga, M.; Albright, T.A.; Lee, T.R. ROMP of t-butyl-substituted ferrocenophanes affords soluble conjugated polymers that contain ferrocene moieties in the backbone. Tetrahedron 2004, 60, 7225–7235. [Google Scholar] [CrossRef]
- Heo, R.W.; Park, J.-S.; Lee, T.R. Synthesis and ring-opening metathesis polymerization of aryl-substituted 1,1’-(1,3-butadienylene)ferrocenes. Macromolecules 2005, 38, 2564–2573. [Google Scholar] [CrossRef]
- Kvaranl, A.; Konradsson, A.E.; Evans, C.; Gerisson, J.K.F. 1H NMR and UV-Vis spectroscopy of chlorine substituted stilbenes: Conformational studies. J. Mol. Struct. 2000, 553, 79–90. [Google Scholar] [CrossRef]
- Mukherjee, N.; Peetz, R.M. Acyclic diene metathesis synthesis of silylene- and siloxane-containing conjugated polymers and macrocycles. Macromolecules 2008, 41, 6677–6685. [Google Scholar] [CrossRef]
- Liu, J.; Gao, B.; Cheng, Y.; Xie, Z.; Geng, Y.; Wang, L.; Jing, X.; Wang, F. Novel white electroluminescent single polymer derived from fluorene and quinacidone. Macromolecules 2008, 41, 1162–1167. [Google Scholar] [CrossRef]


| Entry | Catalyst | Solvent | Temp. (°C) | t (h) | Yield b (%) | Mn c (Da) | Mw c (Da) | Ðc |
|---|---|---|---|---|---|---|---|---|
| Monomer A | ||||||||
| 1 | G2 | toluene | 80 | 12 | 73 | 2001 | 2207 | 1.10 |
| 2 | G2 | toluene | 100 | 12 | 82 | 1714 | 1802 | 1.05 |
| 3 | G2 | toluene d | 80 | 24 | 81 | 1614 | 1666 | 1.03 |
| 4 | G2 | DCB | 100 | 12 | 83 | 2021 | 2250 | 1.06 |
| 5 | G-H2 | toluene | 80 | 12 | 78 | 1672 | 1745 | 1.04 |
| 6 | G-H2 | toluene | 100 | 12 | 80 | 1705 | 1788 | 1.05 |
| 7 | G2 | toluene/DCB | 80 | 24 | 76 | 1867 | 2029 | 1.09 |
| Monomer B | ||||||||
| 8 | G2 | toluene | 80 | 24 | 81 | 1714 | 1791 | 1.04 |
| 9 | G-H2 | toluene | 80 | 24 | 83 | 1372 | 1377 | 1.00 |
| 10 | G2 | toluene/DCB | 80 | 24 | 79 | 2160 | 2374 | 1.10 |
| Monomer C | ||||||||
| 11 | G2 | toluene | 80 | 24 | 76 | 2752 | 3558 | 1.29 |
| 12 | G3 | toluene/DCB | 60 | 24 | 79 | 3355 | 3724 | 1.11 |
| 13 | G3 | toluene/DCB | 60 | 48 | 75 | 3360 | 4066 | 1.21 |
| Entry | Substance | A | ε (Lmol−1cm−1) | Equation a |
|---|---|---|---|---|
| 1 | Oligomer A | 0.39 | 7800 | ε = A/BC (1) |
| 2 | Oligomer B | 0.388 | 7760 | |
| 3 | Oligomer C | 0.386 | 7720 | |
| 4 | Copolymer 1 | 1.05 | 21000 |
| Compound | Oxidation | Reduction | ||
|---|---|---|---|---|
| I (μA) | E (V) | I (μA) | E (V) | |
| Oligomer A | 1.02 | 0.57 | 0.99 | 0.43 |
| Oligomer B | 0.55 | 0.55 | 0.53 | 0.48 |
| Oligomer C | 0.57 | 0.54 | 0.56 | 0.47 |
| Entry | MA/MD | Yield (%) b | Mn (Da) c | Mw (Da) c | Ð c | x d | y d |
|---|---|---|---|---|---|---|---|
| 1 | 1:4 | 76 | 13453 | 23824 | 1.77 | 5 | 25 |
| 2 | 1:2 | 80 | 6602 | 10893 | 1.65 | 2 | 12 |
| 3 | 1:1 | 78 | 2610 | 3973 | 1.52 | 2 | 4 |
| 4 | 2:1 | 79 | 2436 | 2901 | 1.19 | 3 | 2 |
| 5 | 4:1 | 75 | 1896 | 2058 | 1.09 | 3 | 1 |
| Entry | x a | y b | E (V) |
|---|---|---|---|
| 1 | 2 | 4 | 0.627 |
| 2 | 2 | 12 | 0.577 |
| 3 | 3 | 1 | 0.663 |
| 4 | 3 | 2 | 0.661 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Deng, L.; Hu, J.; Zhang, H. Ferrocene-Containing Conjugated Oligomers Synthesized by Acyclic Diene Metathesis Polymerization. Polymers 2019, 11, 1334. https://doi.org/10.3390/polym11081334
Gao X, Deng L, Hu J, Zhang H. Ferrocene-Containing Conjugated Oligomers Synthesized by Acyclic Diene Metathesis Polymerization. Polymers. 2019; 11(8):1334. https://doi.org/10.3390/polym11081334
Chicago/Turabian StyleGao, Xin, Lei Deng, Jianfeng Hu, and Hao Zhang. 2019. "Ferrocene-Containing Conjugated Oligomers Synthesized by Acyclic Diene Metathesis Polymerization" Polymers 11, no. 8: 1334. https://doi.org/10.3390/polym11081334
APA StyleGao, X., Deng, L., Hu, J., & Zhang, H. (2019). Ferrocene-Containing Conjugated Oligomers Synthesized by Acyclic Diene Metathesis Polymerization. Polymers, 11(8), 1334. https://doi.org/10.3390/polym11081334