Dynamic Self-Assembly of Polyelectrolyte Composite Nanomaterial Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NFC Generation
2.3. Preparation of NFC/PVA Substrate
2.4. Preparation of PAH and PSS Film
3. Results and Discussion
3.1. Surface Charge
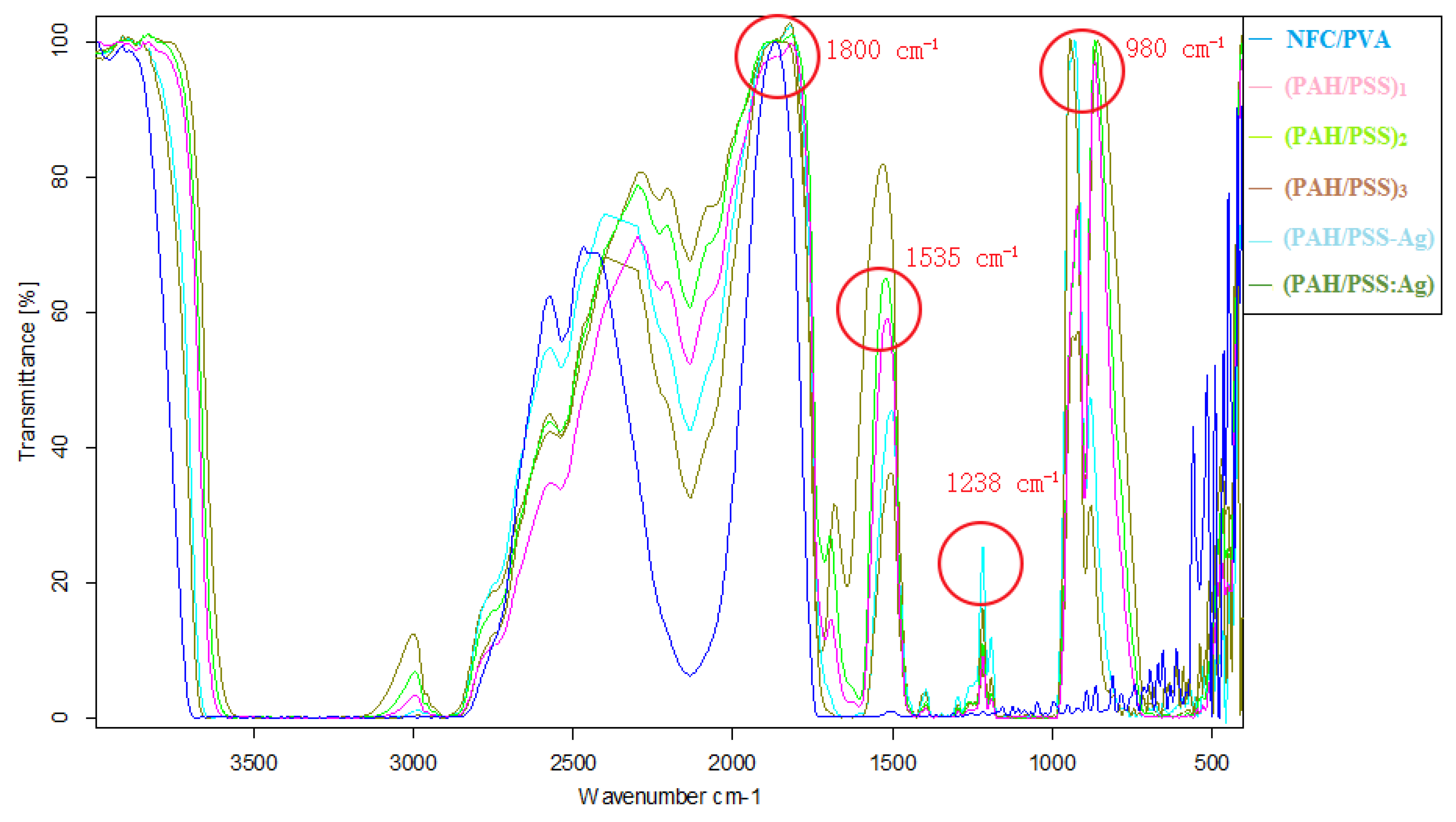
3.2. Fourier-Transform Infrared Spectrometry (FT-IR)
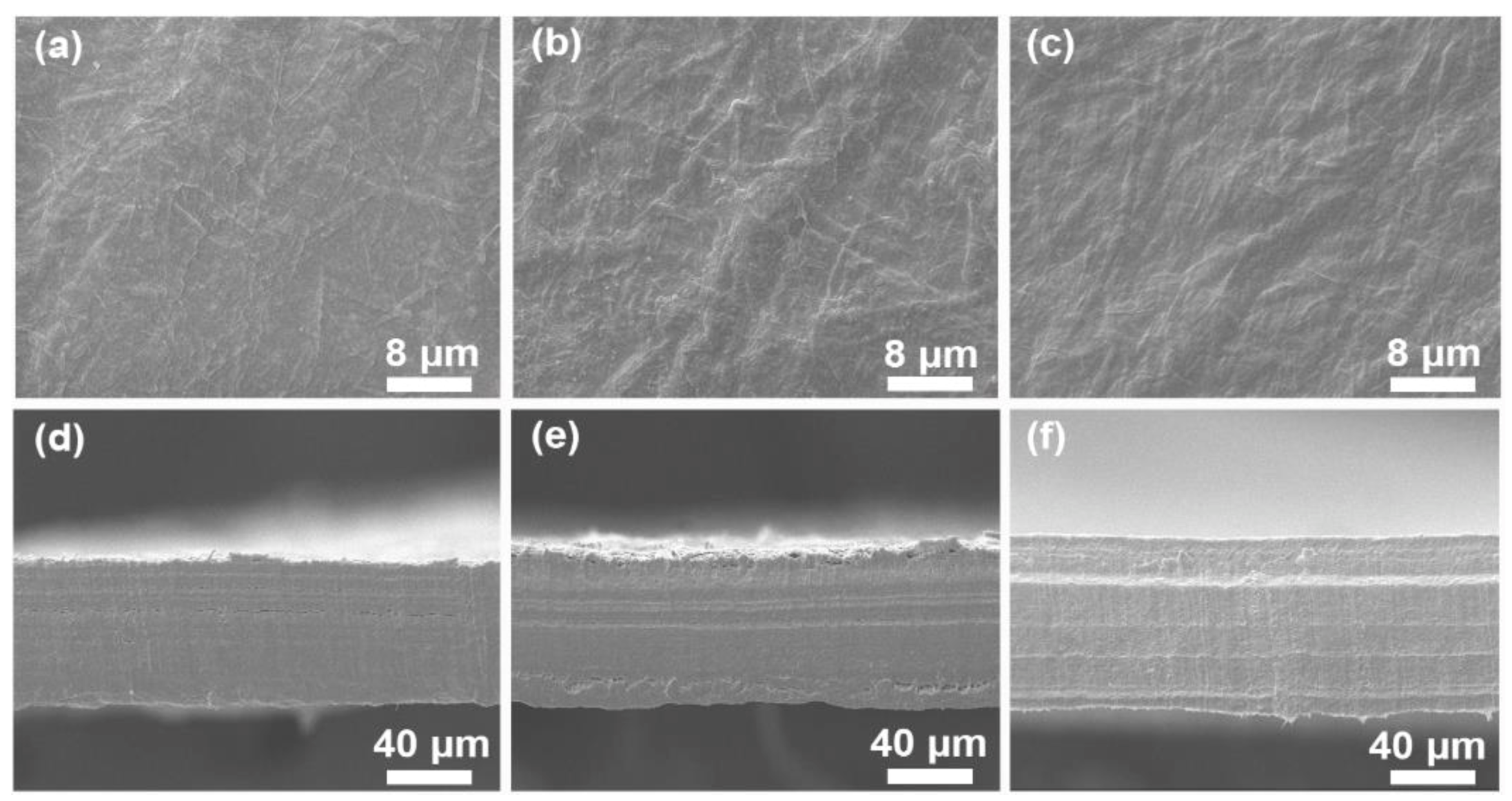
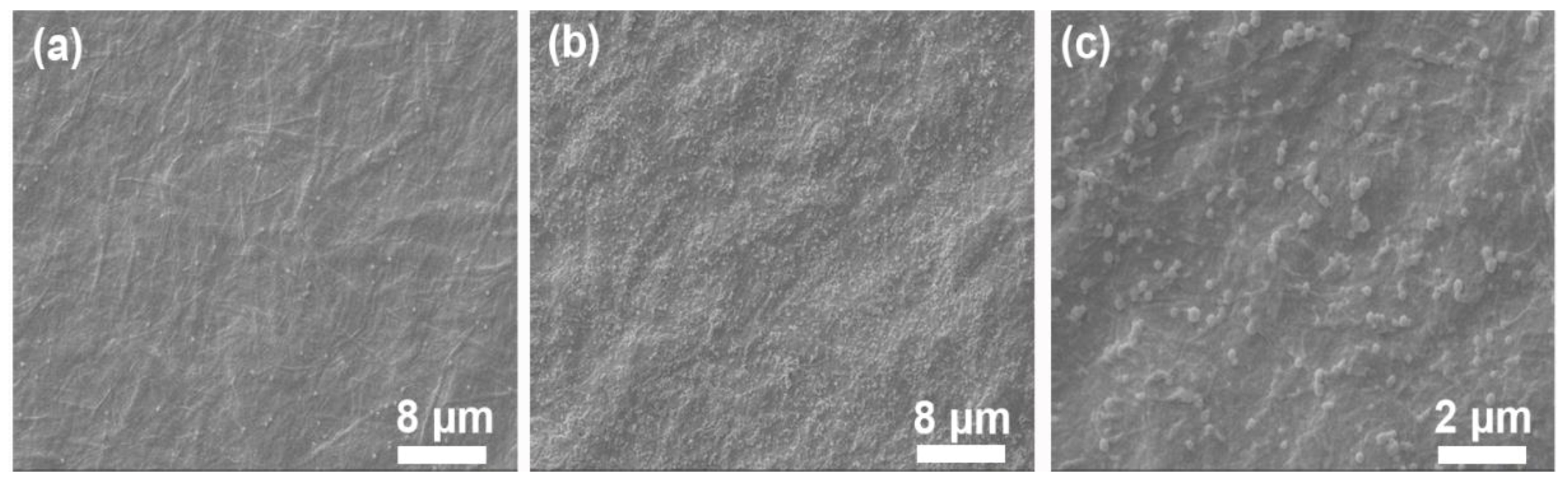
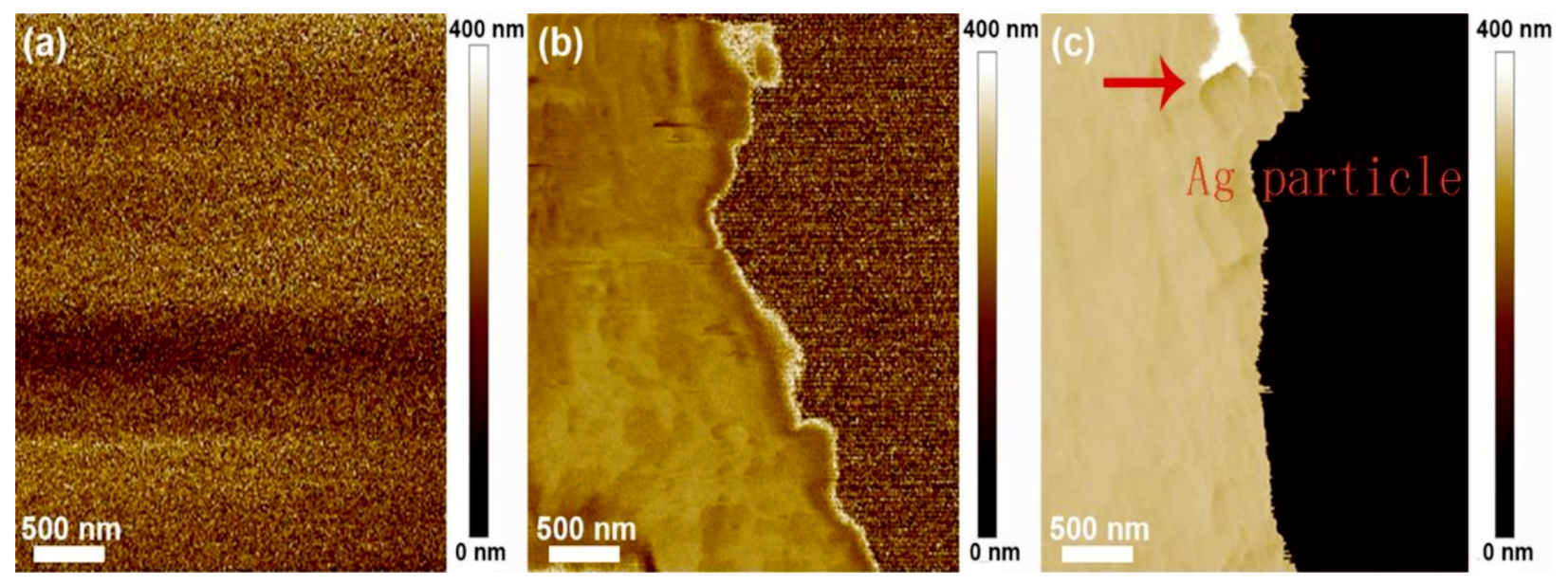
3.3. Morphology Observation
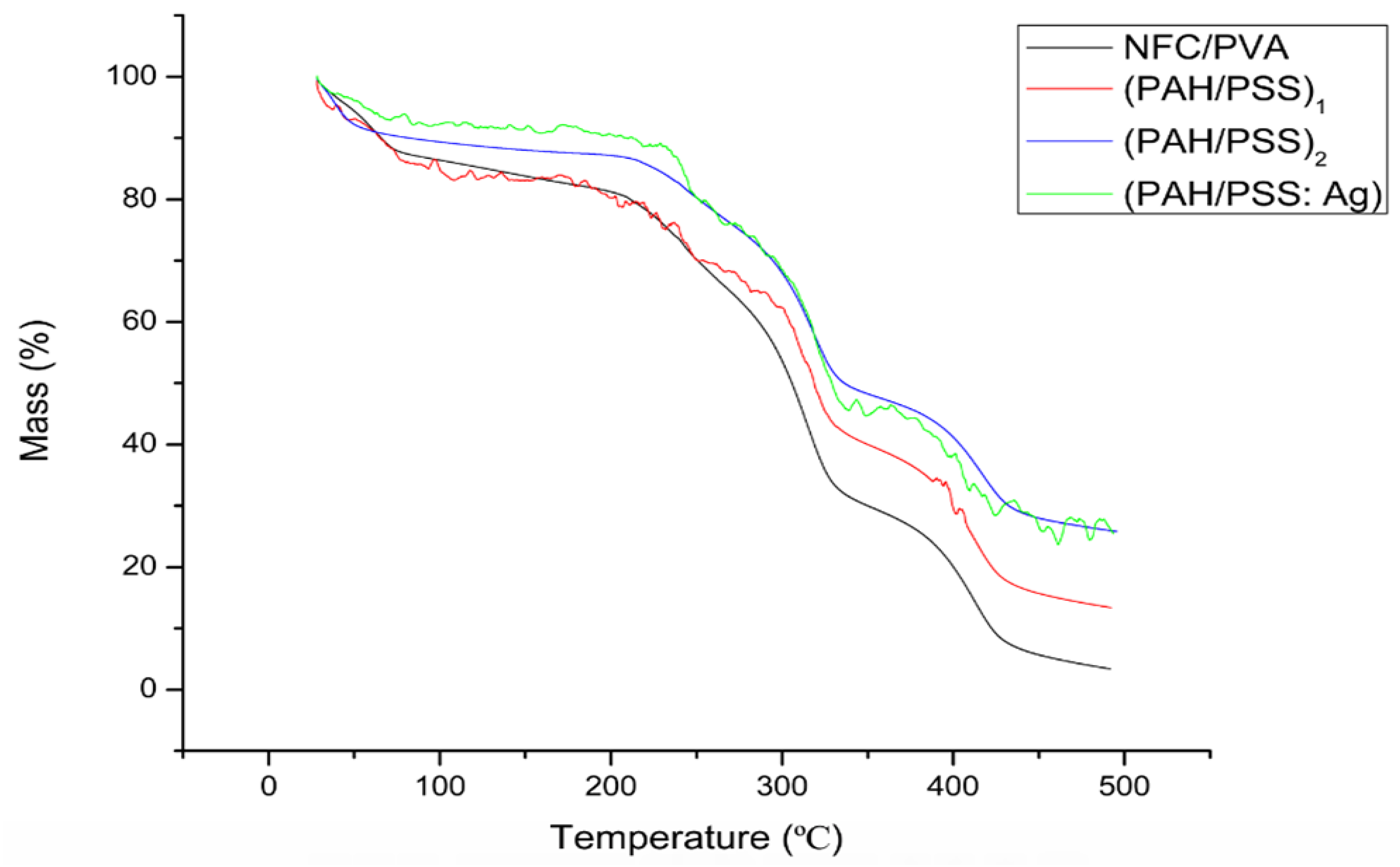
3.4. Thermal and Physical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–410. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W. Research needs in the membrane separation industry: Looking back, looking forward. J. Membr. Sci. 2010, 362, 134–136. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1233–1237. [Google Scholar] [CrossRef]
- Vaca, C.F.; Schönhoff, M. Pore size distribution in polyelectrolyte multilayers determined by nuclear magnetic cryoporometry. J. Chem. Phys. 2007, 126, 104705. [Google Scholar] [CrossRef] [PubMed]
- Adusumili, M.; Bruening, M.L. Variation of ion-exchange capacity, zeta potential, and ion-transport selectivities with the number of layers in a multilayer polyelectrolyte film. Langmuir 2009, 25, 7478–7485. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.Y.; Mohammad, A.W.; Ng, C.Y. A review on nanofiltration membrane fabrication and modification using polyelectrolytes: Effective ways to develop membrane selective barriers and rejection capability. Adv. Colloid Interface Sci. 2003, 197, 85–107. [Google Scholar] [CrossRef]
- Li, X.; Feyter, S.D.; Chen, D.j.; Aldea, S.; Vandezande, P.; Prez, F.D.; Vankelecom, I.F.J. Solvent-resistant nanofiltration membranes based on multilayered polyelectrolyte complexes. Chem. Mater. 2008, 20, 3876–3883. [Google Scholar] [CrossRef]
- Stammen, J.A.; Williams, S.; Ku, D.N.; Guldberg, R.E. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 2001, 22, 799–806. [Google Scholar] [CrossRef]
- Borges, J.; Mano, J.F. Molecular interactions driving the layer-by-layer assembly of multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef]
- Deng, H.B.; Wang, X.Y.; Liu, P.; Ding, B.; Du, Y.M.; Li, G.X.; Hu, X.W.; Yang, J.H. Enhanced bacterial inhibition activity of layer-by-layer structured polysaccharide film-coated cellulose nanofibrous mats via addition of layered silicate Carbohydr. Polymers 2011, 83, 239–245. [Google Scholar] [CrossRef]
- Müller, K.; Quinn, J.F.; Johnston, A.P.R.; Becker, M.; Greiner, A.; Caruso, F. Polyelectrolyte Functionalization of Electrospun Fibers. Chem. Mater. 2006, 18, 2397–2403. [Google Scholar] [CrossRef]
- Hong, S.U.; Bruening, M.L. Separation of amino acid mixtures using multilayer polyelectrolyte nanofiltration membranes. J. Membr. Sci. 2006, 280, 1–5. [Google Scholar] [CrossRef]
- Miller, M.D.; Bruening, M.L. Controlling the nanofiltration properties of multilayer polyelectrolyte membranes through variation of film composition. Langmuir 2004, 20, 11545–11551. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.A.S.; Alloin, F.; Sanchez, J.Y.; Dufresne, A. Cross-Linked nanocomposite polymer electrolytes reinforced with cellulose whiskers. Macromolecules 2004, 4839–4844. [Google Scholar] [CrossRef]
- Stanton, B.W.; Harris, J.J.; Miller, M.D.; Bruening, M.L. Ultrathin, multilayered polyelectrolyte films as nanofiltration membranes. Langmuir 2003, 19, 7038–7042. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, G.S.; Lin, H.S.; Chen, Z.H.; Shi, J.L.; Lin, C.J. Study on bio-safety for Nano-Ag/HAp as anti-bacterial materials. Stomatology 2010, 9, 30–31. [Google Scholar]
- Chavan, D.N.; Patil, G.E.; Kajale, D.D.; Gaikwad, V.B.; Khanna, P.K.; Jain, G.H. Nano Ag-Doped Thick Film: A Low-Temperature Gas Sensor. J. Sens. 2011, 116, 539–556. [Google Scholar]
- He, H.; Chen, Z.; Wang, F.; Zhu, W.H. Investigation on Graphene/Ag Nano-Particles composite ink for flexible electronics. In Proceedings of the 2016 17th International Conference on Electronic Packaging Technology, Wuhan, China, 16–19 August 2016; pp. 612–615. [Google Scholar]
- Shchukin, D.G.; Sukhorukov, G.B.; Möhwald, H. Biomimetic Fabrication of Nanoengineered Hydroxyapatite/Polyelectrolyte Composite Shell. Chem. Mater. 2003, 15, 3947–3950. [Google Scholar] [CrossRef]
- Cui, X.; Li, C.M.; Bao, H. In situ fabrication of silver nanoarrays in hyaluronan/PDDA layer-by-layer assembled structure. J. Colloid Interface Sci. 2008, 327, 459–465. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Armelin, E.; Puiggalí, J.; Alemán, C. Insulating and semiconducting polymeric free-standing nanomembranes with biomedical applications. J. Mater. Chem. B 2015, 3, 5904–5932. [Google Scholar] [CrossRef]
- Najeeb, M.A.; Abdullah, S.M.; Aziz, F.; Ahmad, Z.; Rafique, S.; Wageh, S.; Al-Ghamdi, A.A.; Sulaiman, K.; Touati, F.; Shakoor, R.A.; et al. Structural, morphological and optical properties of PEDOT:PSS/QDs nano-composite films prepared by spin-casting. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 83, 64–68. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.; Kai, W.; Isogai, A.; Iwata, T. Elastic modulus of single cellulose microfibrils from tunicate measured by atomic force microscopy. Biomacromolecules 2009, 10, 2570–2571. [Google Scholar] [CrossRef] [PubMed]
- Anis, S.F.; Lalia, B.S.; Hashaikeh, R. Controlling swelling behavior of poly(vinyl) alcohol via networked cellulose and its application as a reverse osmosis membrane. Desalination 2014, 336, 138–145. [Google Scholar] [CrossRef]
- Schyrr, B.; Pasche, S.; Voirin, G. Biosensors based on porous cellulose nanocrystal—poly(vinyl alcohol) scaffolds. ACS Appl. Mater. Interfaces. 2014, 6, 12674–12683. [Google Scholar] [CrossRef]
- Luxbacher, T. Electrokinetic characterization of flat sheet membranes by streaming current measurement. Desalination 2006, 199, 376–377. [Google Scholar] [CrossRef]
- Bauman, M.; Košak, A.; Lobnik, A.; Petrinić, I.; Luxbacher, T. Nanofiltration membranes modified with alkoxysilanes: Surface characterization using zeta-potential. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 110–117. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, F.; Chen, Z.C.; Lin, X.F. Hepatic-targeting microcapsules construction by Layer-by-layer self-assembly of lactose-branched polyelectrolyte. Chem. J. Chin. Univ. 2011, 32, 957–963. [Google Scholar]
- Liao, K.C.; Lu, F.S.; Liu, C.P.; Fu, D.L. Preparation and research of butylene fipronil microencapsulation by layer-by-layer polyelectrolyte self-assembly. J. Macromol. Sci. A 2015, 52, 374–380. [Google Scholar] [CrossRef]
- Tian, Y.C.; Li, L.; Han, H.Y. Modification of spherical polyelectrolyte brushes by layer-by-layer self-assembly as observed by small angle X-ray scattering. Polymers 2016, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Haitami, A.E.; Martel, D.; Ball, V.; Nguyen, H.C.; Gonthier, E.; Labbe, P.; Voegel, J.C.; Schaaf, P.; Senger, B.; Boulmedais, F. Effect of the supporting electrolyte anion on the thickness of PSS/PAH multilayer films and on their permeability to an electroactive probe. Langmuir ACS J. Surf. Colloids 2009, 25, 2282–2289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ricotti, L.; Taccola, S.; Bernardeschi, I.; Pensabene, V.; Dario, P.; Menciassi, A. Quantification of growth and differentiation of C2C12 skeletal muscle cells on PSS-PAH-based polyelectrolyte layer-by-layer nanofilms. Biomed. Mater. 2011, 6, 031001. [Google Scholar] [CrossRef] [PubMed]
- Vincent, B. Permeabilty of silver cations through (PAH-PSS) m polyelectrolyte multilayer films to deposit silver in underlying (PAH-tannic acid) n film without external reducing agent at pH 5.0. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 70–74. [Google Scholar] [CrossRef]
- Aoki, P.H.; Volpati, D.; Cabrera, F.C.; Trombini, V.L.; Jr, A.R.; Constantino, C.J.L. Spray layer-by-layer films based on phospholipid vesicles aiming sensing application via e-tongue system. Mater. Sci. Eng. C 2012, 32, 862–871. [Google Scholar] [CrossRef]
- Aoki, P.H.; Volpati, D.; Jr, R.A.; Caetano, W.; Constantino, C.J.L. Layer-by-layer technique as a new approach to produce nanostructured films containing phospholipids as transducers in sensing applications. Langmuir ACS J. Surf. Colloids 2009, 25, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.J.; Jiang, X.G.; Lv, G.J.; Ma, X.J.; Jin, Y.Q.; Wang, F.; Chi, Y.; Yan, J.H. Investigation into particle size influence on PAH formation during dry sewage sludge pyrolysis: TG-FTIR analysis and batch scale research. J. Anal. Appl. Pyrolysis 2015, 112, 388–393. [Google Scholar] [CrossRef]
- Awada, H.; Monplaisir, D.; Daneauly, C. Growth of polyelectrolyte on lignocellulosic fibres: Study by ζ-potential, FTIR, and XPS. Bioresources 2012, 7, 2090–2104. [Google Scholar] [CrossRef]
- Jiang, M.; Nölting, B.; Stayton, P.S.; Sligar, S.G. Surface-Linked Molecular Monolayers of an Engineered Myoglobin: Structure, Stability, and Function. Langmuir 1996, 12, 1278–1283. [Google Scholar] [CrossRef]
- Akar, A. Reaction of some polymers containing carbonyl groups with phenol in the presence of mineral acids. Angew. Makromol. Chem. 2003, 160, 83–90. [Google Scholar] [CrossRef]
- Wilson, J.T.; Cui, W.; Chaikof, E.L. Layer-by-Layer Assembly of a Conformal Nanothin PEG Coating for Intraportal Islet Transplantation. Nano Lett. 2008, 8, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Cock, L.D.; Stefaan, D.; Geest, B.D.; Grooten, J.; Vervaet, C.; Remon, J.P.; Sukhorukov, G.B.; Antipina, M.N. Polymeric multilayer capsules in drug delivery. Angew. Chem. Int. Ed. 2010, 49, 6954–6973. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Bhattacharyya, A. Nanostructured Functional Coatings for High Performance Textiles. Bull. Mater. Sci. 2012, 35, 933–938. [Google Scholar] [CrossRef]
- Boiso, V.; Dubreuil, F.; Bogdanovic, G.; Fery, A. Interactions between silica surfaces coated by polyelectrolyte multilayers in aqueous environment: Comparison between precursor and multilayer regime. Colloids Surf. A Physicochem. Eng. Asp. 2004, 243, 147–155. [Google Scholar] [CrossRef]
- Kolasińska, M.; Krastev, R.; Warszyński, P. Characteristics of polyelectrolyte multilayers: Effect of PEI anchoring layer and posttreatment after deposition. J. Colloid Interface Sci. 2007, 305, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Balzer, B.N.; Micciulla, S.; Dodoo, S.; Zerball, M.; Gallei, M.; Rehahn, M.; Klitzing, R.V.; Hugel, T. Adhesion property profiles of supported thin polymer films. ACS Appl. Mater. Interfaces 2013, 5, 6300–6306. [Google Scholar] [CrossRef] [PubMed]
- Lösche, M.; Schmitt, J.; Decher, G.; Bouwman, W.G.; Kjaer, K. Detailed Structure of Molecularly Thin Polyelectrolyte Multilayer Films on Solid Substrates as Revealed by Neutron Reflectometry. Macromolecules 1998, 31, 8893–8906. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Rhee, K.Y.; Park, S.J. Nanodiamond nanocluster-decorated graphene oxide/epoxy nanocomposites with enhanced mechanical behavior and thermal stability. Compos. Part B Eng. 2017, 114, 111–120. [Google Scholar] [CrossRef]
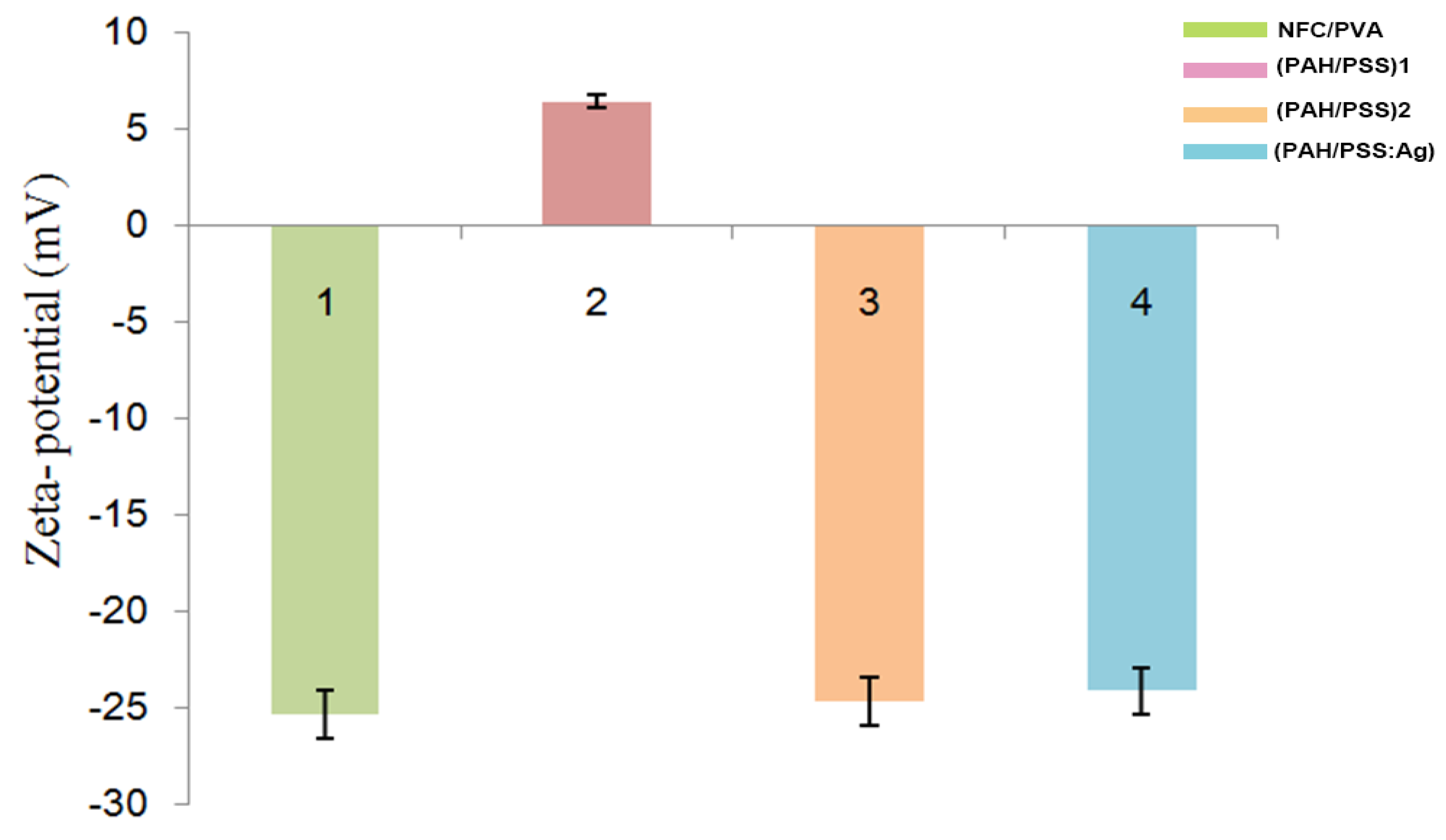
| Multilayer Film | Mean Pore Size (μm) | Elastic Modulus (GPa) | Tensile Strength (MPa) |
|---|---|---|---|
| NFC/PVA | 7.53 | 0.25 | 12.94 |
| (PAH/PSS) 1 | 6.75 | 0.83 | 6.82 |
| (PAH/PSS) 2 | 5.54 | 4.29 | 21.90 |
| (PAH/PSS: Ag) 2 | 0.28 | 3.62 | 28.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Q.; Wang, X.; Ragauskas, A.J. Dynamic Self-Assembly of Polyelectrolyte Composite Nanomaterial Film. Polymers 2019, 11, 1258. https://doi.org/10.3390/polym11081258
Hou Q, Wang X, Ragauskas AJ. Dynamic Self-Assembly of Polyelectrolyte Composite Nanomaterial Film. Polymers. 2019; 11(8):1258. https://doi.org/10.3390/polym11081258
Chicago/Turabian StyleHou, Qiupeng, Xiwen Wang, and Arthur J. Ragauskas. 2019. "Dynamic Self-Assembly of Polyelectrolyte Composite Nanomaterial Film" Polymers 11, no. 8: 1258. https://doi.org/10.3390/polym11081258
APA StyleHou, Q., Wang, X., & Ragauskas, A. J. (2019). Dynamic Self-Assembly of Polyelectrolyte Composite Nanomaterial Film. Polymers, 11(8), 1258. https://doi.org/10.3390/polym11081258






