An Immunosensor Based on Au-Ag Bimetallic NPs Patterned on a Thermal Resistant Flexible Polymer Substrate for In-Vitro Protein Detection
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
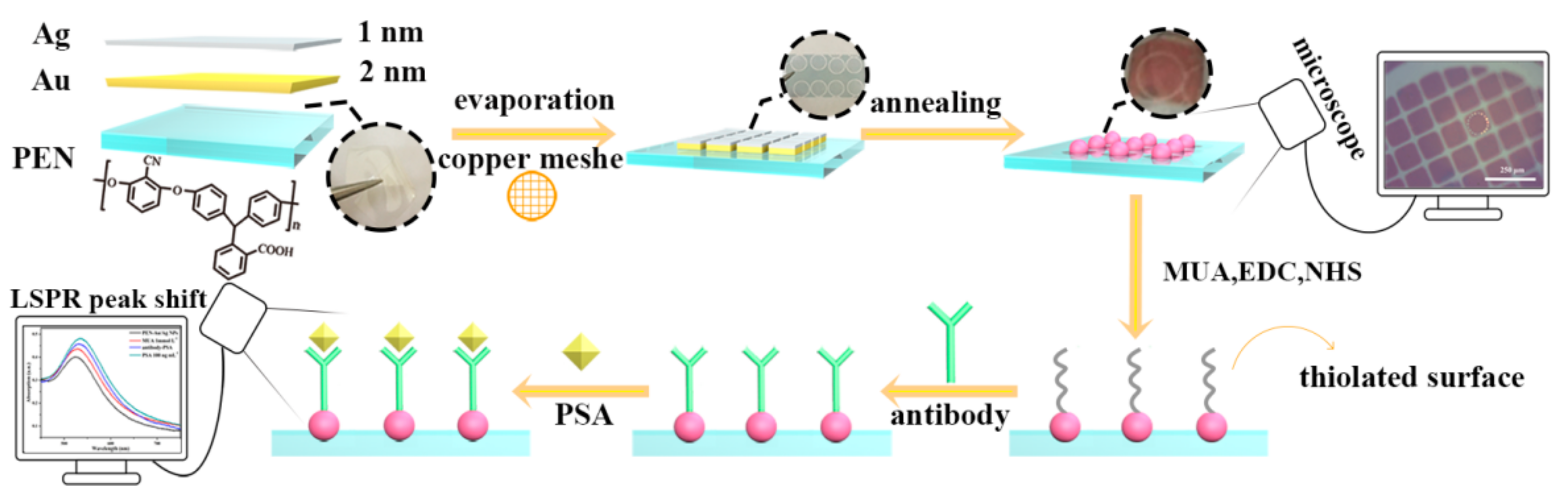
2.2. Preparation of PEN Casting Film
2.3. Preparation of Au-Ag NPs Patterns on PEN Substrates
2.4. Preparation of Optical Probes for Specific Immunodetection of Prostate Specific Antigen
2.5. Thermo Analysis, Molecular Analysis, Alloy Structure, Location Spectroscopy, and Morphology Characterization
3. Results and Discussion
3.1. Influence of an Additional Ag Layer
3.2. Influence of Rapid Annealing Temperature on Au-Ag NPs
3.3. Immunodetectionof the PSA Antigen Based on a PEN/Au-Ag NPs LSPR Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bao, Z.; Chen, X. Flexible and Stretchable Devices. Adv. Mater. 2016, 28, 4177–4179. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, A.S.; Opoku, C.; Poulin-Vittrant, G.; Camara, N.; Daumont, C.; Barbagiovanni, E.G.; Franzò, G.; Mirabella, S.; Alquier, D. Flexible Organic/Inorganic Hybrid Field-Effect Transistors with High Performance and Operational Stability. ACS Appl. Mater. Interfaces 2016, 9, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.W.; Yoon, S.M.; Yu, S.M.; Ju, Y.; Kim, D. Solution-shearing-processed flexible polymer solar mini sub-modules fabricated on an embedded silver-grid substrate. Sol. Energy Mater. Sol. Cells 2019, 193, 169–177. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, Y.; Cheng, D.; Li, X.; Huang, Y.; Rogers, J.A. All-Elastomeric, Strain-Responsive Thermochromic Color Indicators. Small 2014, 10, 1266–1271. [Google Scholar] [CrossRef]
- Shou, H.; Jia, K.; Zhou, X.; Gao, L.; He, X.; Zhou, X.; Zhang, D.; Liu, X. Large scale synthesis of an amorphous polyester elastomer with tunable mechanoluminescence and preliminary application in optical strain sensing. J. Mater. Chem. C 2017, 5, 4134–4138. [Google Scholar] [CrossRef]
- Nassar, J.M.; Mishra, K.; Lau, K.; Aguirre-Pablo, A.A.; Hussain, M.M. Recyclable Nonfunctionalized Paper-Based Ultralow-Cost Wearable Health Monitoring System. Adv. Mater. Technol. 2017, 2, 1600228. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, X.; Shou, H.; He, X.; Jia, K.; Liu, X. Immobilization of Ag nanowire into zinc phthalocyanine doped copolyester elastomer for optoelectric flexible strain sensor. Chem. Phys. Lett. 2018, 693, 55–59. [Google Scholar] [CrossRef]
- Basker, D.K.; Saravanamuttu, K. Spontaneous Formation of Fractal Aggregates of Au Nanoparticles in Epoxy-Siloxane Films and Their Application as Substrates for NIR Surface Enhanced Raman Spectroscopy. Polymers 2017, 9, 507. [Google Scholar] [CrossRef]
- You, Y.; Wang, Y.; Tu, L.; Tong, L.; Wei, R.; Liu, X. Interface Modulation of Core-Shell Structured BaTiO3@polyaniline for Novel Dielectric Materials from Its Nanocomposite with Polyarylene Ether Nitrile. Polymers 2018, 10, 1378. [Google Scholar] [CrossRef]
- Wei, R.; Tu, L.; You, Y.; Zhan, C.; Wang, Y.; Liu, X. Fabrication of crosslinked single-component polyarylene ether nitrile composite with enhanced dielectric properties. Polymers 2019, 161, 162–169. [Google Scholar] [CrossRef]
- Wang, P.; Jia, K.; Zhou, X.; Guan, X.; Wang, L.; Tian, Y.; Wu, C.; Liu, X. Ca2+ Induced Crosslinking of AIE-Active Polyarylene Ether Nitrile into Fluorescent Polymeric Nanoparticles for Cellular Bioimaging. Macromol. Rapid Commun. 2017, 38, 1700360. [Google Scholar] [CrossRef]
- Tang, H.; Pu, Z.; Wei, J.; Guo, H.; Huang, X.; Liu, X. Fluorescence-color-tunable and transparent polyarylene ether nitrile films with high thermal stability and mechanical strength based on polymeric rare-earth complexes for roll-up displays. Mater. Lett. 2013, 91, 235–238. [Google Scholar] [CrossRef]
- Jia, K.; Wang, P.; Wei, S.; Huang, Y.; Liu, X. Scalable creation of gold nanostructures on high performance engineering polymeric substrate. Appl. Surf. Sci. 2017, 426, 579–586. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-encapsulated metal nanoparticles: Synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 2001, 32, 181–190. [Google Scholar] [CrossRef]
- Son, Y.; Yeo, J.; Moon, H.; Lim, T.W.; Hong, S.; Nam, K.H.; Yoo, S.; Grigoropoulos, C.P.; Yang, D.Y.; Ko, S.H. Nanoscale electronics: Digital fabrication by direct femtosecond laser processing of metal nanoparticles. Adv. Mater. 2011, 23, 3176–3181. [Google Scholar] [CrossRef]
- Hu, M.; Novo, C.; Funston, A.; Wang, H.; Staleva, H.; Zou, S.; Mulvaney, P.; Xia, Y.; Hartland, G.V. Dark-field microscopy studies of single metal nanoparticles: Understanding the factors that influence the linewidth of the localized surface plasmon resonance. J. Mater. Chem. 2008, 18, 1949–1960. [Google Scholar] [CrossRef]
- Fong, K.E.; Yung, L.Y.L. Localized surface plasmon resonance: A unique property of plasmonic nanoparticles for nucleic acid detection. Nanoscale 2013, 5, 12043. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M.; Kreibig, P.D.U.; Vollmer, P.D.M. Optical Properties of Metal Clusters; Springer Science and Business Media LLC: Berlin, Germany, 1995; Volume 25. [Google Scholar]
- Weng, G.; Yang, Y.; Zhao, J.; Zhu, J.; Li, J.; Zhao, J. Preparation and SERS performance of Au NP/paper strips based on inkjet printing and seed mediated growth: The effect of silver ions. Solid State Commun. 2018, 272, 67–73. [Google Scholar] [CrossRef]
- Chen, C.W.; Chen, Y.J.; Thomas, S.R.; Yen, Y.T.; Cheng, L.T.; Wang, Y.C.; Su, T.Y.; Lin, H.; Hsu, C.H.; Ho, J.C.; et al. Enhanced power conversion efficiency in solution-processed rigid CuIn (S, Se)2 and flexible Cu (In, Ga)Se2 solar cells utilizing plasmonic Au-SiO2 core-shell nanoparticles. Solar RRL 2019, 3, 1800343. [Google Scholar] [CrossRef]
- Kim, K.; Lee, J.W.; Lee, H.B.; Shin, K.S. Novel Fabrication of Au Nanoparticle Films on Planar and Curved Surfaces of Glass and Fiber Materials. Langmuir 2009, 25, 9697–9702. [Google Scholar] [CrossRef]
- Gwo, S.; Chen, H.-Y.; Sun, L.; Lin, M.-H.; Li, X. Nanomanipulation and controlled self-assembly of metal nanoparticles and nanocrystals for plasmonics. Chem. Soc. Rev. 2016, 45, 5672–5716. [Google Scholar] [CrossRef]
- Nsimama, P.D.; Herz, A.; Wang, D.; Schaaf, P. Influence of the substrate on the morphological evolution of gold thin films during solid-state dewetting. Appl. Surf. Sci. 2016, 388, 475–482. [Google Scholar] [CrossRef]
- Do, M.T.; Tong, Q.C.; Lidiak, A.; Luong, M.H.; Ledoux-Rak, I.; Lai, N.D. Nano-patterning of gold thin film by thermal annealing combined with laser interference techniques. Appl. Phys. A 2016, 122, 360. [Google Scholar] [CrossRef]
- Karakouz, T.; Tesler, A.B.; Bendikov, T.A.; Vaskevich, A.; Rubinstein, I. Highly Stable Localized Plasmon Transducers Obtained by Thermal Embedding of Gold Island Films on Glass. Adv. Mater. 2008, 20, 3893–3899. [Google Scholar] [CrossRef]
- Khan, Y.; Li, A.; Chang, L.; Li, L.; Guo, L. Gold nano disks arrays for localized surface plasmon resonance based detection of PSA cancer marker. Sens. Actuators B 2018, 255, 1298–1307. [Google Scholar] [CrossRef]
- Cataldi, U.; Caputo, R.; Kurylyak, Y.; Klein, G.; Chekini, M.; Umeton, C.; Bürgi, T. Growing gold nanoparticles on a flexible substrate to enable simple mechanical control of their plasmonic coupling. J. Mater. Chem. C 2014, 2, 7927–7933. [Google Scholar] [CrossRef]
- Kracker, M.; Worsch, C.; Bocker, C.; Rüssel, C. Optical properties of dewetted thin silver/gold multilayer films on glass substrates. Thin Solid Film. 2013, 539, 47–54. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tatsuma, T. Electrodeposition of thermally stable gold and silver nanoparticle ensembles through a thin alumina nanomask. Nanoscale 2010, 2, 1494. [Google Scholar] [CrossRef]
- Durán-Álvarez, J.C.; Avella, E.; Ramírez-Zamora, R.M.; Zanella, R. Photocatalytic degradation of ciprofloxacin using mono-(Au, Ag and Cu) and bi-(Au–Ag and Au–Cu) metallic nanoparticles supported on TiO 2 under UV-C and simulated sunlight. Catal. Today 2016, 266, 175–187. [Google Scholar] [CrossRef]
- Zhao, C.; Li, B.; Du, J.; Chen, J.; Li, Y. Microstructure and optical absorption property of Au nanoparticles and Au, Ag bimetal nanoparticles separately dispersed Al2O3 composite films. J. Alloy. Compd. 2016, 691, 772–777. [Google Scholar] [CrossRef]
- Jia, K.; Khaywah, M.Y.; Li, Y.; Bijeon, J.L.; Adam, P.M.; Deturche, R.; Guelorget, B.; Francois, M.; Louarn, G.; Ionescu, R.E. Strong improvements of localized surface plasmon resonance sensitivity by using Au/Ag bimetallic nanostructures modified with polydopamine films. ACS Appl. Mater. Interfaces 2014, 6, 219–227. [Google Scholar] [CrossRef]
- Puchalski, M.; Kowalczyk, P.; Zasada, I.; Krukowski, P.; Olejniczak, W.; Kowalczyk, P. Alloying process at the interface of silver nanoparticles deposited on Au(111) substrate due to the high-temperature treatments. J. Alloy. Compd. 2009, 481, 486–491. [Google Scholar] [CrossRef]
- Kariuki, N.N.; Luo, J.; Maye, M.M.; Hassan, S.A.; Menard, T.; Naslund, H.R.; Lin, Y.; Wang, C.; Engelhard, M.H.; Zhong, C.-J. Composition-controlled synthesis of bimetallic gold−silver nanoparticles. Langmuir 2004, 20, 11240–11246. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L.; Shou, H.; Wang, J.; Pan, L.; Jia, K.; Liu, X. Chain conformation dependent fluorescence of blue-emitting poly(arylene ether nitrile). J. Lumin. 2016, 179, 622–628. [Google Scholar] [CrossRef]
- Furmanski, J.; Cady, C.M.; Brown, E.N. Time–temperature equivalence and adiabatic heating at large strains in high density polyethylene and ultrahigh molecular weight polyethylene. Polymers 2013, 54, 381–390. [Google Scholar] [CrossRef]
- Tominaga, M.; Shimazoe, T.; Nagashima, M.; Kusuda, H.; Kubo, A.; Kuwahara, Y.; Taniguchi, I. Electrocatalytic oxidation of glucose at gold–silver alloy, silver and gold nanoparticles in an alkaline solution. J. Electroanal. Chem. 2006, 590, 37–46. [Google Scholar] [CrossRef]
- Seguini, G.; Curi, J.; Spiga, S.; Tallarida, G.; Wiemer, C.; Perego, M. Solid-state dewetting of ultra-thin Au films on SiO2 and HfO2. Nanotechnology 2014, 25, 495603. [Google Scholar] [CrossRef]
- Jonous, Z.A.; Shayeh, J.S.; Yazdian, F.; Yadegari, A.; Hashemi, M.; Omidi, M. An electrochemical biosensor for prostate cancer biomarker detection using graphene oxide-gold nanostructures. Eng. Life Sci. 2019, 19, 206–216. [Google Scholar] [CrossRef]
- Jia, K.; Bijeon, J.L.; Adam, P.M.; Ionescu, R.E. A facile and cost-effective TEM grid approach to design gold nano-structured substrates for high throughput plasmonic sensitive detection of biomolecules. Analyst 2013, 138, 1015. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Wei, S.; Tong, L.; He, X.; Bai, Y.; Jia, K.; Liu, X. An Immunosensor Based on Au-Ag Bimetallic NPs Patterned on a Thermal Resistant Flexible Polymer Substrate for In-Vitro Protein Detection. Polymers 2019, 11, 1257. https://doi.org/10.3390/polym11081257
Wang P, Wei S, Tong L, He X, Bai Y, Jia K, Liu X. An Immunosensor Based on Au-Ag Bimetallic NPs Patterned on a Thermal Resistant Flexible Polymer Substrate for In-Vitro Protein Detection. Polymers. 2019; 11(8):1257. https://doi.org/10.3390/polym11081257
Chicago/Turabian StyleWang, Pan, Shiliang Wei, Lifen Tong, Xiaohong He, Yun Bai, Kun Jia, and Xiaobo Liu. 2019. "An Immunosensor Based on Au-Ag Bimetallic NPs Patterned on a Thermal Resistant Flexible Polymer Substrate for In-Vitro Protein Detection" Polymers 11, no. 8: 1257. https://doi.org/10.3390/polym11081257
APA StyleWang, P., Wei, S., Tong, L., He, X., Bai, Y., Jia, K., & Liu, X. (2019). An Immunosensor Based on Au-Ag Bimetallic NPs Patterned on a Thermal Resistant Flexible Polymer Substrate for In-Vitro Protein Detection. Polymers, 11(8), 1257. https://doi.org/10.3390/polym11081257




