The Preparation and Properties of Terephthalyl-Alcohol-Modified Phenolic Foam with High Heat Aging Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Modified Phenolic Resin
2.3. Preparation of Modified Phenolic Resin Foam
3. Characterization
3.1. Characterization of Resin
3.2. Characterization of Foam
4. Results and Discussion
4.1. Characterization of Modified Phenolic Resin
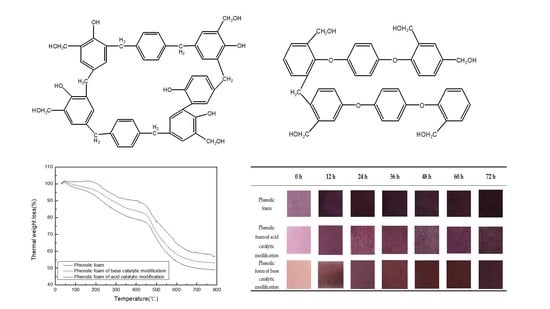
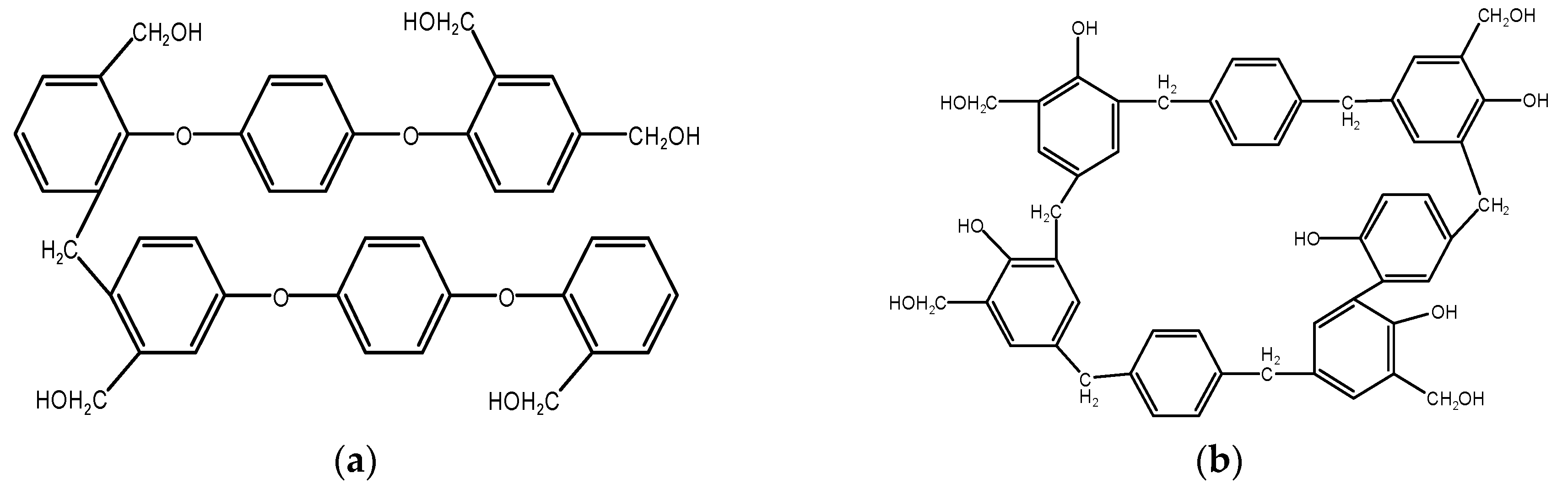
4.1.1. Mechanism of Terephthalyl Alcohol-Modified Phenol
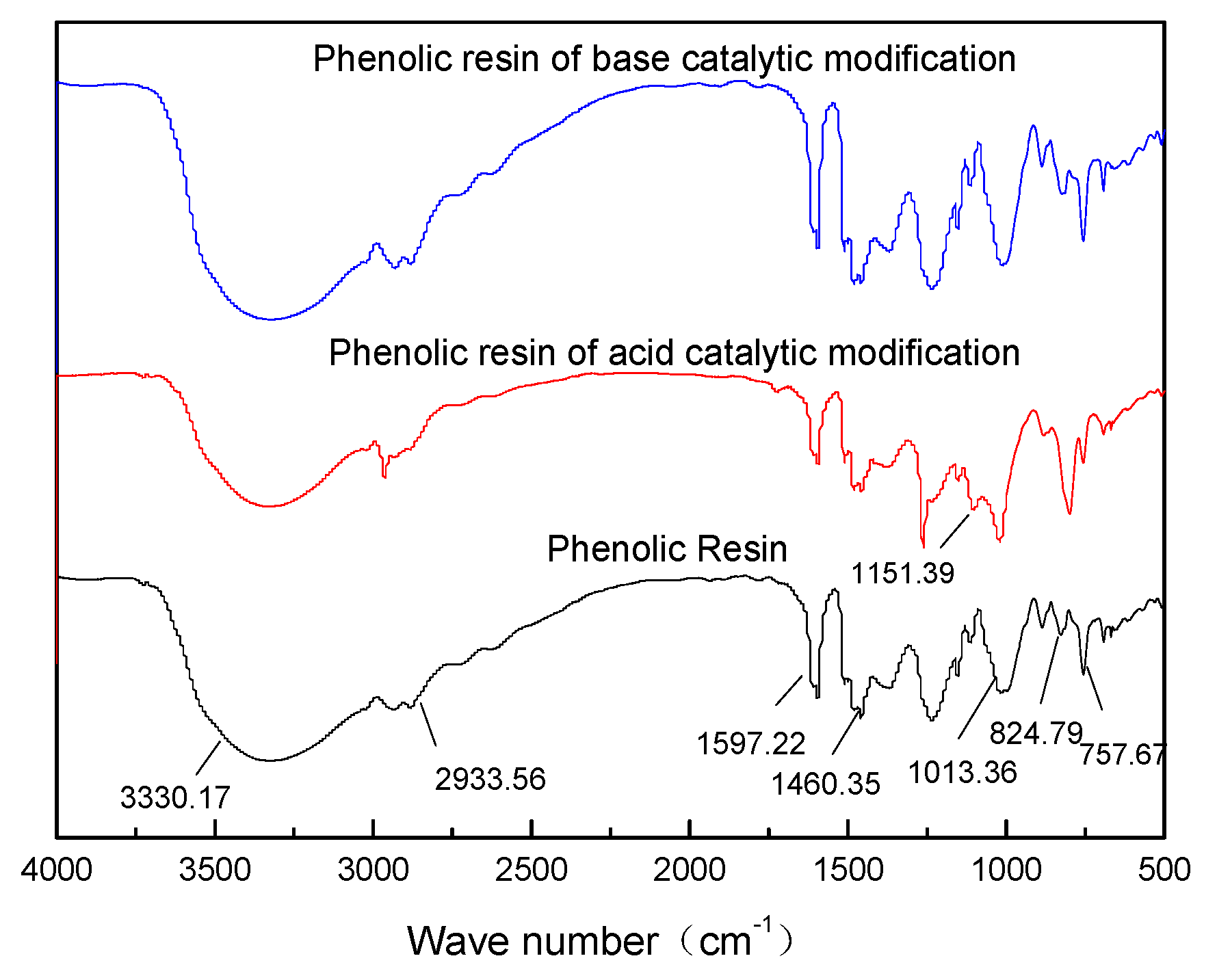
4.1.2. Infrared Spectrum of Modified Phenolic Resin
4.2. Modified Phenolic Foam Heat Resistance and Aging Resistance Analysis
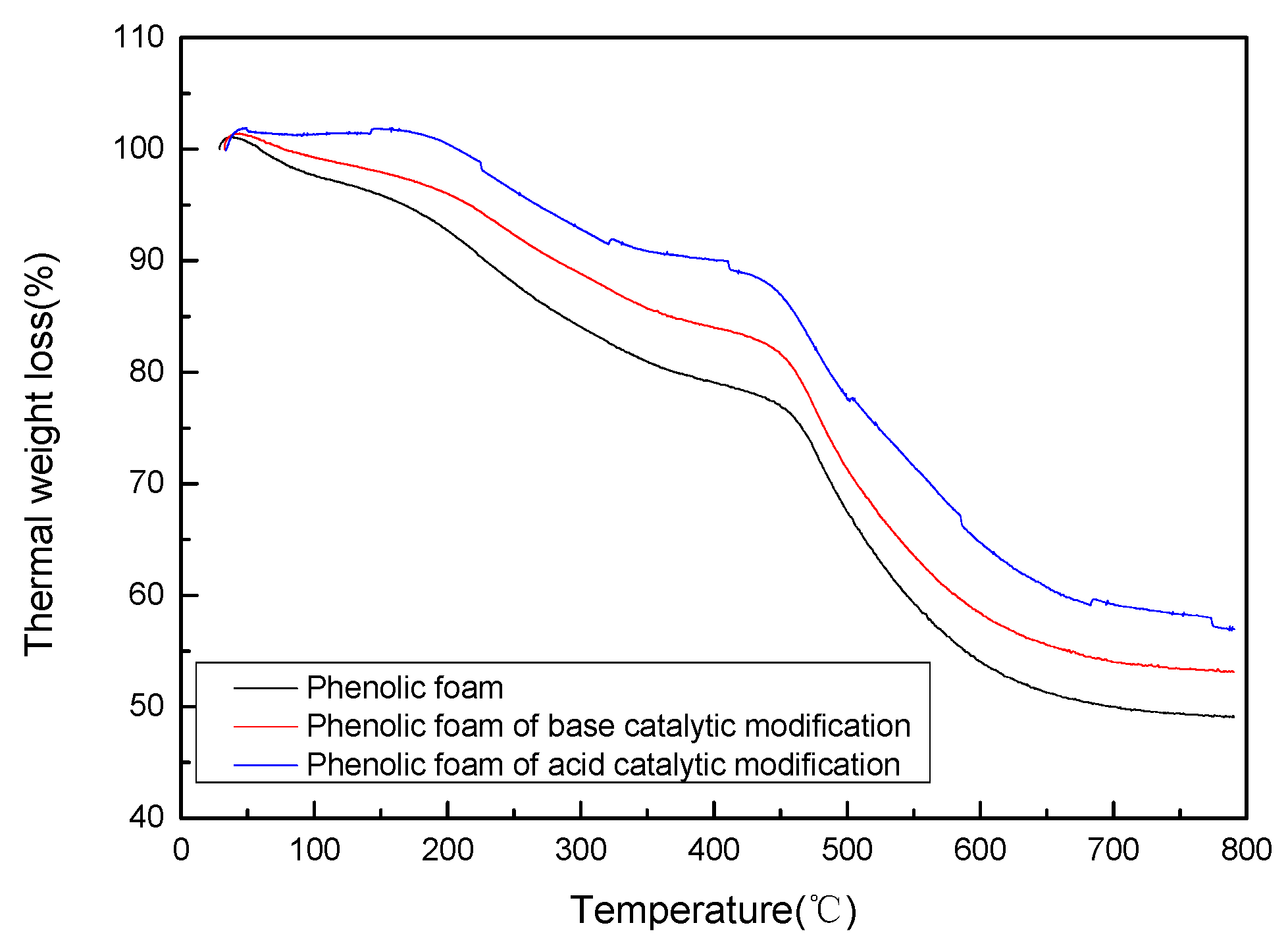
4.2.1. Thermogravimetric Analysis of Modified Phenolic Foam
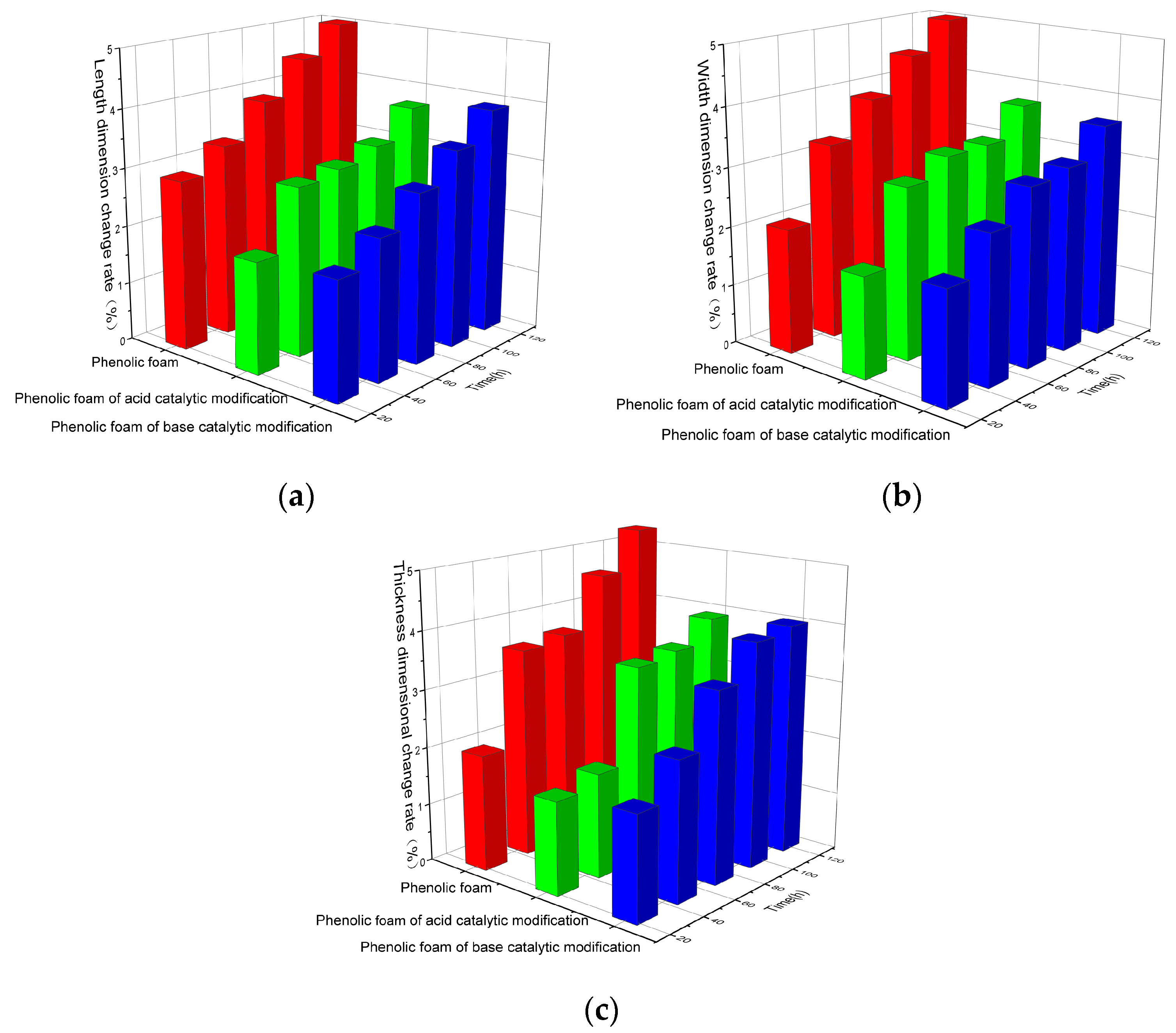
4.2.2. Dimensional Change Rate of Modified Phenolic Foam under Thermal Action
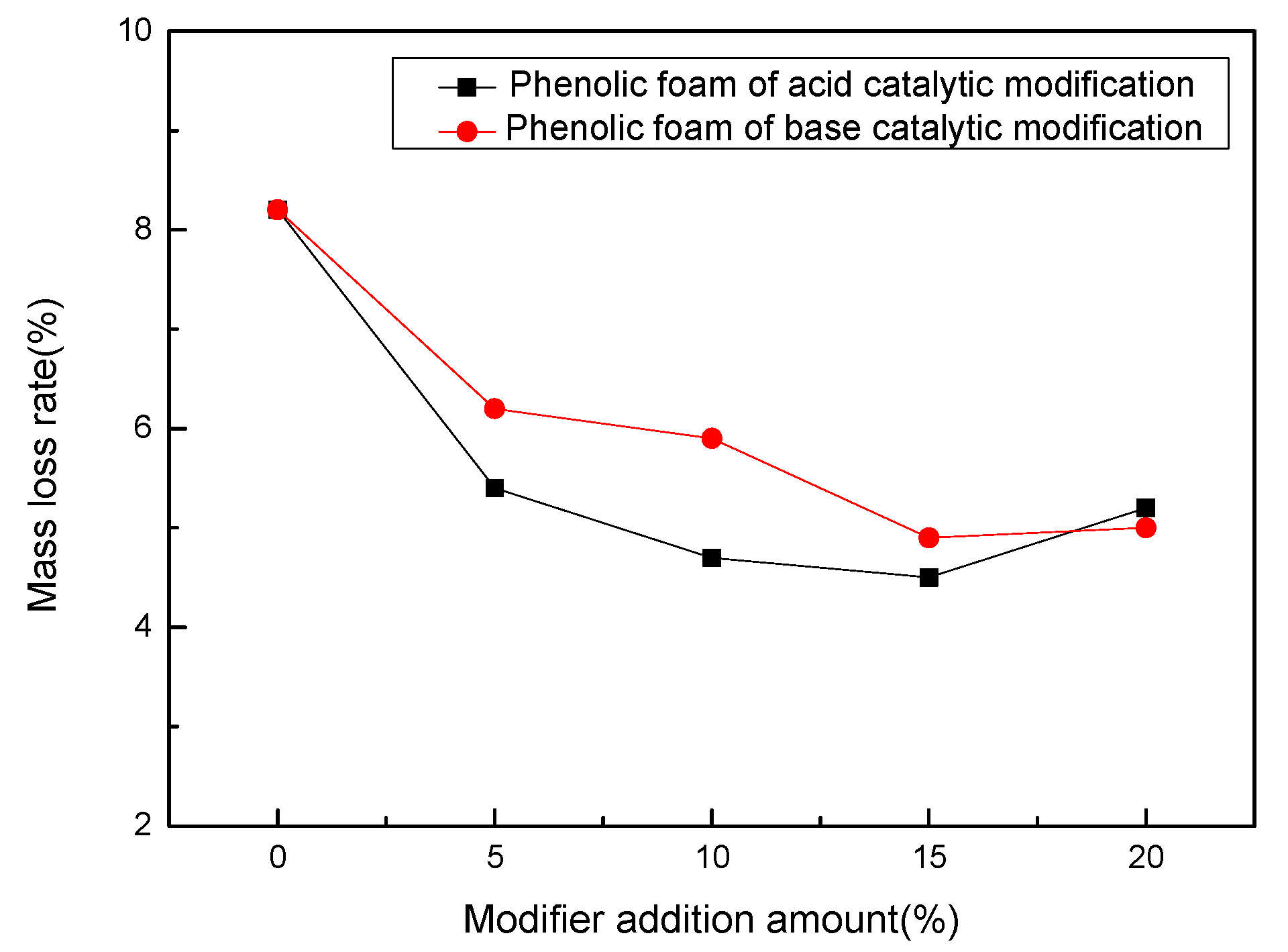
4.2.3. Mass Loss Rate of Modified Phenolic Foam
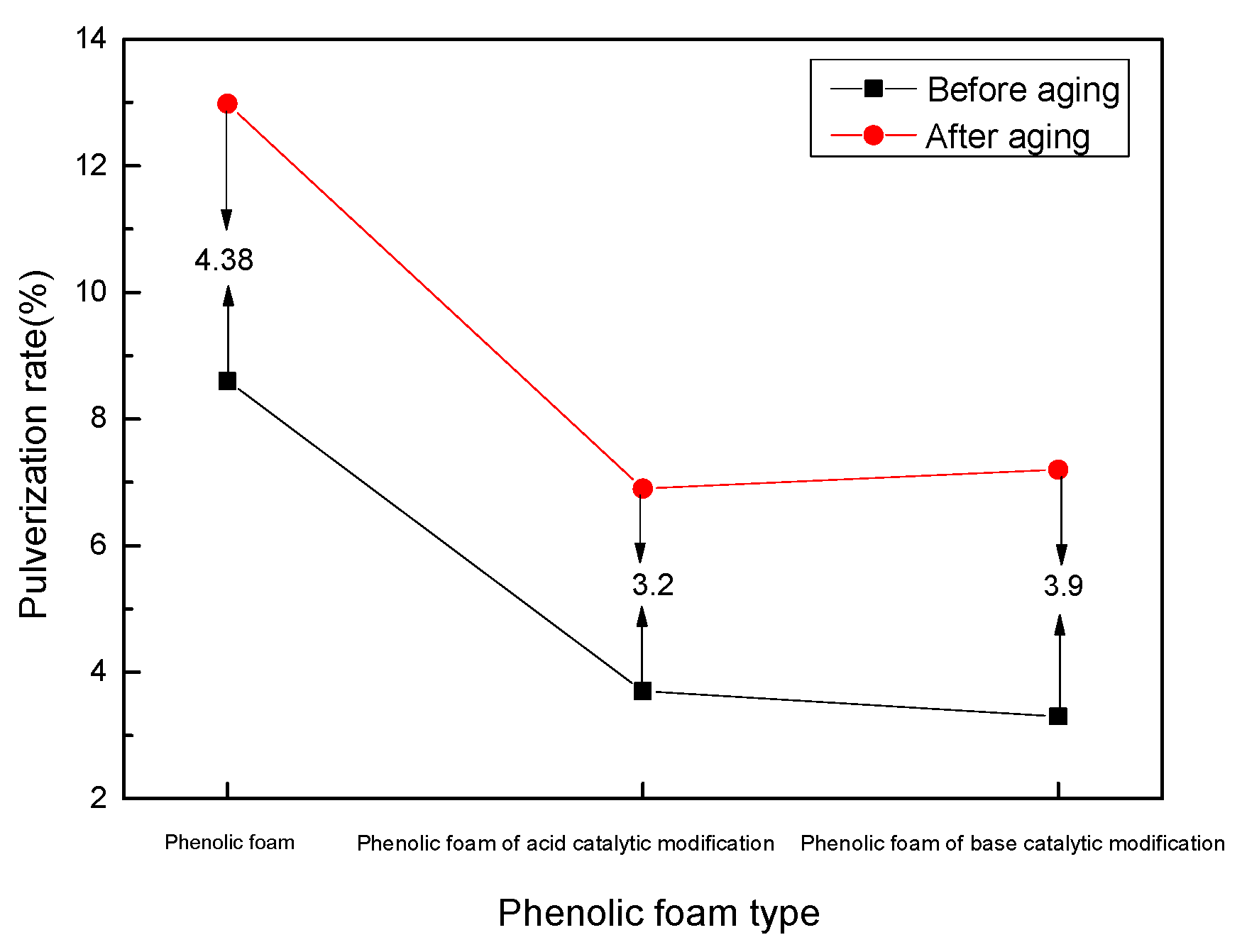
4.2.4. Effect of Heat Aging on the Degree of Phenolic Foaming
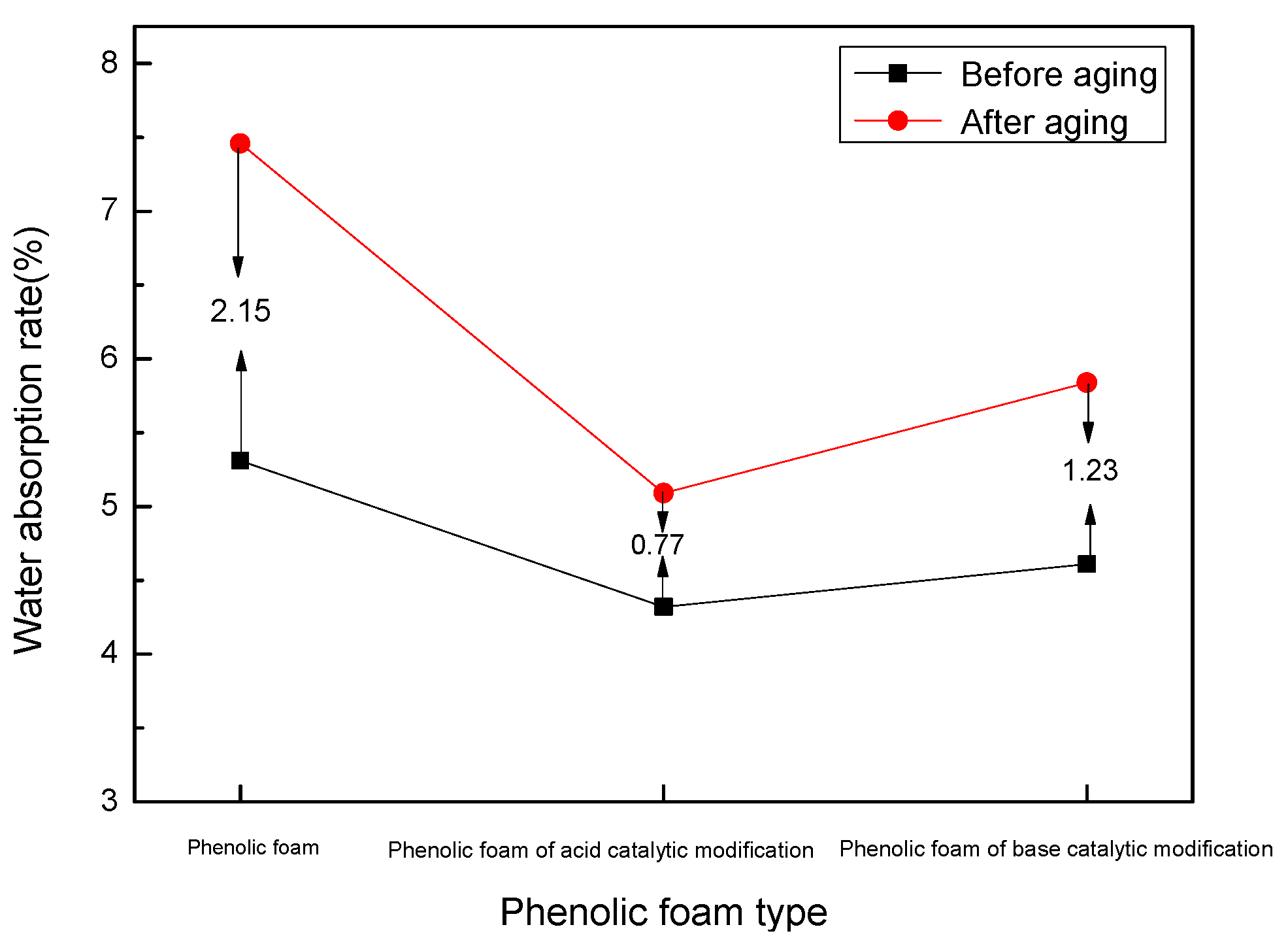
4.2.5. Effect of Heat Aging on Water Absorption of Modified Phenolic Foam
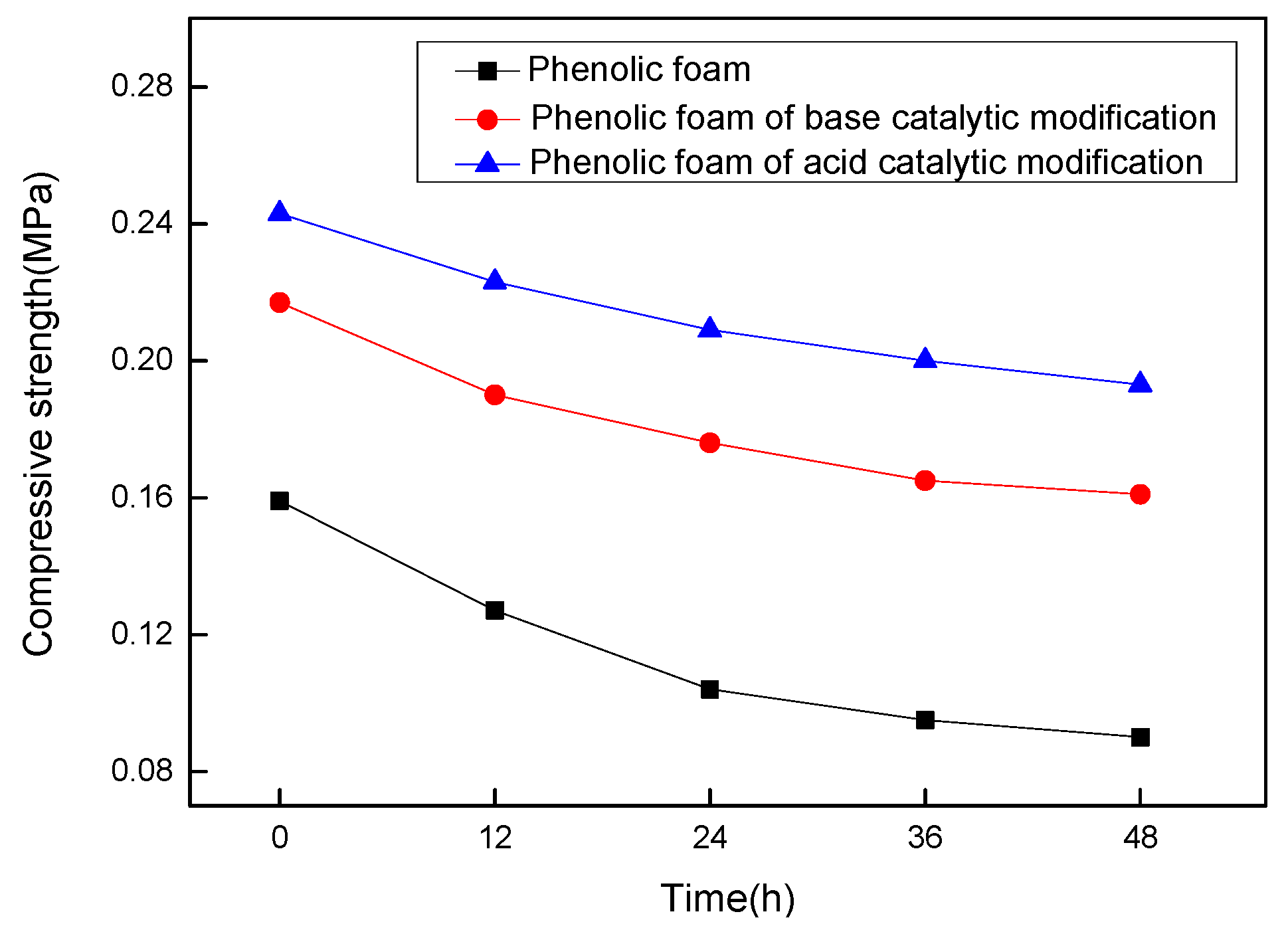
4.2.6. Effect of Heat Aging on the Compressive Strength of Modified Phenolic Foam
4.2.7. Modified Phenolic Foam Heat Aging Color Change
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Yao, Z.; Chen, Y.; Wei, D.; Wu, Y. Thermomechanical analyses of phenolic foam reinforced with glass fiber mat. Mater. Des. 2013, 131–135. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Wu, L. Preparation of a silica nanospheres/graphene oxide hybrid and its application in phenolic foams with improved mechanical strengths, friability and flame retardancy. RSC Adv. 2015, 5, 99907–99913. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Yuan, H.; Song, L.; Hu, Y. Fire performance and mechanical properties of phenolic foams modified by phosphorus-containing polyethers. J. Polym. Res. 2012, 19, 9831. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, W.; Li, C.; Wang, G.; Lin, X.; Liu, Z. Effects of surfactants on the mechanical properties, microstructure, and flame resistance of phenol–urea–formaldehyde foam. Polym. Bull. 2016, 73, 1–20. [Google Scholar] [CrossRef]
- Liu, S.; Wu, D.; Li, X.; Liu, P. Synthesis and foaming properties of resole phenolic resin. Plast. Sci. Technol. 2014, 42, 9–33. [Google Scholar] [CrossRef]
- Liu, R.; Tan, W.; Li, H.; Hu, Z. Effect of the ratio of paraformaldehyde to phenolic substance on the properties of expandable resole phenolic resin. Thermosetting Resin 2013, 28, 6–10, 19. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Mu, Y.; Zhang, J.; Zhang, S.; Li, J.; Zhang, W. Structural Properties and Copolycondensation Mechanism of Valonea Tannin-Modified Phenol-formaldehyde Resin. J. Polym. Environ. 2018, 26, 1297–1309. [Google Scholar] [CrossRef]
- Zhang, M. Study on Silicone Modified Phenolic Resin. Chem. Des. Commun. 2017, 43, 116. [Google Scholar]
- Yang, Y.; He, J. Toughening Method and Toughening Mechanism Analysis of Phenolic Resin. Plast. Sci. Technol. 2013, 41, 108–113. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, L.; Jia, P. Enhancement of thermal stability and chemical reactivity of phenolic resin ameliorated by nanoSiO2. Korean J. Chem. Eng. 2018, 35, 298–302. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G.; Zhang, W.; Yin, H.; Xu, Y. Synthesis of humin-phenol-formaldehyde adhesive. Polymers 2017, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Geng, W.; You, H.; Wang, Y.; Loy, D. Modification of a phenolic resin with epoxy- and methacrylate-functionalized silica sols to improve the ablation resistance of their glass fiber-reinforced composites. Polymers 2014, 6, 105–113. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Tong, K.; Zhang, Q.; Liu, Y.; Zhang, J.; Liu, J.; Shu, S.; Xu, J.; Hu, Y. Study on the preparation of carbon nanotubes by iron modified phenolic resin pyrolysis. Mater. Sci. Eng. 2018, 423, 012088. [Google Scholar] [CrossRef]
- You, Y.; Dai, Y.; Dong, X.; Li, L.; Chen, Y.; Cao, X. Synthesis of phenolic resin modified by silane coupling agent. Synth. Resin Plast. 2017, 34, 17–19. [Google Scholar]
- Hu, X.; Zeng, J.; Dai, W.; Shi, W.; Li, L.; Han, C. EPDM/vinyl triethoxysilane modified phenol formaldehyde resin composite. Polym. Bull. 2011, 66, 703–710. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Wang, L.; Yao, Y.; Wu, J.; Sun, Y.; Tian, M.; Liu, J. Phenolic resin modified by boron-silicon with high char yield. Polym. Test. 2019, 73, 208–213. [Google Scholar] [CrossRef]
- Gao, M.; Wu, W.; Wang, Y.; Wang, Y.; Wang, H. Phenolic foam modified with dicyandiamide as toughening agent. J. Therm. Anal. Calorim. 2016, 124, 189–195. [Google Scholar] [CrossRef]
- Bo, C.; Hu, L.; Chen, Y.; Yang, X.; Zhang, M.; Zhou, Y. Synthesis of a novel cardanol-based compound and environmentally sustainable production of phenolic foam. J. Mater. Sci. 2018, 53, 10784–10797. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, L.; Bo, C.; Qian, Q.; Feng, G.; Jia, P.; Zhang, B.; Zhou, Y. Mechanical property of lignin-modified phenolic foam enhanced by nano-SiO2 via a novel method. Chem. Pap. 2018, 72, 763–767. [Google Scholar] [CrossRef]
- Sang, M.; Meng, Y.; Wang, S. Graphene/cardanol modified phenolic resin for the development of carbon fiber paper-based composites. RSC Adv. 2018, 8, 24464–24469. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Xu, Y.; Wang, C.; Chu, F. Preparation and characterization of phenolic foams with eco-friendly halogen-free flame retardant. J. Therm. Anal. Calorim. 2013, 114, 1143–1151. [Google Scholar] [CrossRef]
- Jing, S.; Li, T.; Li, X.; Xu, Q.; Hu, J.; Li, R. Phenolic foams modified by cardanol through bisphenol modification. J. Appl. Polym. Sci. 2014, 131, 39942. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; An, Y.; Zhang, F.; Zhang, Y.; Liu, T.; Dong, L.; Yin, Y. Polyimide modified phenolic foam. Acta Polym. Sin. 2013, 8, 1072–1079. [Google Scholar] [CrossRef]
- Luan, J.; Wei, X.; Zhou, X.; Lu, G.; Liu, C.; Li, G.; Na, Y.; Wang, H.; Qi, X. Study on phenolic foams modified by graphene oxide. Adv. Mater. Res. 2015, 1095, 510–513. [Google Scholar] [CrossRef]
- Guan, Z.; Jaeseon, Y.; Shim, J.; Lee, J.; Choi, W. Photocatalytic hydroxylation of benzene to phenol over titanium oxide entrapped into hydrophobically modified siliceous foam. Appl. Catal. B-Environ. 2011, 102, 132–139. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.; Gai, G. Performance of Modified Aramid Fiber Reinforced Phenolic Foam. Adv. Mater. Res. 2012, 1, 258–261. [Google Scholar] [CrossRef]
- Ge, T.; Tang, K.; Yu, Y.; Tan, X. Preparation and Properties of the 3-pentadecyl-phenolInSituModifiedFoamable Phenolic Resin. Polymers 2018, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Tang, K.; Tang, X. Preparation and Properties of Acetoacetic Ester-Terminated Polyether Pre-Synthesis Modified Phenolic Foam. Materials 2019, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Duan, Y.; Zhu, N. Foaming toughening and low acid modification of PEA copolymer modified phenolic resin. Plastic 2016, 45, 13–15, 20. [Google Scholar]
- Ge, T.; Wang, J.; Xiao, S. Effect of Nucleating Agent on Microstructure and Properties of Phenolic Foam. Plast. Sci. Technol. 2016, 44, 41–46. [Google Scholar] [CrossRef]
- Ge, T.; Yang, X.; Li, Y. Synthesis and foaming of urea toughened modified resole phenolic resin. Plastic 2013, 42, 55–58. [Google Scholar]
- Yang, G.; Gao, L.; Cheng, K. Preparation and properties of quartz cloth–reinforced Xylok composites fabricated by vacuum bag only process. High Perform. Polym. 2013, 25, 493–501. [Google Scholar] [CrossRef]
- Satheesh Chandran, M.; Temina, M.; Sunitha, K.; Dona, M.; Reghunadhan Nair, C.P. Allyl ether of aralkyl phenolic resin with low melt viscosity and its Alder-ene blends with bismaleimide: Synthesis, curing, and laminate properties. Polym. Adv. Technol. 2014, 25, 881–890. [Google Scholar] [CrossRef]
- Satheesh Chandran, M.; Sanil, K.; Sunitha, K. Alder-ene polymers derived from allyl aralkyl phenolic resin and bismaleimides: Carbon fiber composites properties. Polym. Adv. Technol. 2016, 27, 984–992. [Google Scholar] [CrossRef]
- Lia, X.; Wang, Z.; Wu, L. One-step in situ synthesis of a novel α-zirconium phosphate/graphene oxide hybrid and its application in phenolic foam with enhanced mechanical strength, flame retardancy and thermal stability. RSC Adv. 2016, 6, 74903–74912. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Zhang, Y.; Fang, J. Preparation and Thermal Properties of 4,4’-Biphenyldiol Modified Thermosetting Phenolic Resin. J. Mater. Sci. Eng. 2018, 578–583. [Google Scholar] [CrossRef]
- Lei, Z.; Ji, J.; Wu, Q.; Zhang, J.; Wang, Y.; Jing, X.; Liu, Y. Curing behavior and microstructure of epoxy-POSS modified novolac phenolic resin with different substitution degree. Polymer 2019, 178, 121587. [Google Scholar] [CrossRef]
- Huang, F.; Wan, L. Phenolic Resin and Its Application; Chemical Industry Press: Beijing, China, 2011; p. 126. [Google Scholar]

| Modified Phenolic Resin (phr) | Tween-80 (phr) | N-Pentane (phr) | P-Toluenesulfonic Acid (phr) |
|---|---|---|---|
| 100 | 4 | 12 | 18 |
| Modified Phenolic Resin | Viscosity (Pa·s) | Water Content (%) | Free Phenol (%) |
|---|---|---|---|
| Phenolic resin for foaming | 3~5 | 6~8 | <5 |
| Phenolic resin of acid catalytic modification | 4.78 | 7.39 | 3.35 |
| Phenolic resin of base catalytic modification | 4.21 | 7.86 | 3.74 |
| Phenolic Foam | Phenolic Foam of Base Catalytic Modification | Phenolic Foam of Acid Catalytic Modification | |
|---|---|---|---|
| Temperature 5% Weight Loss (°C) | 161.76 | 216.36 | 267.58 |
| Rapid Weight Loss Temperature (°C) | 475.34 | 479.86 | 481.54 |
| Residual Carbon Rate at 800 (°C) | 49.06 | 53.13 | 56.98 |
| 0 h | 12 h | 24 h | 36 h | 48 h | 60 h | 72 h | |
|---|---|---|---|---|---|---|---|
| Phenolic foam |  |  |  |  |  |  |  |
| Phenolic foam of acid catalytic modification |  |  |  |  |  |  |  |
| Phenolic foam of base catalytic modification |  |  |  |  |  |  |  |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, T.; Hu, X.; Tang, K.; Wang, D. The Preparation and Properties of Terephthalyl-Alcohol-Modified Phenolic Foam with High Heat Aging Resistance. Polymers 2019, 11, 1267. https://doi.org/10.3390/polym11081267
Ge T, Hu X, Tang K, Wang D. The Preparation and Properties of Terephthalyl-Alcohol-Modified Phenolic Foam with High Heat Aging Resistance. Polymers. 2019; 11(8):1267. https://doi.org/10.3390/polym11081267
Chicago/Turabian StyleGe, Tiejun, Xiaoqi Hu, Kaihong Tang, and Dongqi Wang. 2019. "The Preparation and Properties of Terephthalyl-Alcohol-Modified Phenolic Foam with High Heat Aging Resistance" Polymers 11, no. 8: 1267. https://doi.org/10.3390/polym11081267
APA StyleGe, T., Hu, X., Tang, K., & Wang, D. (2019). The Preparation and Properties of Terephthalyl-Alcohol-Modified Phenolic Foam with High Heat Aging Resistance. Polymers, 11(8), 1267. https://doi.org/10.3390/polym11081267