Electrically Conductive, Transparent Polymeric Nanocomposites Modified by 2D Ti3C2Tx (MXene)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation coPA/MXene Composites
2.3. Characterization
3. Results and Discussion
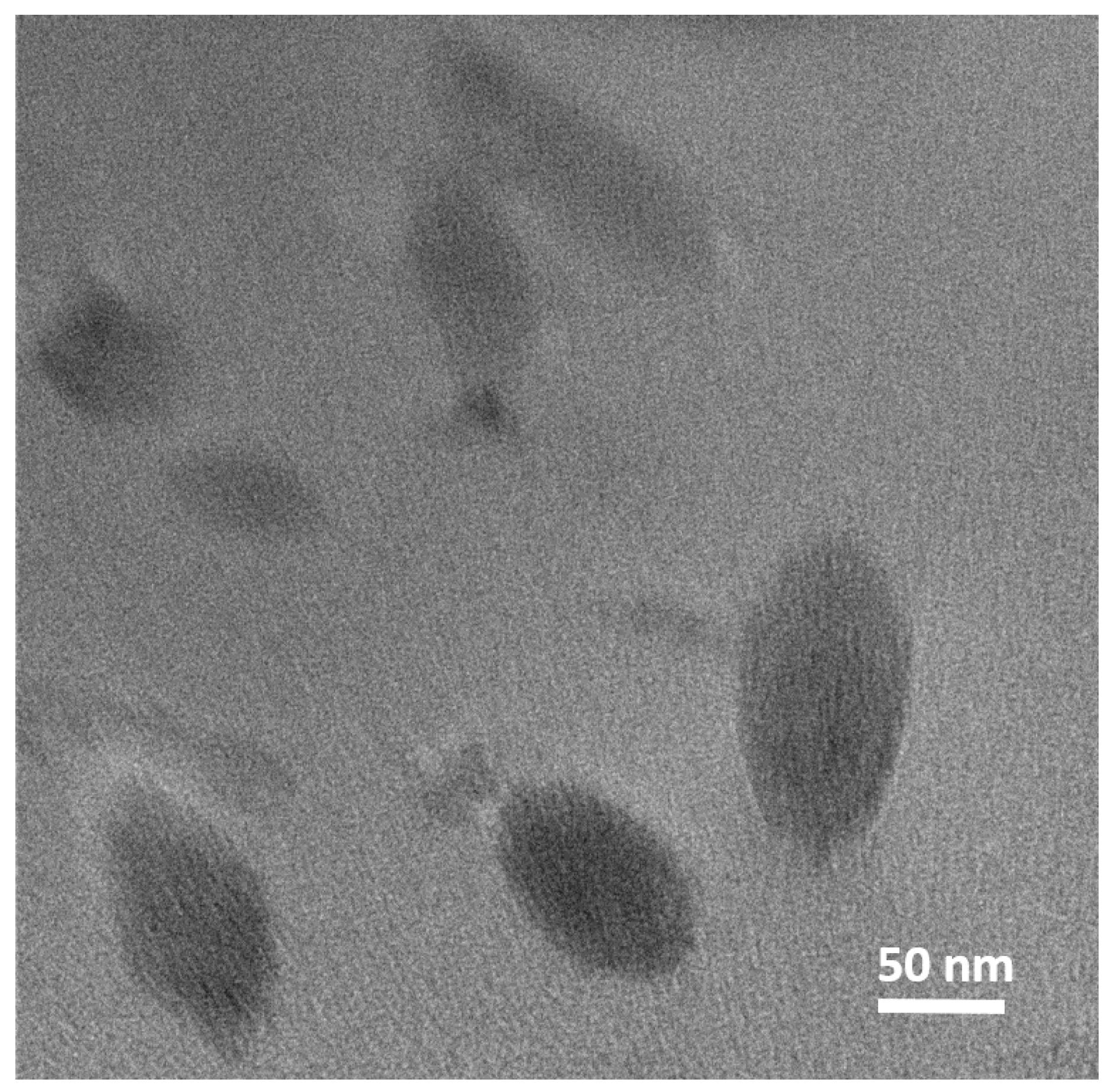
3.1. MXene Characterization
3.2. Composite Characterization
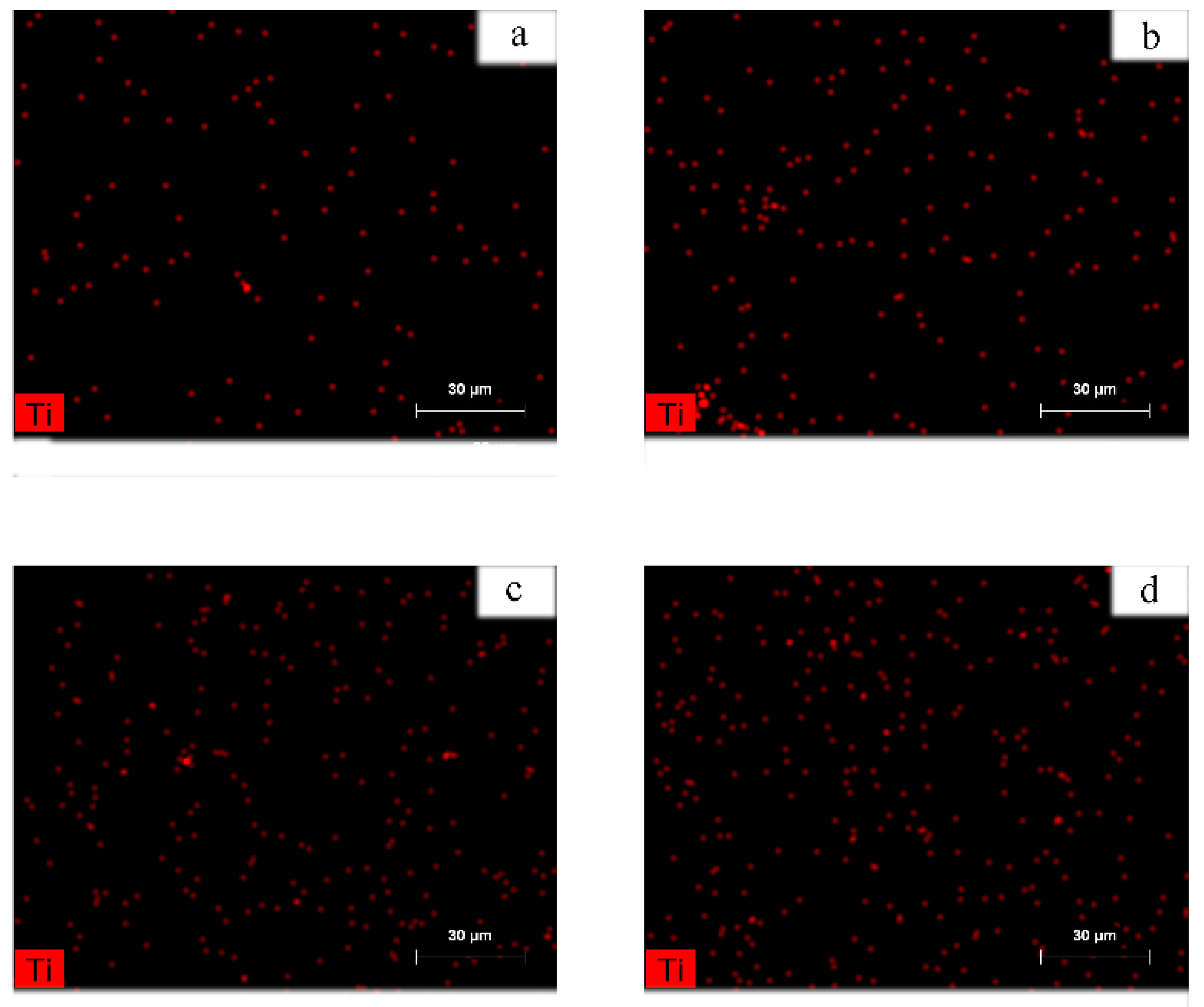
3.2.1. MXene Dispersion
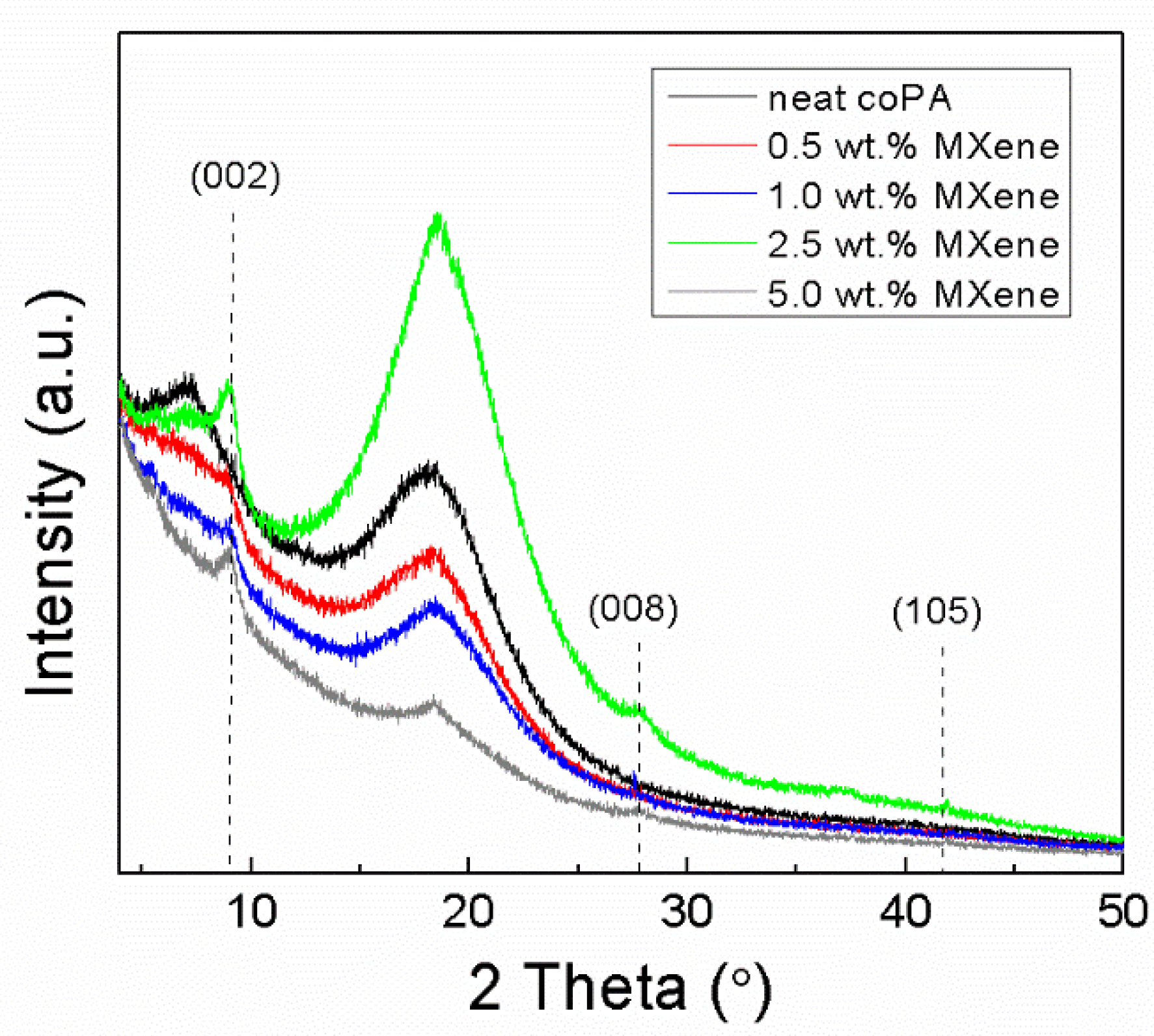
3.2.2. XRD Analysis of Composite Films
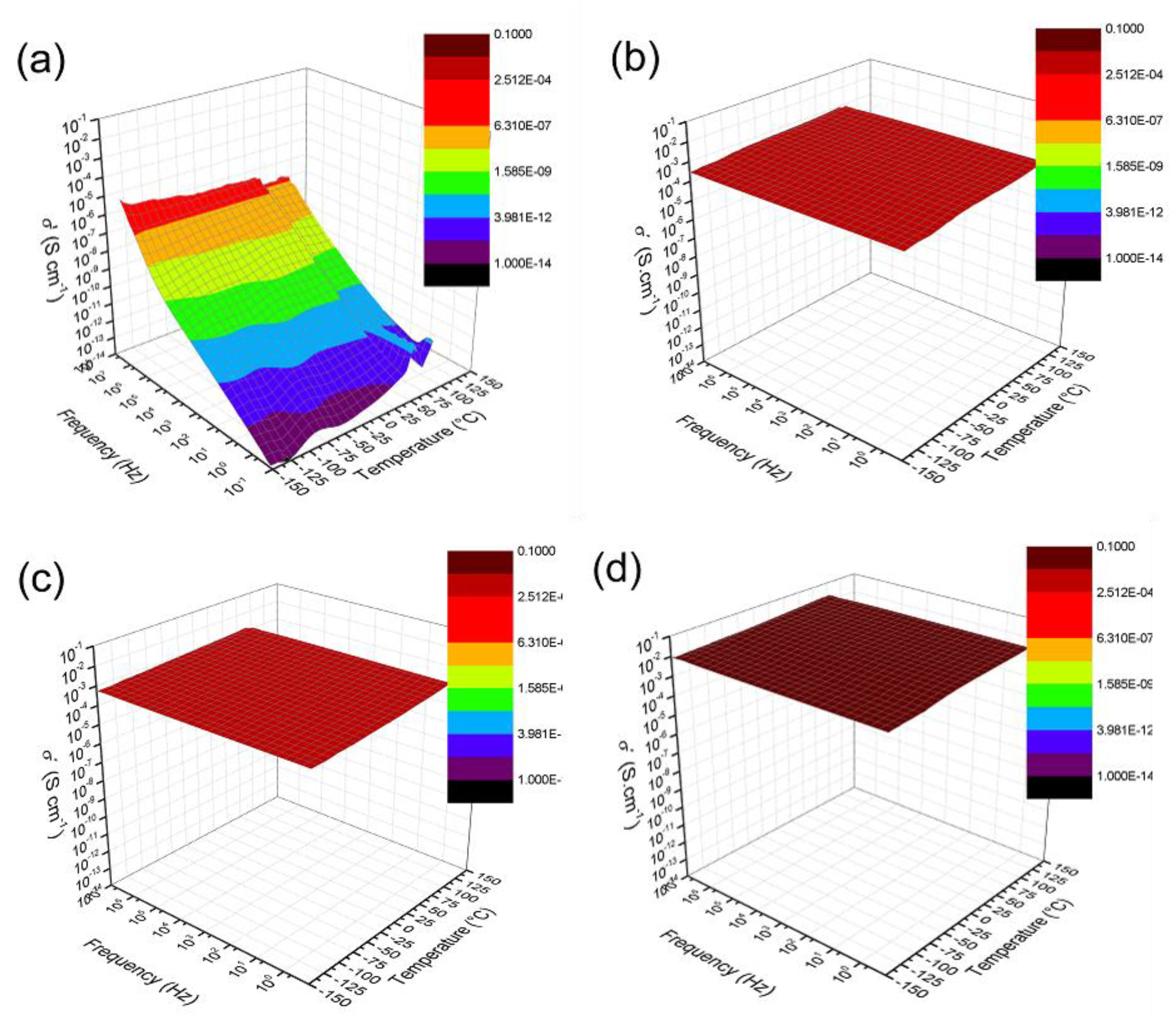
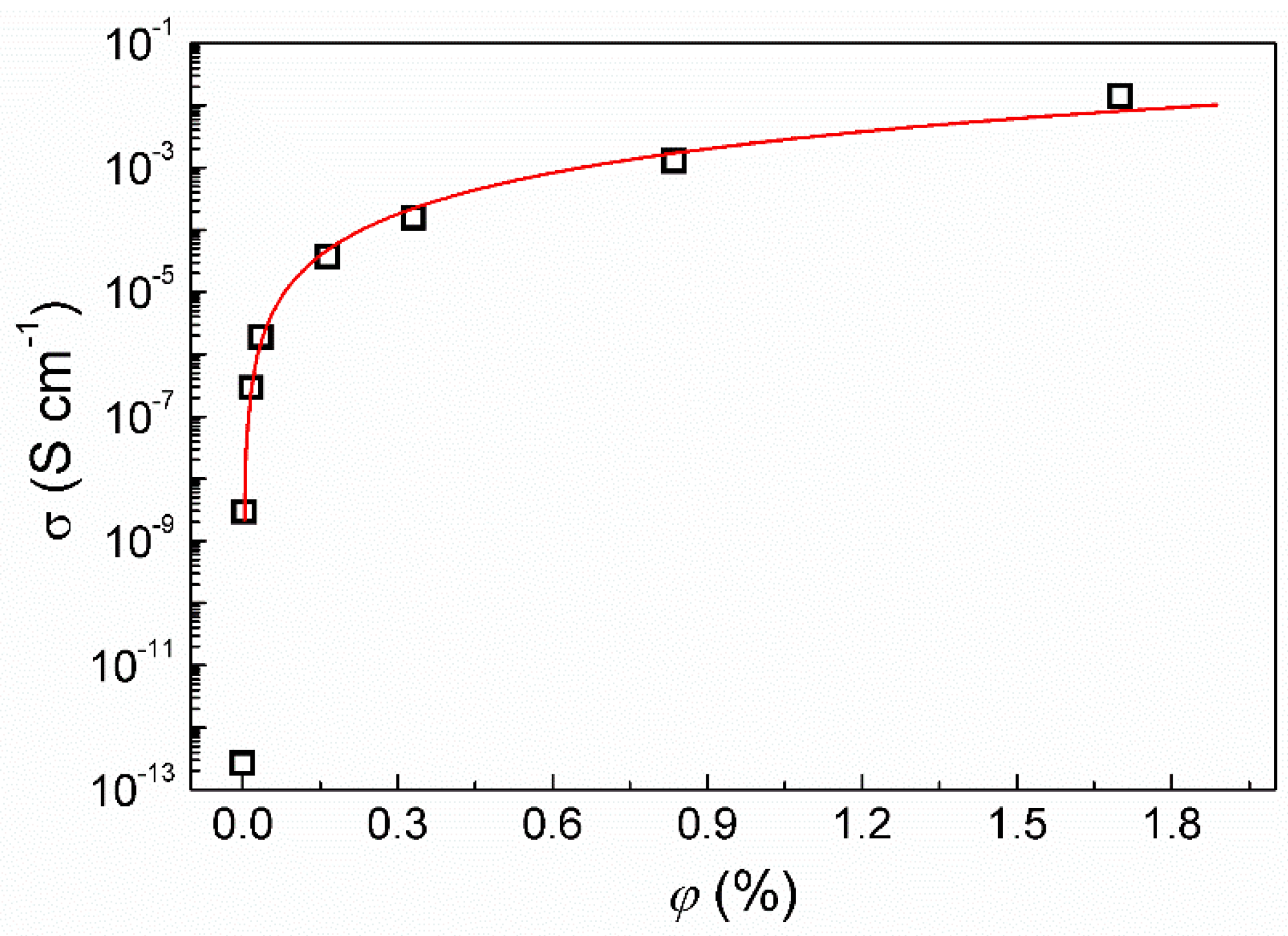
3.2.3. Electrical Conductivity Measurements
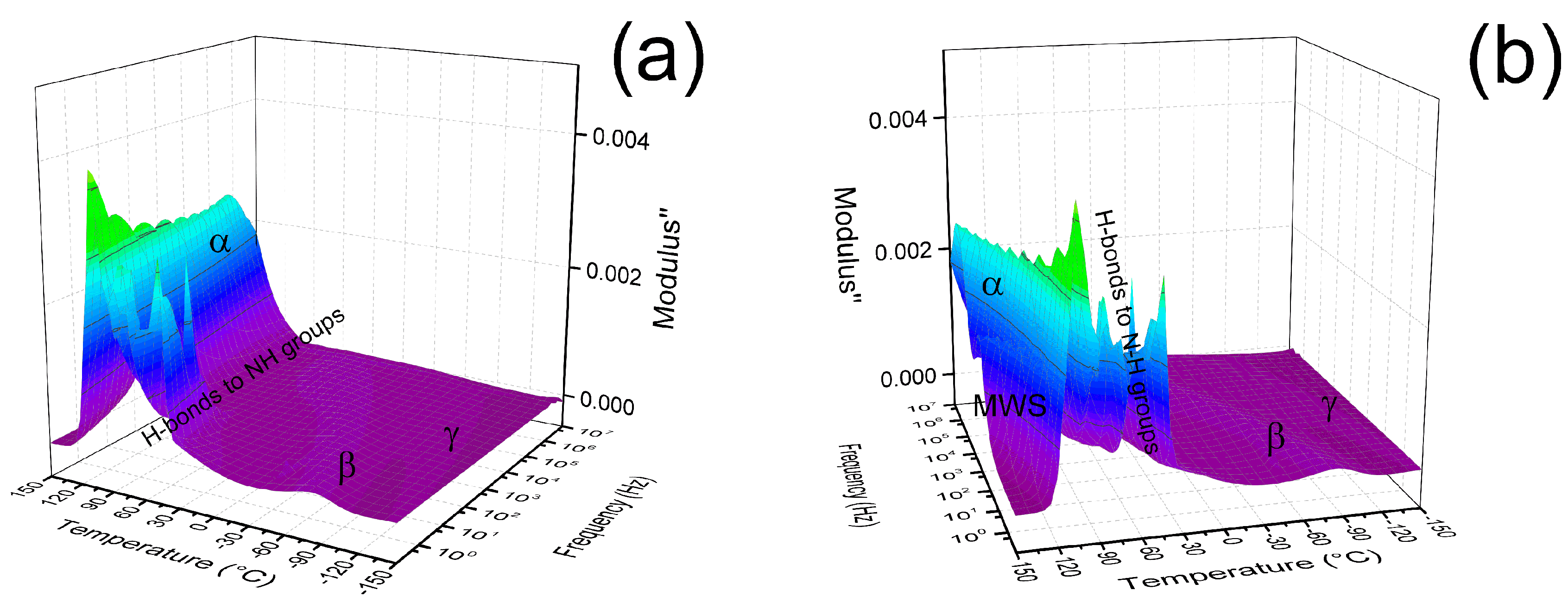
3.2.4. Dielectric Properties
3.2.5. Charge Transport Mechanism
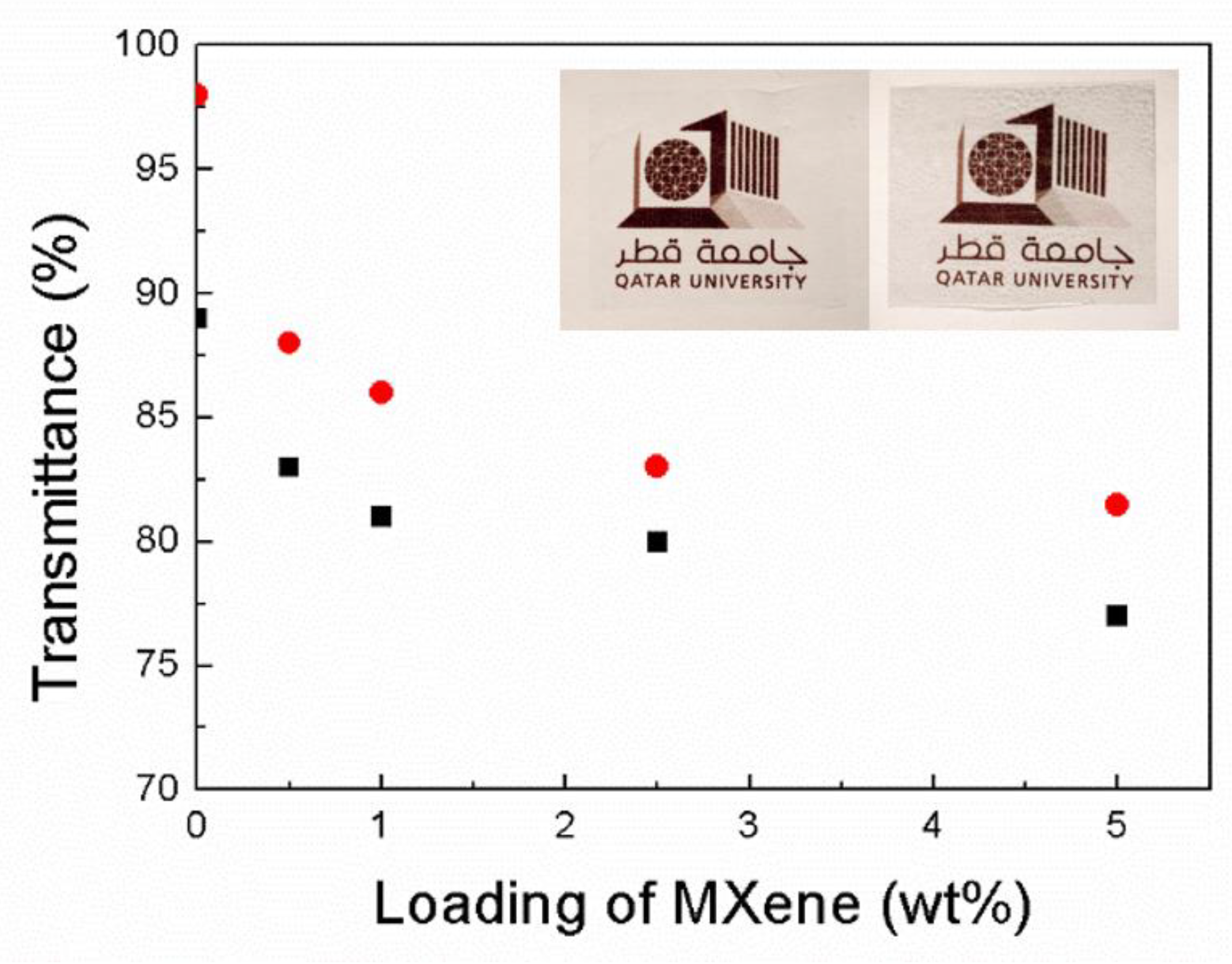
3.2.6. UV/Vis Characterization
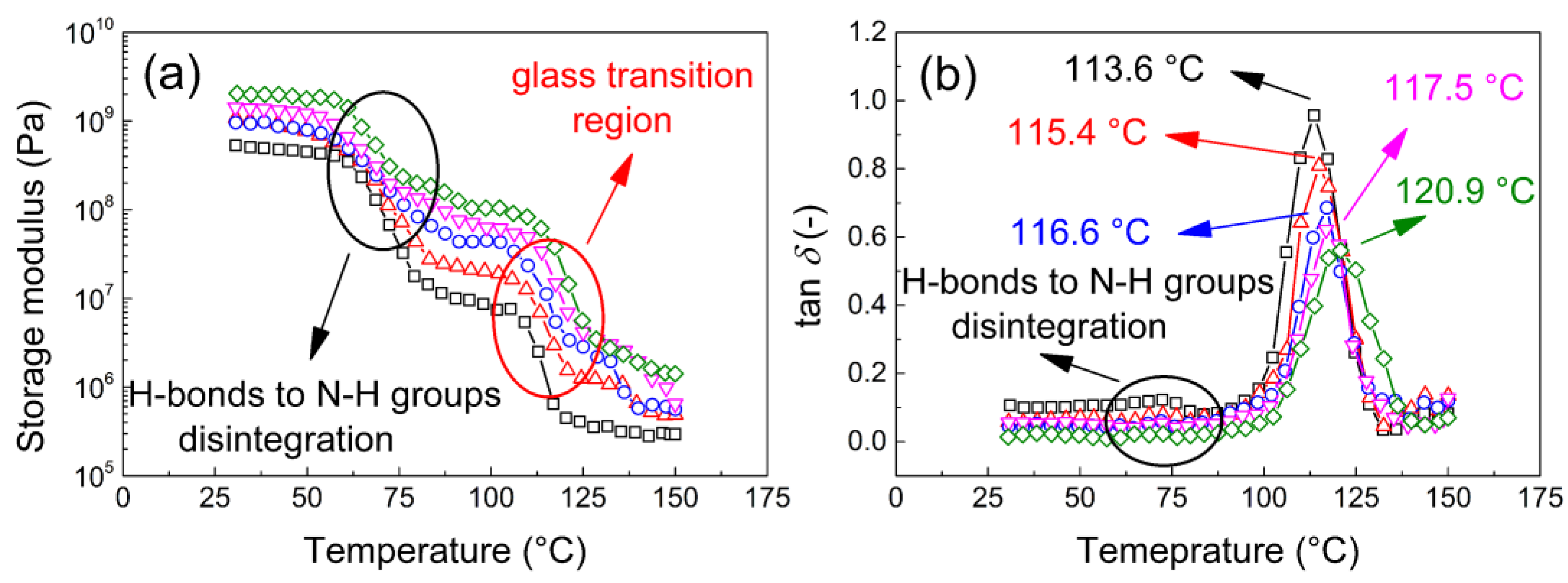
3.2.7. Dynamic Mechanical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Chen, F.; Wan, P.; Xu, H.; Sun, X. Flexible Transparent Supercapacitors Based on Hierarchical Nanocomposite Films. ACS Appl. Mater. Interfaces 2017, 9, 17865–17871. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-W.; Xiao, H.-M.; Fu, S.-Y. Wearable Electronics of Silver-Nanowire/Poly(dimethylsiloxane) Nanocomposite for Smart Clothing. Sci. Rep. 2015, 5, 13971. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-K.; Son, D.; Kim, J.; Yoo, Y.J.; Lee, G.J.; Wang, L.; Choi, M.K.; Yang, J.; Lee, M.; Do, K.; et al. Wearable Force Touch Sensor Array Using a Flexible and Transparent Electrode. Adv. Funct. Mater. 2017, 27, 1605286. [Google Scholar] [CrossRef]
- Kumar, A.; Zhou, C. The Race To Replace Tin-Doped Indium Oxide: Which Material will Win? ACS Nano 2010, 4, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Xu, P.; Faghaninia, A.; Shukla, S.; Ager, J.W.; Lo, C.S.; Jalan, B. Wide bandgap BaSnO3 films with room temperature conductivity exceeding 104 S cm−1. Nat. Commun. 2017, 8, 15167. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, B.; Journal, K.A.-S. Carbon nanotubes and nanofibers in composite materials. SAMPE 2002, 38, 59–70. [Google Scholar]
- Ong, B.S.; Wu, Y.; Liu, P.; Gardner, S. High-Performance Semiconducting Polythiophenes for Organic Thin-Film Transistors. J. Am. Chem. Soc. 2004, 126, 3378–3379. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, I.; Heeney, M.; Bailey, C.; Genevicius, K.; MacDonald, I.; Shkunov, M.; Sparrowe, D.; Tierney, S.; Wagner, R.; Zhang, W.; et al. Liquid-crystalline semiconducting polymers with high charge-carrier mobility. Nat. Mater. 2006, 5, 328–333. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Z.; Sun, H.; Gao, C. Highly Electrically Conductive Ag-Doped Graphene Fibers as Stretchable Conductors. Adv. Mater. 2013, 25, 3249–3253. [Google Scholar] [CrossRef]
- Sui, D.; Huang, Y.; Huang, L.; Liang, J.; Ma, Y.; Chen, Y. Flexible and Transparent Electrothermal Film Heaters Based on Graphene Materials. Small 2011, 7, 3186–3192. [Google Scholar] [CrossRef]
- Lagrève, C.; Feller, J.F.; Linossier, I.; Levesque, G. Poly(butylene terephthalate)/poly(ethylene-co-alkyl acrylate)/carbon black conductive composites: Influence of composition and morphology on electrical properties. Polym. Eng. Sci. 2001, 41, 1124–1132. [Google Scholar] [CrossRef]
- Feller, J.F. Conductive polymer composites: Influence of extrusion conditions on positive temperature coefficient effect of poly(butylene terephthalate)/poly(olefin)-carbon black blends. J. Appl. Polym. Sci. 2004, 91, 2151–2157. [Google Scholar] [CrossRef]
- Weber, I.; Schwartz, P. Monitoring bending fatigue in carbon-fibre/epoxy composite strands: A comparison between mechanical and resistance techniques. Compos. Sci. Technol. 2001, 61, 849–853. [Google Scholar] [CrossRef]
- Park, J.; Shin, K.S. Novel method of polymer/low-melting-point metal alloy/light metal fiber composite fabrication. Express Polym. Lett. 2016, 10, 526–536. [Google Scholar] [CrossRef]
- Boiteux, G.; Fournier, J.; Issotier, D.; Scytre, G.; Marichy, G. Conductive thermoset composites: PTC effect. Synth. Met. 1999, 102, 1234–1235. [Google Scholar] [CrossRef]
- Sadej, M.; Gojzewski, H.; Gajewski, P.; Vancso, G.J.; Andrzejewska, E. Photocurable acrylate-based composites with enhanced thermal conductivity containing boron and silicon nitrides. Express Polym. Lett. 2018, 12, 790–807. [Google Scholar] [CrossRef]
- Lin, C.W.; Hwang, B.J.; Lee, C.R. Characteristics and sensing behavior of electrochemically codeposited polypyrrole-poly(vinyl alcohol) thin film exposed to ethanol vapors. J. Appl. Polym. Sci. 1999, 73, 2079–2087. [Google Scholar] [CrossRef]
- Qiu, L.; Lim, J.A.; Wang, X.; Lee, W.H.; Hwang, M.; Cho, K. Versatile Use of Vertical-Phase-Separation-Induced Bilayer Structures in Organic Thin-Film Transistors. Adv. Mater. 2008, 20, 1141–1145. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.-Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel Electronic and Magnetic Properties of Two-Dimensional Transition Metal Carbides and Nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Zhou, A.; Li, Z.; Chen, J.; Bala, H.; Hu, Q.; Cao, X. Hydrothermal synthesis of TiO2/Ti3C2 nanocomposites with enhanced photocatalytic activity. Mater. Lett. 2015, 150, 62–64. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Dall’Agnese, Y.; Lukatskaya, M.R.; Cook, K.M.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. High capacitance of surface-modified 2D titanium carbide in acidic electrolyte. Electrochem. Commun. 2014, 48, 118–122. [Google Scholar] [CrossRef]
- Xin, Y.; Yu, Y.-X. Possibility of bare and functionalized niobium carbide MXenes for electrode materials of supercapacitors and field emitters. Mater. Des. 2017, 130, 512–520. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, S.; Ding, W.; Nie, Y.; Wei, Z. An extraordinarily stable catalyst: Pt NPs supported on two-dimensional Ti3C2X2 (X = OH, F) nanosheets for oxygen reduction reaction. Chem. Commun. 2013, 49, 10112. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, L.; Li, Z.; Zhou, A.; Hu, Q.; Cao, X. Preparation of MXene-Cu2O nanocomposite and effect on thermal decomposition of ammonium perchlorate. Solid State Sci. 2014, 35, 62–65. [Google Scholar] [CrossRef]
- Peng, Q.; Guo, J.; Zhang, Q.; Xiang, J.; Liu, B.; Zhou, A.; Liu, R.; Tian, Y. Unique Lead Adsorption Behavior of Activated Hydroxyl Group in Two-Dimensional Titanium Carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef] [PubMed]
- Mashtalir, O.; Cook, K.M.; Mochalin, V.N.; Crowe, M.; Barsoum, M.W.; Gogotsi, Y. Dye adsorption and decomposition on two-dimensional titanium carbide in aqueous media. J. Mater. Chem. A 2014, 2, 14334–14338. [Google Scholar] [CrossRef]
- Lorencova, L.; Gajdosova, V.; Hroncekova, S.; Bertok, T.; Blahutova, J.; Vikartovska, A.; Parrakova, L.; Gemeiner, P.; Kasak, P.; Tkac, J. 2D MXenes as Perspective Immobilization Platforms for Design of Electrochemical Nanobiosensors. Electroanalysis 2019. [Google Scholar] [CrossRef]
- Lorencova, L.; Bertok, T.; Dosekova, E.; Holazova, A.; Paprckova, D.; Vikartovska, A.; Sasinkova, V.; Filip, J.; Kasak, P.; Jerigova, M.; et al. Electrochemical performance of Ti3C2Tx MXene in aqueous media: Towards ultrasensitive H2O2 sensing. Electrochim. Acta 2017, 235, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Rasool, K.; Mahmoud, K.A.; Johnson, D.J.; Helal, M.; Berdiyorov, G.R.; Gogotsi, Y. Efficient Antibacterial Membrane based on Two-Dimensional Ti3C2Tx (MXene) Nanosheets. Sci. Rep. 2017, 7, 1598. [Google Scholar] [CrossRef] [PubMed]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.-L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Wang, Y.; Jiang, F.; Yu, Y.; Mi, L.; Song, L. Two-dimensional MXene Ti3C2 produced by exfoliation of Ti3AlC2. Mater. Des. 2017, 114, 161–166. [Google Scholar] [CrossRef]
- An, H.; Habib, T.; Shah, S.; Gao, H.; Radovic, M.; Green, M.J.; Lutkenhaus, J.L. Surface-agnostic highly stretchable and bendable conductive MXene multilayers. Sci. Adv. 2018, 4, eaaq0118. [Google Scholar] [CrossRef]
- Feng, Y.; Deng, Q.; Peng, C.; Hu, J.; Li, Y.; Wu, Q.; Xu, Z. An ultrahigh discharged energy density achieved in an inhomogeneous PVDF dielectric composite filled with 2D MXene nanosheets via interface engineering. J. Mater. Chem. C 2018, 6, 13283–13292. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, H.-B.; Liu, J.; Xie, X.; Yang, R.; Li, Y.; Hong, S.; Yu, Z.-Z. Highly Conductive Transition Metal Carbide/Carbonitride(MXene)@polystyrene Nanocomposites Fabricated by Electrostatic Assembly for Highly Efficient Electromagnetic Interference Shielding. Adv. Funct. Mater. 2017, 27, 1702807. [Google Scholar] [CrossRef]
- Zhang, J.; Seyedin, S.; Qin, S.; Wang, Z.; Moradi, S.; Yang, F.; Lynch, P.A.; Yang, W.; Liu, J.; Wang, X.; et al. Highly Conductive Ti3C2Tx MXene Hybrid Fibers for Flexible and Elastic Fiber-Shaped Supercapacitors. Small 2019, 15, 1804732. [Google Scholar] [CrossRef]
- Liu, R.; Miao, M.; Li, Y.; Zhang, J.; Cao, S.; Feng, X. Ultrathin Biomimetic Polymeric Ti3C2Tx MXene Composite Films for Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2018, 10, 44787–44795. [Google Scholar] [CrossRef]
- Ling, Z.; Ren, C.E.; Zhao, M.-Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Chen, Q.; Li, P.; Zhou, A.; Cao, X.; Hu, Q. Preparation, mechanical and anti-friction performance of MXene/polymer composites. Mater. Des. 2016, 92, 682–689. [Google Scholar] [CrossRef]
- Sobolčiak, P.; Ali, A.; Hassan, M.K.; Helal, M.I.; Tanvir, A.; Popelka, A.; Al-Maadeed, M.A.; Krupa, I.; Mahmoud, K.A. 2D Ti3C2Tx (MXene)-reinforced polyvinyl alcohol (PVA) nanofibers with enhanced mechanical and electrical properties. PLoS ONE 2017, 12, e0183705. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Deng, Q.; Liu, Z.; Shen, D.; Wang, T.; Huang, Q.; Du, S.; Jiang, N.; Lin, C.-T.; Yu, J. Enhanced thermal properties of poly(vinylidene fluoride) composites with ultrathin nanosheets of MXene. RSC Adv. 2017, 7, 20494–20501. [Google Scholar] [CrossRef]
- Tu, S.; Jiang, Q.; Zhang, X.; Alshareef, H.N. Large Dielectric Constant Enhancement in MXene Percolative Polymer Composites. ACS Nano 2018, 12, 3369–3377. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Saito, T.; Lai, S.; Rager, M.S.; Aytug, T.; Parans Paranthaman, M.; Zhao, M.-Q.; Gogotsi, Y. Ti3C2Tx (MXene)–polyacrylamide nanocomposite films. RSC Adv. 2016, 6, 72069–72073. [Google Scholar] [CrossRef]
- Lipatov, A.; Alhabeb, M.; Lukatskaya, M.R.; Boson, A.; Gogotsi, Y.; Sinitskii, A. Effect of Synthesis on Quality, Electronic Properties and Environmental Stability of Individual Monolayer Ti3C2 MXene Flakes. Adv. Electron. Mater. 2016, 2, 1600255. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Thermo Scientific. XPS: Knowledge Base. Available online: https://xpssimplified.com/periodictable.php (accessed on 28 June 2019).
- Boča, M.; Barborík, P.; Mičušík, M.; Omastová, M. X-ray photoelectron spectroscopy as detection tool for coordinated or uncoordinated fluorine atoms demonstrated on fluoride systems NaF, K2TaF7, K3TaF8, K2ZrF6, Na7Zr6F31 and K3ZrF7. Solid State Sci. 2012, 14, 828–832. [Google Scholar] [CrossRef]
- Ramesh, C. New Crystalline Transitions in Nylons 4,6, 6,10, and 6,12 Using High Temperature X-ray Diffraction Studies. Macromolecules 1999, 32, 3721–3726. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory; Taylor & Francis: Abingdon, UK, 1985; ISBN 978-0-203-29042-2. [Google Scholar]
- Hoseini, A.H.A.; Arjmand, M.; Sundararaj, U.; Trifkovic, M. Significance of interfacial interaction and agglomerates on electrical properties of polymer-carbon nanotube nanocomposites. Mater. Des. 2017, 125, 126–134. [Google Scholar] [CrossRef]
- Logakis, E.; Pandis, C.; Peoglos, V.; Pissis, P.; Pionteck, J.; Pötschke, P.; Mičušík, M.; Omastová, M. Electrical/dielectric properties and conduction mechanism in melt processed polyamide/multi-walled carbon nanotubes composites. Polymer 2009, 50, 5103–5111. [Google Scholar] [CrossRef]
- Kirkpatrick, S. Percolation and Conduction. Rev. Mod. Phys. 1973, 45, 574–588. [Google Scholar] [CrossRef]
- Tchmutin, I.A.; Ponomarenko, A.T.; Shevchenko, V.G.; Godovski, D.Y. Analysis of peculiarities in percolation behavior of some conducting polymer composites. Synth. Met. 1994, 66, 19–23. [Google Scholar] [CrossRef]
- Moucka, R.; Mrlik, M.; Ilcikova, M.; Spitalsky, Z.; Kazantseva, N.; Bober, P.; Stejskal, J. Electrical transport properties of poly(aniline-co-p-phenylenediamine) and its composites with incorporated silver particles. Chem. Pap. 2013, 67, 1012–1019. [Google Scholar] [CrossRef]
- Preda, F.-M.; Alegría, A.; Bocahut, A.; Fillot, L.-A.; Long, D.R.; Sotta, P. Investigation of Water Diffusion Mechanisms in Relation to Polymer Relaxations in Polyamides. Macromolecules 2015, 48, 5730–5741. [Google Scholar] [CrossRef]
- Füllbrandt, M.; Wellert, S.; von Klitzing, R.; Schönhals, A. Thermal and corrosion (in)stability of polyamide 6 studied by broadband dielectric spectroscopy. Polymer 2015, 75, 34–43. [Google Scholar] [CrossRef]
- Zhang, J.; Mine, M.; Zhu, D.; Matsuo, M. Electrical and dielectric behaviors and their origins in the three-dimensional polyvinyl alcohol/MWCNT composites with low percolation threshold. Carbon 2009, 47, 1311–1320. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Qin, W.; Yang, Z.; Yang, D.; Xing, Y.; Liu, S.F.; Li, C. Perovskite as an effective Voc switcher for high efficiency polymer solar cells. Nano Energy 2016, 20, 126–133. [Google Scholar] [CrossRef]
- Mrlík, M.; Moučka, R.; Ilčíková, M.; Bober, P.; Kazantseva, N.; Špitálský, Z.; Trchová, M.; Stejskal, J. Charge transport and dielectric relaxation processes in aniline-based oligomers. Synth. Met. 2014, 192, 37–42. [Google Scholar] [CrossRef]
- Petzelt, J.; Nuzhnyy, D.; Bovtun, V.; Savinov, M.; Kempa, M.; Rychetsky, I. Broadband dielectric and conductivity spectroscopy of inhomogeneous and composite conductors. Phys. Status Solidi 2013, 210, 2259–2271. [Google Scholar] [CrossRef]
- Varga, M.; Kopecká, J.; Morávková, Z.; Křivka, I.; Trchová, M.; Stejskal, J.; Prokeš, J. Effect of oxidant on electronic transport in polypyrrole nanotubes synthesized in the presence of methyl orange. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1147–1159. [Google Scholar] [CrossRef]
- Sobolciak, P.; Mrlík, M.; AlMaadeed, M.A.; Krupa, I. Calorimetric and dynamic mechanical behavior of phase change materials based on paraffin wax supported by expanded graphite. Thermochim. Acta 2015, 617, 111–119. [Google Scholar] [CrossRef]
- Chen, D.; Tang, C.; Chan, K.; Tsui, C.; Yu, P.; Leung, M.; Uskokovic, P. Dynamic mechanical properties and in vitro bioactivity of PHBHV/HA nanocomposite. Compos. Sci. Technol. 2007, 67, 1617–1626. [Google Scholar] [CrossRef]
- Pagacz, J.; Raftopoulos, K.N.; Leszczyńska, A.; Pielichowski, K. Bio-polyamides based on renewable raw materials. J. Therm. Anal. Calorim. 2016, 123, 1225–1237. [Google Scholar] [CrossRef]
- Ilčíková, M.; Mrlík, M.; Sedláček, T.; Šlouf, M.; Zhigunov, A.; Koynov, K.; Mosnáček, J. Synthesis of Photoactuating Acrylic Thermoplastic Elastomers Containing Diblock Copolymer-Grafted Carbon Nanotubes. ACS Macro Lett. 2014, 3, 999–1003. [Google Scholar] [CrossRef]
- Cvek, M.; Mrlík, M.; Ilčíková, M.; Mosnáček, J.; Münster, L.; Pavlínek, V. Synthesis of Silicone Elastomers Containing Silyl-Based Polymer-Grafted Carbonyl Iron Particles: An Efficient Way To Improve Magnetorheological, Damping, and Sensing Performances. Macromolecules 2017, 50, 2189–2200. [Google Scholar] [CrossRef]






| Sample | Surface Chemical Composition [at. %] | |||||
|---|---|---|---|---|---|---|
| C1s carbide/sp2/sp3/CO/OC=O | O1s oxide/C=O/CO | Ti2p TiC/Ti2+/Ti3+/TiO2 | Al2p Al/AlOx | F1s F−/X-F/X-F-X | N1s | |
| MAX phase | 33.5 5.2/3.3/19.8/2.8/2.4 | 38.2 16.1/14.5/7.5 | 11.6 4.4/1.5/1.2/4.6 | 12.0 2.1/9.9 | 3.0 0.8/1.6/0.6 | 1.8 |
| MXene | 42.9 9.6/9.4/14.9/6.1/2.9 | 19.7 8.1/6.9/4.7 | 18.3 6.8/5.3/2.6/3.6 | 3.8 0.8/3.0 | 13.5 2.5/7.6/3.4 | 2.0 |
| Sample Composition | Tg Values of coPA/MXene Films (°C) | Ea (kJ/mol) | |||
|---|---|---|---|---|---|
| 0.5 Hz | 1 Hz | 2.5 Hz | 5 Hz | ||
| neat coPA | 112.0 | 113.6 | 114.4 | 115.4 | 373 |
| 0.5 wt. % MXene | 114.3 | 115.4 | 116.3 | 117.5 | 405 |
| 1 wt. % MXene | 115.5 | 116.6 | 117.3 | 118.6 | 423 |
| 2.5 wt. % MXene | 116.5 | 117.5 | 118.4 | 119.3 | 464 |
| 5 wt. % MXene | 120.1 | 120.9 | 121.8 | 122.7 | 505 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanvir, A.; Sobolčiak, P.; Popelka, A.; Mrlik, M.; Spitalsky, Z.; Micusik, M.; Prokes, J.; Krupa, I. Electrically Conductive, Transparent Polymeric Nanocomposites Modified by 2D Ti3C2Tx (MXene). Polymers 2019, 11, 1272. https://doi.org/10.3390/polym11081272
Tanvir A, Sobolčiak P, Popelka A, Mrlik M, Spitalsky Z, Micusik M, Prokes J, Krupa I. Electrically Conductive, Transparent Polymeric Nanocomposites Modified by 2D Ti3C2Tx (MXene). Polymers. 2019; 11(8):1272. https://doi.org/10.3390/polym11081272
Chicago/Turabian StyleTanvir, Aisha, Patrik Sobolčiak, Anton Popelka, Miroslav Mrlik, Zdenko Spitalsky, Matej Micusik, Jan Prokes, and Igor Krupa. 2019. "Electrically Conductive, Transparent Polymeric Nanocomposites Modified by 2D Ti3C2Tx (MXene)" Polymers 11, no. 8: 1272. https://doi.org/10.3390/polym11081272
APA StyleTanvir, A., Sobolčiak, P., Popelka, A., Mrlik, M., Spitalsky, Z., Micusik, M., Prokes, J., & Krupa, I. (2019). Electrically Conductive, Transparent Polymeric Nanocomposites Modified by 2D Ti3C2Tx (MXene). Polymers, 11(8), 1272. https://doi.org/10.3390/polym11081272







