Synthetic Environmentally Friendly Castor Oil Based-Polyurethane with Carbon Black as a Microphase Separation Promoter
Abstract
1. Introduction
2. Experimental
2.1. Materials
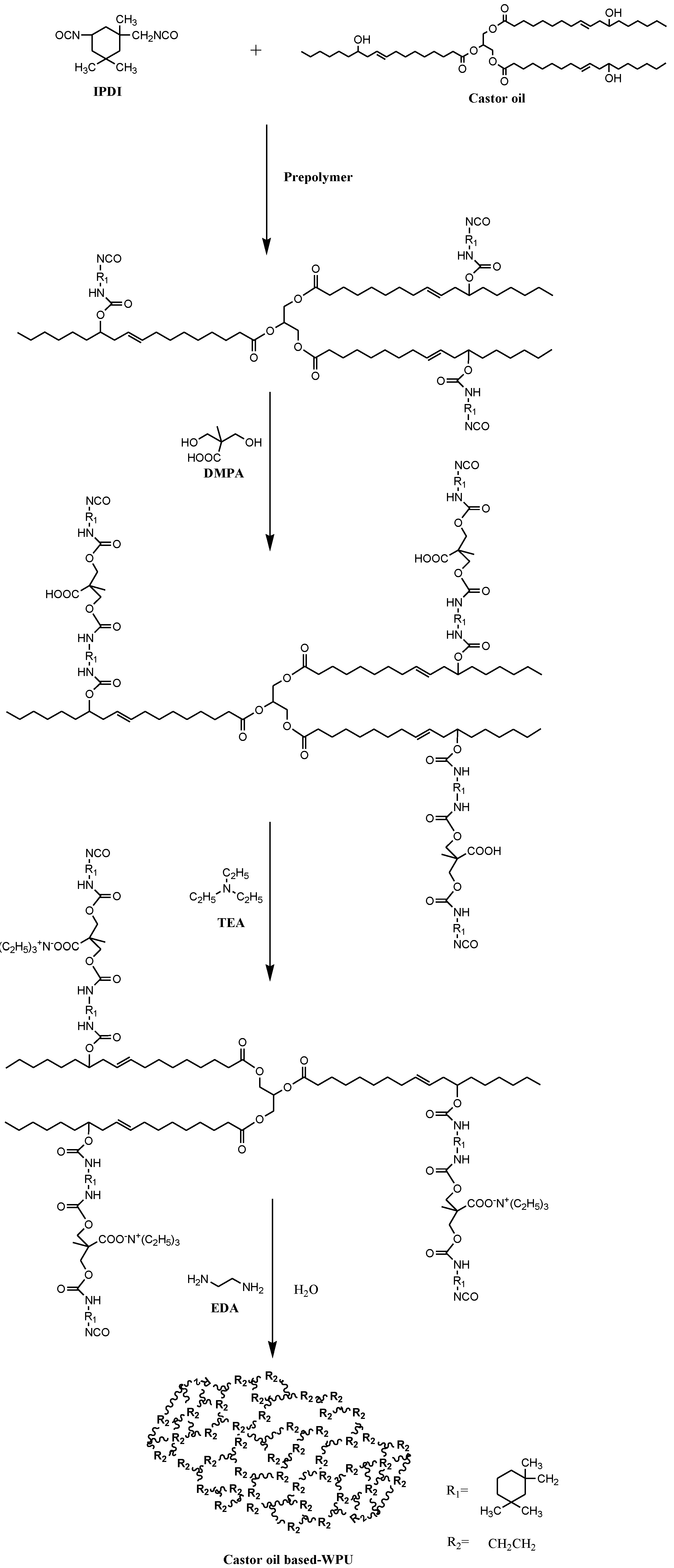
2.2. Synthesis of CB/WPUs
2.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4. Surface Roughness Analysis
2.5. Thermogravimetric Analysis (TGA)
2.6. Conductivities
2.7. Dynamic Mechanical Analysis (DMA)
2.8. Stress–Strain Testing
2.9. Hydrolytic Degradation Tests
2.10. Morphology Analysis
3. Results and Discussion
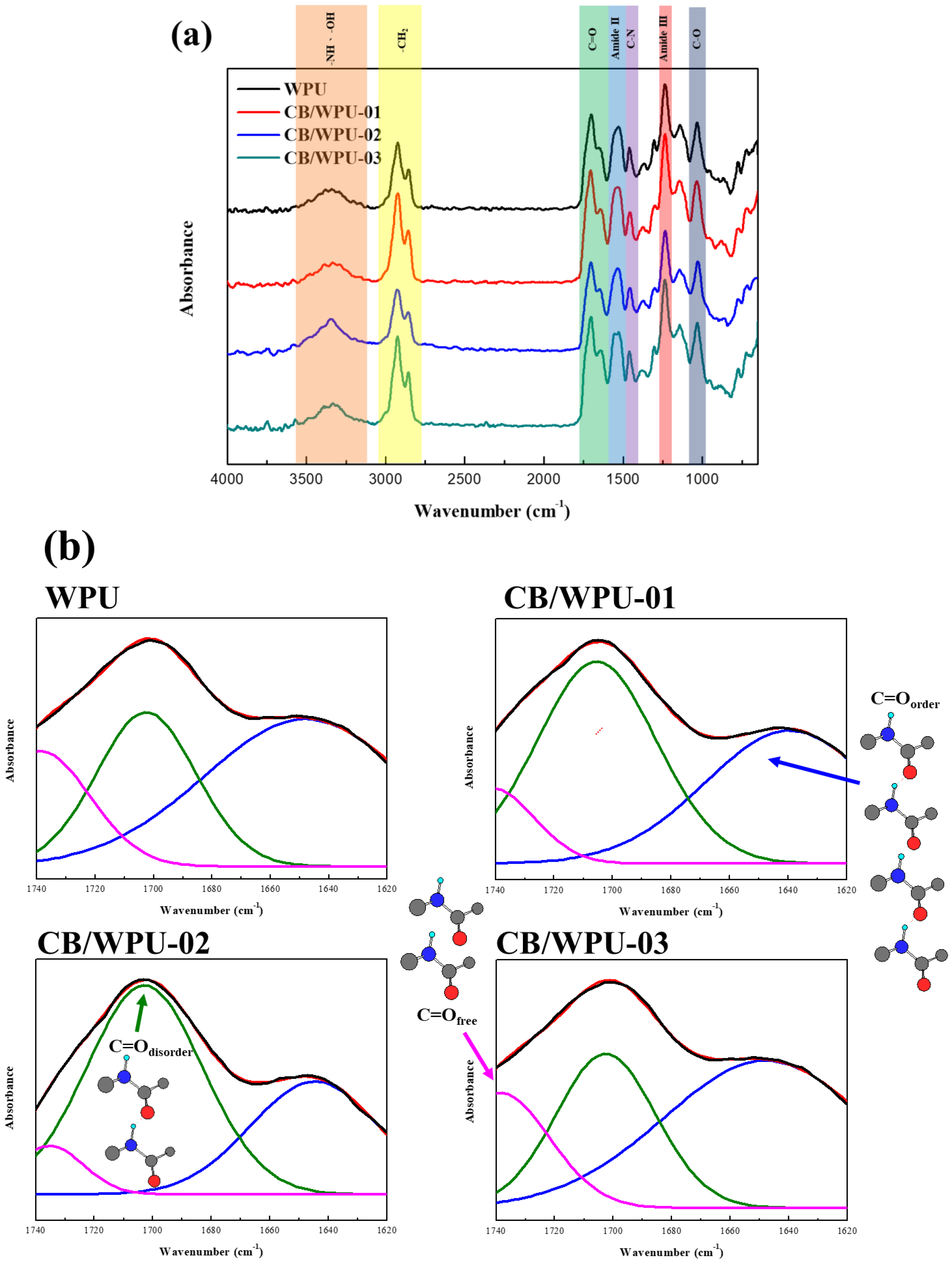
3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
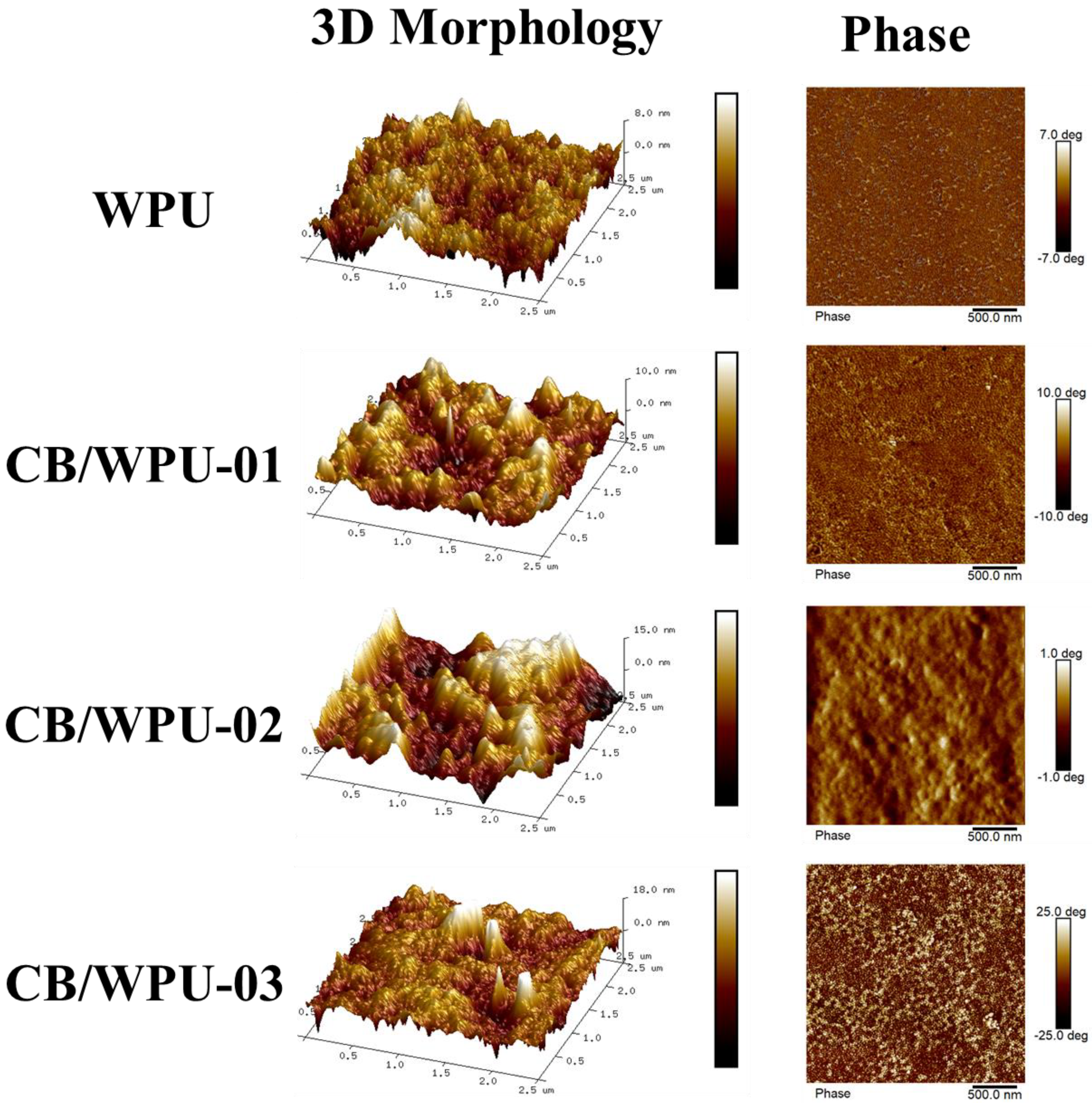
3.2. Surface Roughness and Electrical Resistance Analysis
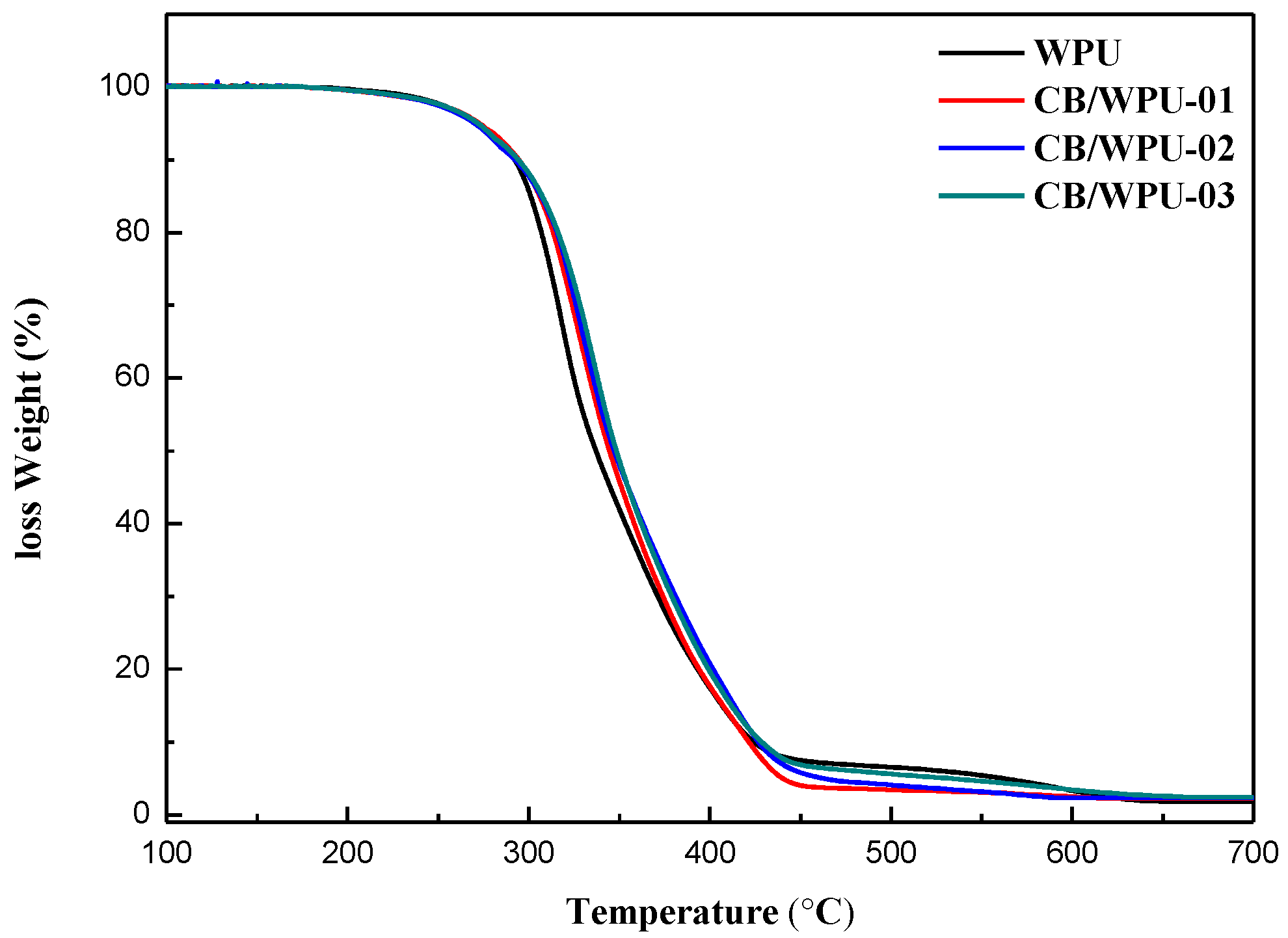
3.3. Thermal Properties
3.4. Dynamic Mechanical Analysis (DMA)
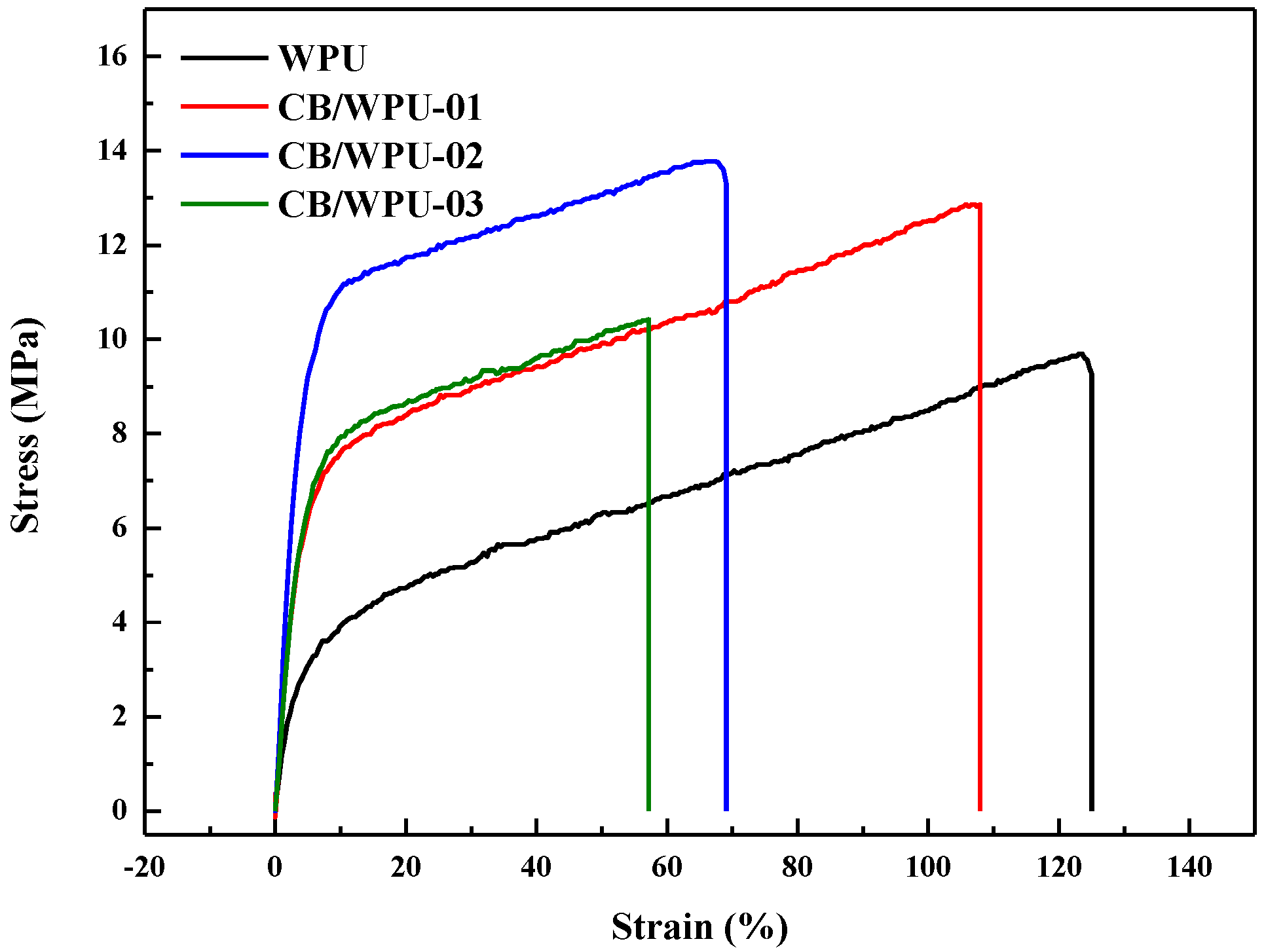
3.5. Tensile Properties
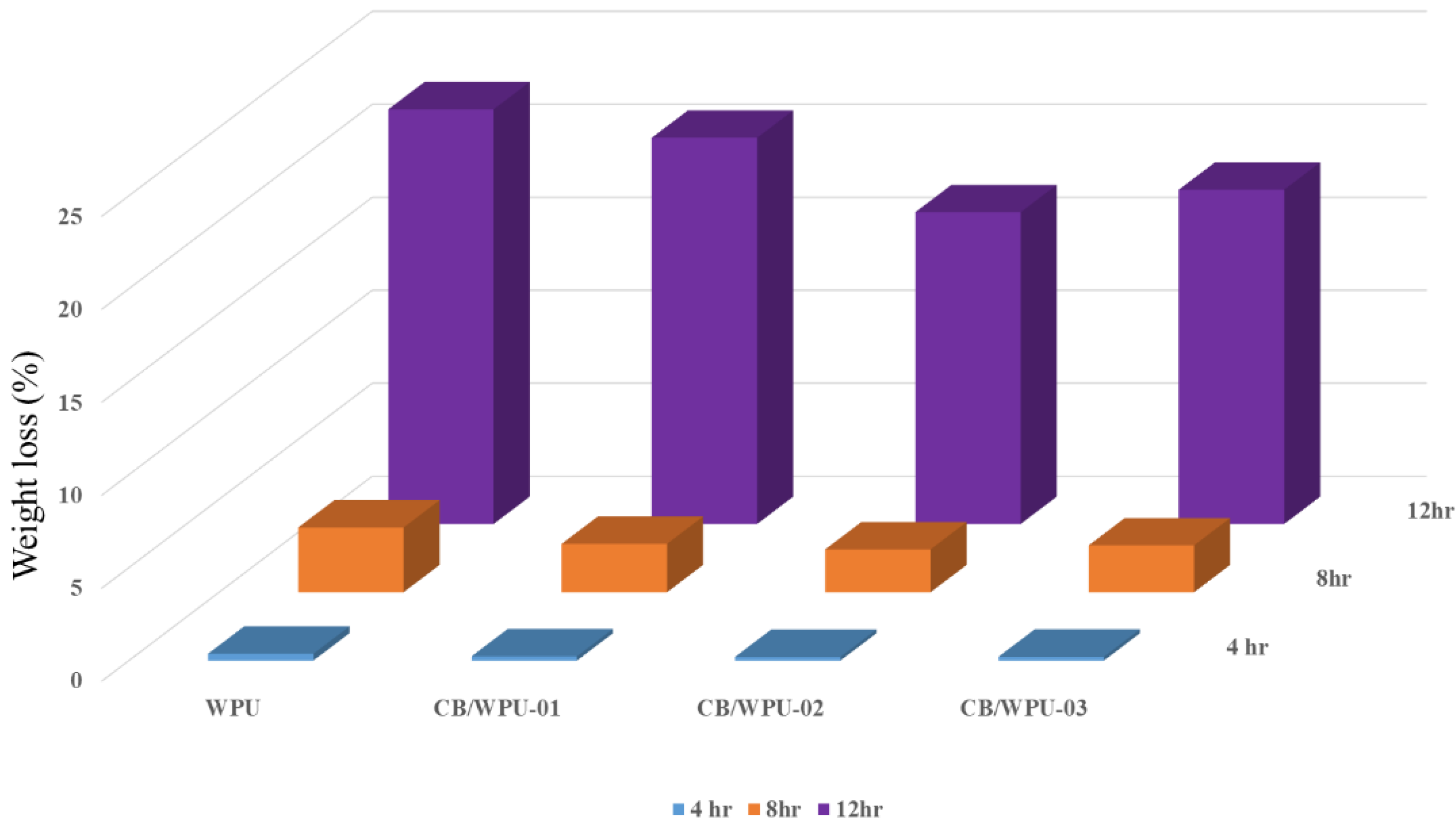
3.6. Hydrolytic Degradation
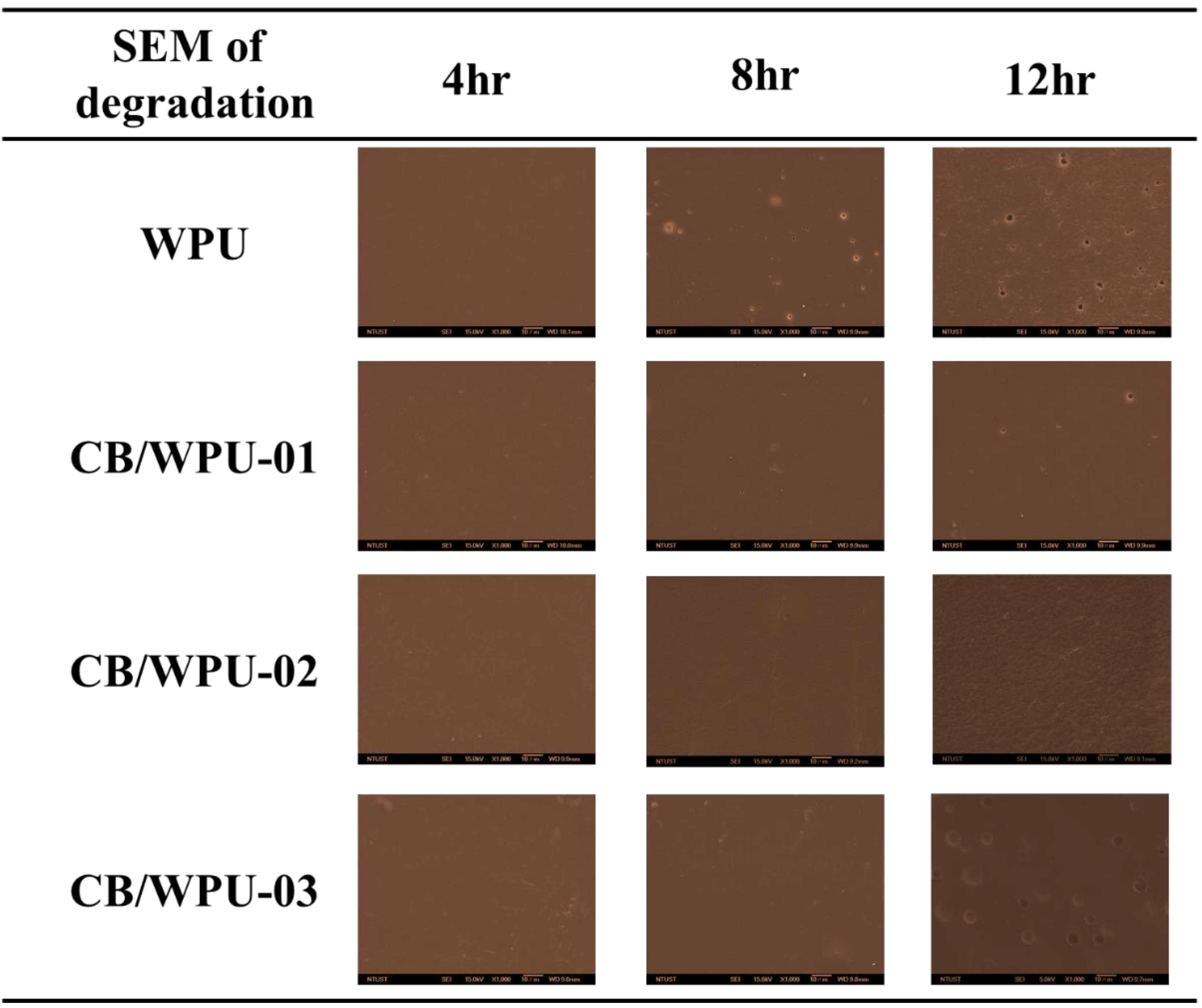
3.7. SEM Morphology Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Samarth, N.B.; Mahanwar, P.A. Modified vegetable oil based additives as a future polymeric material. Open. J. Org. Polym. Mater. 2015, 5, 1–22. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Wound healing bionanocomposites based on castor oil polymeric films reinforced with chitosan-modified ZnO nanoparticles. Biomacromolecules 2015, 16, 2631–2644. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Ahmad, A.; Mohamed, N. Characterization of novel castor oil-based polyurethane polymer electrolytes. Polymers 2015, 7, 747–759. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, L.; Wang, Z.; Wilbon, P.A.; Wang, C.; Chu, F.; Tang, C. Lignin and soy oil-derived polymeric biocomposites by “grafting from” RAFT polymerization. Green Chem. 2016, 18, 4974–4981. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Isocyanate terminated castor oil-based polyurethane prepolymer: Synthesis and characterization. Prog. Org. Coat. 2015, 80, 39–48. [Google Scholar] [CrossRef]
- Rojas, M.F.; Miranda, L.P.; Ramirez, A.M.; Quintero, K.P.; Bernard, F.; Einloft, S.; Carreño Díaz, L.A. New biocomposites based on castor oil polyurethane foams and ionic liquids for CO2 capture. Fluid Phase Equilib. 2017, 452, 103–112. [Google Scholar] [CrossRef]
- Nguyen Dang, L.; Le Hoang, S.; Malin, M.; Weisser, J.; Walter, T.; Schnabelrauch, M.; Seppälä, J. Synthesis and characterization of castor oil-segmented thermoplastic polyurethane with controlled mechanical properties. Eur. Polym. J. 2016, 81, 129–137. [Google Scholar] [CrossRef]
- Dave, V.J.; Patel, H.S. Synthesis and characterization of interpenetrating polymer networks from transesterified castor oil based polyurethane and polystyrene. J. Saudi Chem. Soc. 2017, 21, 18–24. [Google Scholar] [CrossRef]
- Li, S.; Xu, C.; Yang, W.; Tang, Q. Thermoplastic Polyurethanes Stemming from Castor Oil: Green Synthesis and Their Application in Wood Bonding. Coatings 2017, 7, 159. [Google Scholar]
- Ionescu, M.; Radojčić, D.; Wan, X.; Shrestha, M.L.; Petrović, Z.S.; Upshaw, T.A. Highly functional polyols from castor oil for rigid polyurethanes. Eur. Polym. J. 2016, 84, 736–749. [Google Scholar] [CrossRef]
- Chen, G.; Guan, X.; Xu, R.; Tian, J.; He, M.; Shen, W.; Yang, J. Synthesis and characterization of UV-curable castor oil-based polyfunctional polyurethane acrylate via photo-click chemistry and isocyanate polyurethane reaction. Prog. Org. Coat. 2016, 93, 11–16. [Google Scholar] [CrossRef]
- Otto, G.P.; Moisés, M.P.; Carvalho, G.; Rinaldi, A.W.; Garcia, J.C.; Radovanovic, E.; Fávaro, S.L. Mechanical properties of a polyurethane hybrid composite with natural lignocellulosic fibers. Compos. Part B 2017, 110, 459–465. [Google Scholar] [CrossRef]
- Ashrafizadeh, H.; Mertiny, P.; McDonald, A. Evaluation of the effect of temperature on mechanical properties and wear resistance of polyurethane elastomers. Wear 2016, 368, 26–38. [Google Scholar] [CrossRef]
- Zia, F.; Zia, K.M.; Zuber, M.; Kamal, S.; Aslam, N. Starch based polyurethanes: A critical review updating recent literature. Carbohydr. Polym. 2015, 134, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Du, Z.; Wang, H.; Cheng, X. Preparation of waterborne polyurethane nanocomposite reinforced with halloysite nanotubes for coating applications. J. Appl. Polym. Sci. 2016, 133, 43949. [Google Scholar] [CrossRef]
- Liu, H.; Huang, W.; Yang, X.; Dai, K.; Zheng, G.; Liu, C.; Shen, C.; Yan, X.; Guo, J.; Guo, Z. Organic vapor sensing behaviors of conductive thermoplastic polyurethane–graphene nanocomposites. J. Mater. Chem. C 2016, 4, 4459–4469. [Google Scholar] [CrossRef]
- Li, J.W.; Lee, H.T.; Tsai, H.A.; Suen, M.C.; Chiu, C.W. Synthesis and Properties of Novel Polyurethanes Containing Long-Segment Fluorinated Chain Extenders. Polymers 2018, 10, 1292. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Q.; Jing, B.; Cai, Y.; Liu, P.; Hu, J. Effect of microphase-separation promoters on the shape-memory behavior of polyurethane. J. Appl. Polym. Sci. 2006, 102, 5224–5231. [Google Scholar] [CrossRef]
- Xia, H.; Song, M.; Zhang, Z.; Richardson, M. Microphase separation, stress relaxation, and creep behavior of polyurethane nanocomposites. J. Appl. Polym. Sci. 2007, 103, 2992–3002. [Google Scholar] [CrossRef]
- Hosseini-Sianaki, T.; Nazockdast, H.; Salehnia, B.; Nazockdast, E. Microphase separation and hard domain assembly in thermoplastic polyurethane/multiwalled carbon nanotube nanocomposites. Polym. Eng. Sci. 2015, 55, 2163–2173. [Google Scholar] [CrossRef]
- Landa, M.; Canales, J.; Fernández, M.; Muñoz, M.E.; Santamaría, A. Effect of MWCNTs and graphene on the crystallization of polyurethane based nanocomposites, analyzed via calorimetry, rheology and AFM microscopy. Polym. Test. 2014, 35, 101–108. [Google Scholar] [CrossRef]
- Ghosh, B.; Gogoi, S.; Thakur, S.; Karak, N. Bio-based waterborne polyurethane/carbon dot nanocomposite as a surface coating material. Prog. Org. Coat. 2016, 90, 324–330. [Google Scholar] [CrossRef]
- Liu, K.; Miao, S.; Su, Z.; Sun, L.; Ma, G.; Zhang, S. Castor oil-based waterborne polyurethanes with tunable properties and excellent biocompatibility. Eur. J. Lipid Sci. Technol. 2016, 118, 1512–1520. [Google Scholar] [CrossRef]
- Wang, G.; Fu, Y.; Guo, A.; Mei, T.; Wang, J.; Li, J.; Wang, X. Reduced graphene oxide–polyurethane nanocomposite foam as a reusable photoreceiver for efficient solar steam generation. Chem. Mater. 2017, 29, 5629–5635. [Google Scholar] [CrossRef]
- Dong, M.; Li, Q.; Liu, H.; Liu, C.; Wujcik, E.K.; Shao, Q.; Ding, T.; Mai, X.; Shen, C.; Guo, Z. Thermoplastic polyurethane-carbon black nanocomposite coating: Fabrication and solid particle erosion resistance. Polymer 2018, 158, 381–390. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Y.; Li, X.; Chen, W. Synthesis of vegetable oil-based waterborne polyurethane/silver-halloysite antibacterial nanocomposites. Compos. Sci. Technol. 2016, 126, 86–93. [Google Scholar] [CrossRef]
- Tsou, C.H.; Wu, C.S.; Hung, W.S.; De Guzman, M.R.; Gao, C.; Wang, R.Y.; Chen, J.; Wan, N.; Peng, Y.J.; Suen, M.C. Rendering polypropylene biocomposites antibacterial through modification with oyster shell powder. Polymer 2019, 160, 265–271. [Google Scholar] [CrossRef]
- Liu, H.; Huang, W.; Gao, J.; Dai, K.; Zheng, G.; Liu, C.; Shen, C.; Yan, X.; Guo, J.; Guo, Z. Piezoresistive behavior of porous carbon nanotube-thermoplastic polyurethane conductive nanocomposites with ultrahigh compressibility. Appl. Phys. Lett. 2016, 108, 011904. [Google Scholar]
- Shearouse, W.C.; Lillie, L.M.; Reineke, T.M.; Tolman, W.B. Sustainable polyesters derived from glucose and castor oil: Building block structure impacts properties. ACS Macro Lett. 2015, 4, 284–288. [Google Scholar] [CrossRef]
- Lillie, L.M.; Tolman, W.B.; Reineke, T.M. Degradable and renewably-sourced poly(ester-thioethers) by photo-initiated thiol–ene polymerization. Polym. Chem. 2018, 9, 3272–3278. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, X.; Wang, H.; Liu, Y.; Du, Z. Surface and mechanical properties of waterborne polyurethane films reinforced by hydroxyl-terminated poly(fluoroalkyl methacrylates). Polymer 2017, 133, 68–77. [Google Scholar] [CrossRef]

| Designation | IPDI (moles) | CO (mole) | DMPA (moles) | EDA (moles) | TEA (moles) | CB (wt.%) |
|---|---|---|---|---|---|---|
| WPU | 4 | 1.2 | 0.8 | 0.8 | 0.6 | 0 |
| CB/WPU-01 | 4 | 1.2 | 0.8 | 0.8 | 0.6 | 1 |
| CB/WPU-02 | 4 | 1.2 | 0.8 | 0.8 | 0.6 | 2 |
| CB/WPU-03 | 4 | 1.2 | 0.8 | 0.8 | 0.6 | 3 |
| Designation | TGA | FTIR | |||||
|---|---|---|---|---|---|---|---|
| Tonset (℃) | T50 (℃) | 700 ℃ Residue | C=Oorder | C=Odiorder | C=Ofree | H-Bond Content | |
| WPU | 290.49 | 337.05 | 1.81% | 53.07% | 26.89% | 20.04% | 79.96% |
| CB/WPU-01 | 294.89 | 344.55 | 2.24% | 42.33% | 45.94% | 11.73% | 88.27% |
| CB/WPU-02 | 297.13 | 347.21 | 2.30% | 34.45% | 57.83% | 7.72% | 92.28% |
| CB/WPU-03 | 299.58 | 348.43 | 2.45% | 57.55% | 27.35% | 15.10% | 84.90% |
| Sample | Tgd from Tanδ (℃) | Tan δmax | Electrical Resistance (ohm) |
|---|---|---|---|
| WPU | 52.66 | 0.403 | -- a |
| CB/WPU-01 | 59.36 | 0.400 | 2.01 106 |
| CB/WPU-02 | 59.41 | 0.398 | 8.41 105 |
| CB/WPU-03 | 55.82 | 0.384 | 3.99 104 |
| Sample | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| WPU | 9.7 | 0.85 | 123 |
| CB/WPU-01 | 12.9 | 1.39 | 108 |
| CB/WPU-02 | 13.8 | 3.64 | 69 |
| CB/WPU-03 | 10.4 | 1.77 | 60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-W.; Tsen, W.-C.; Tsou, C.-H.; Suen, M.-C.; Chiu, C.-W. Synthetic Environmentally Friendly Castor Oil Based-Polyurethane with Carbon Black as a Microphase Separation Promoter. Polymers 2019, 11, 1333. https://doi.org/10.3390/polym11081333
Li J-W, Tsen W-C, Tsou C-H, Suen M-C, Chiu C-W. Synthetic Environmentally Friendly Castor Oil Based-Polyurethane with Carbon Black as a Microphase Separation Promoter. Polymers. 2019; 11(8):1333. https://doi.org/10.3390/polym11081333
Chicago/Turabian StyleLi, Jia-Wun, Wen-Chin Tsen, Chi-Hui Tsou, Maw-Cherng Suen, and Chih-Wei Chiu. 2019. "Synthetic Environmentally Friendly Castor Oil Based-Polyurethane with Carbon Black as a Microphase Separation Promoter" Polymers 11, no. 8: 1333. https://doi.org/10.3390/polym11081333
APA StyleLi, J.-W., Tsen, W.-C., Tsou, C.-H., Suen, M.-C., & Chiu, C.-W. (2019). Synthetic Environmentally Friendly Castor Oil Based-Polyurethane with Carbon Black as a Microphase Separation Promoter. Polymers, 11(8), 1333. https://doi.org/10.3390/polym11081333