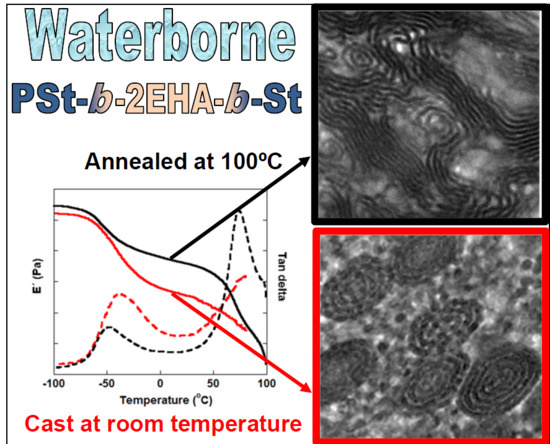
Mechanical and Morphological Properties of Waterborne ABA Hard-Soft-Hard Block Copolymers Synthesized by Means of RAFT Miniemulsion Polymerization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
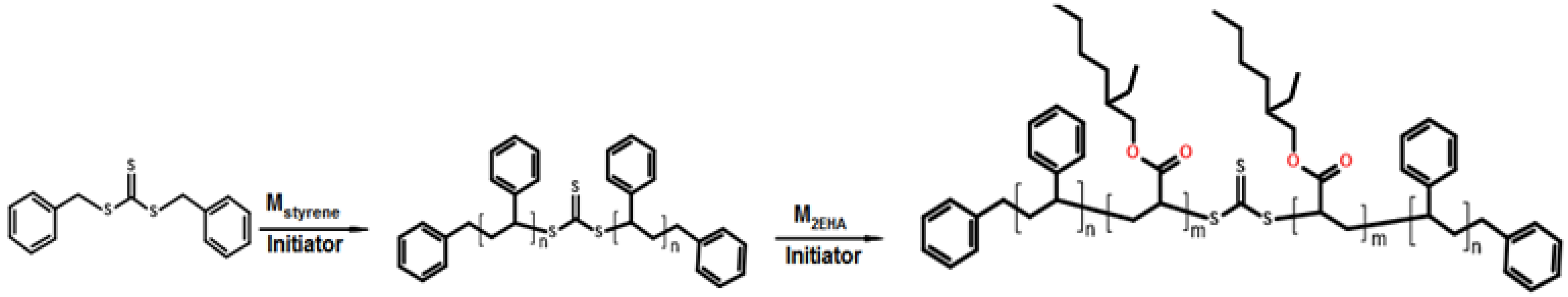
2.2.1. Synthesis of the First A Block: Batch Miniemulsion Polymerization
2.2.2. Synthesis of the Second Block: Semi-batch Emulsion Polymerization
3. Results and Discussion
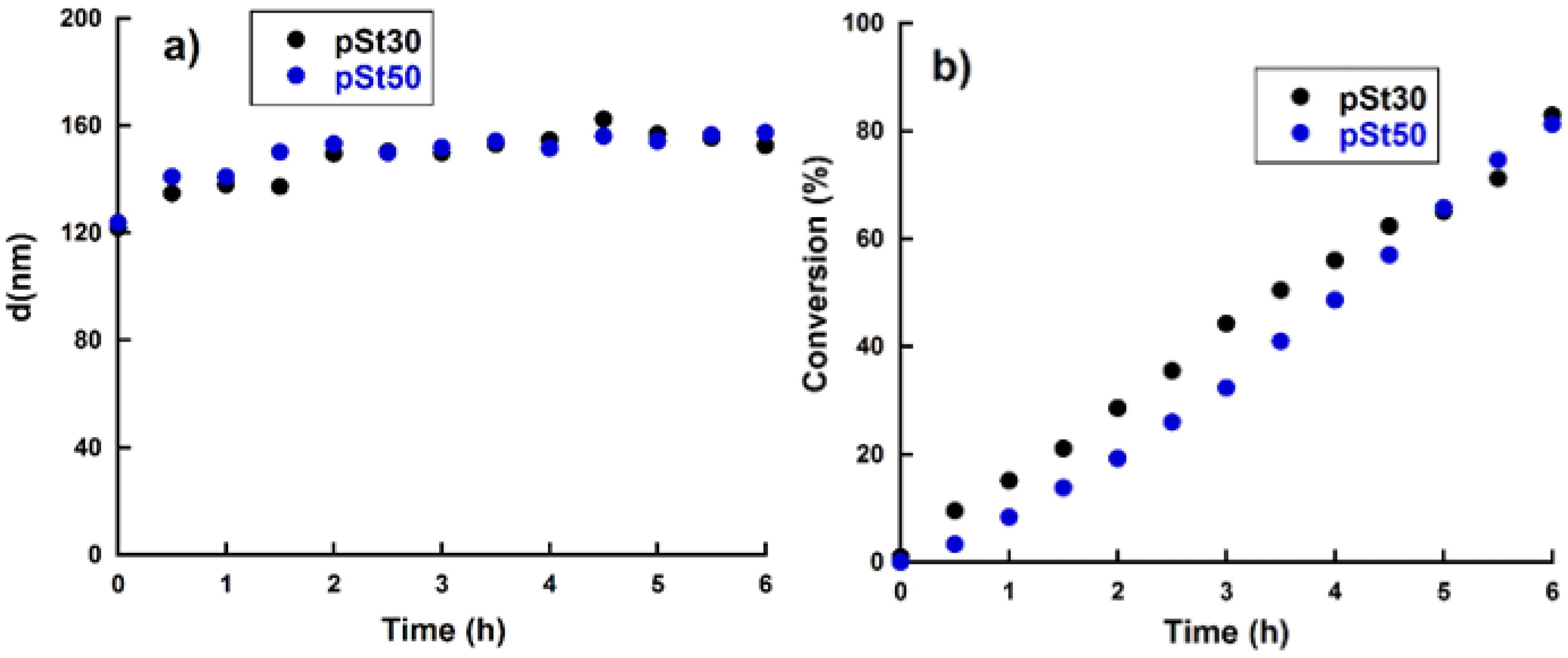
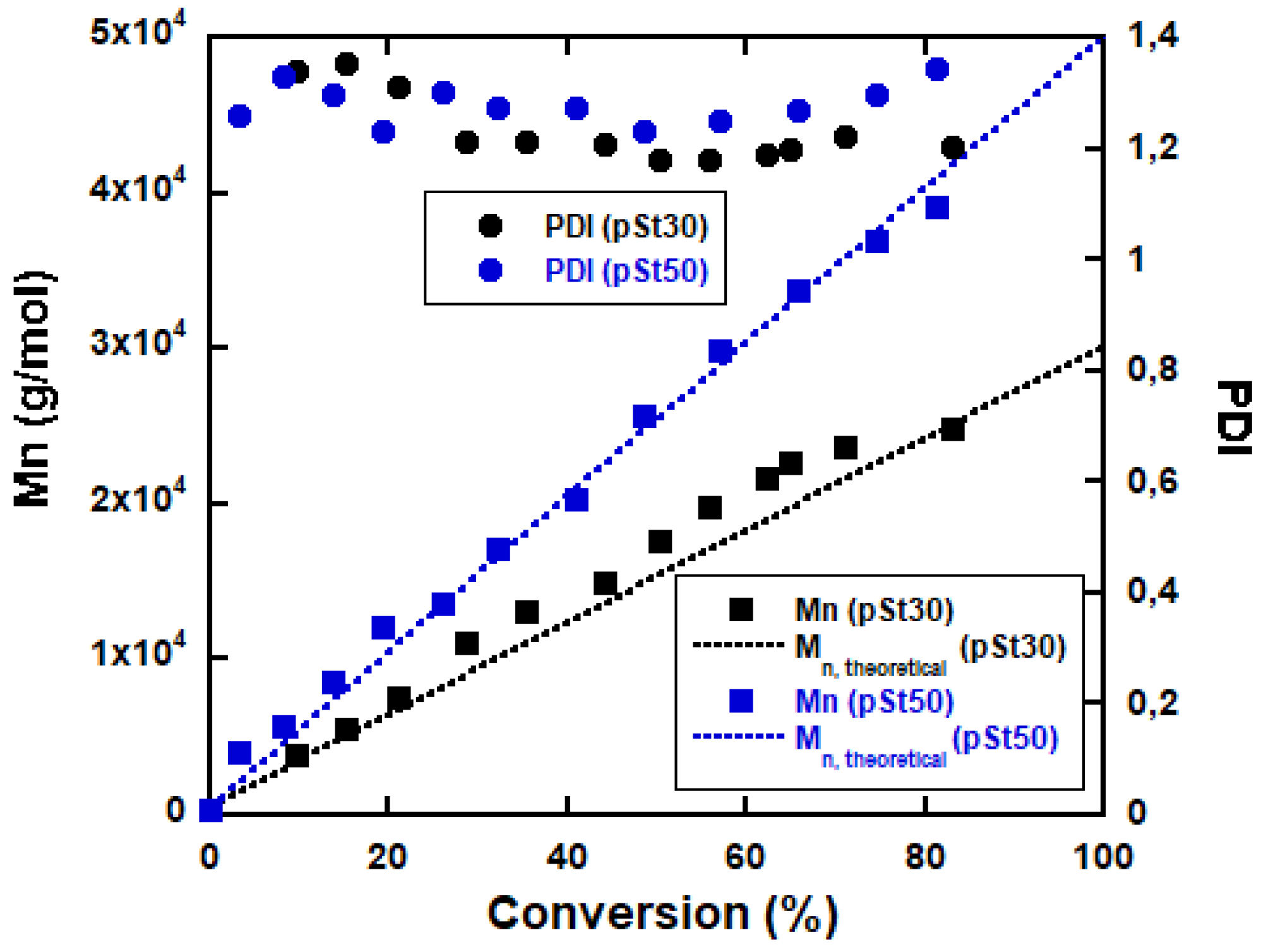
3.1. RAFT Mediated Miniemulsion Polymerization
3.2. ABA Triblock Copolymer Latex
3.3. Thermal Properties of the Initial Homopolymers and Final Block Copolymers
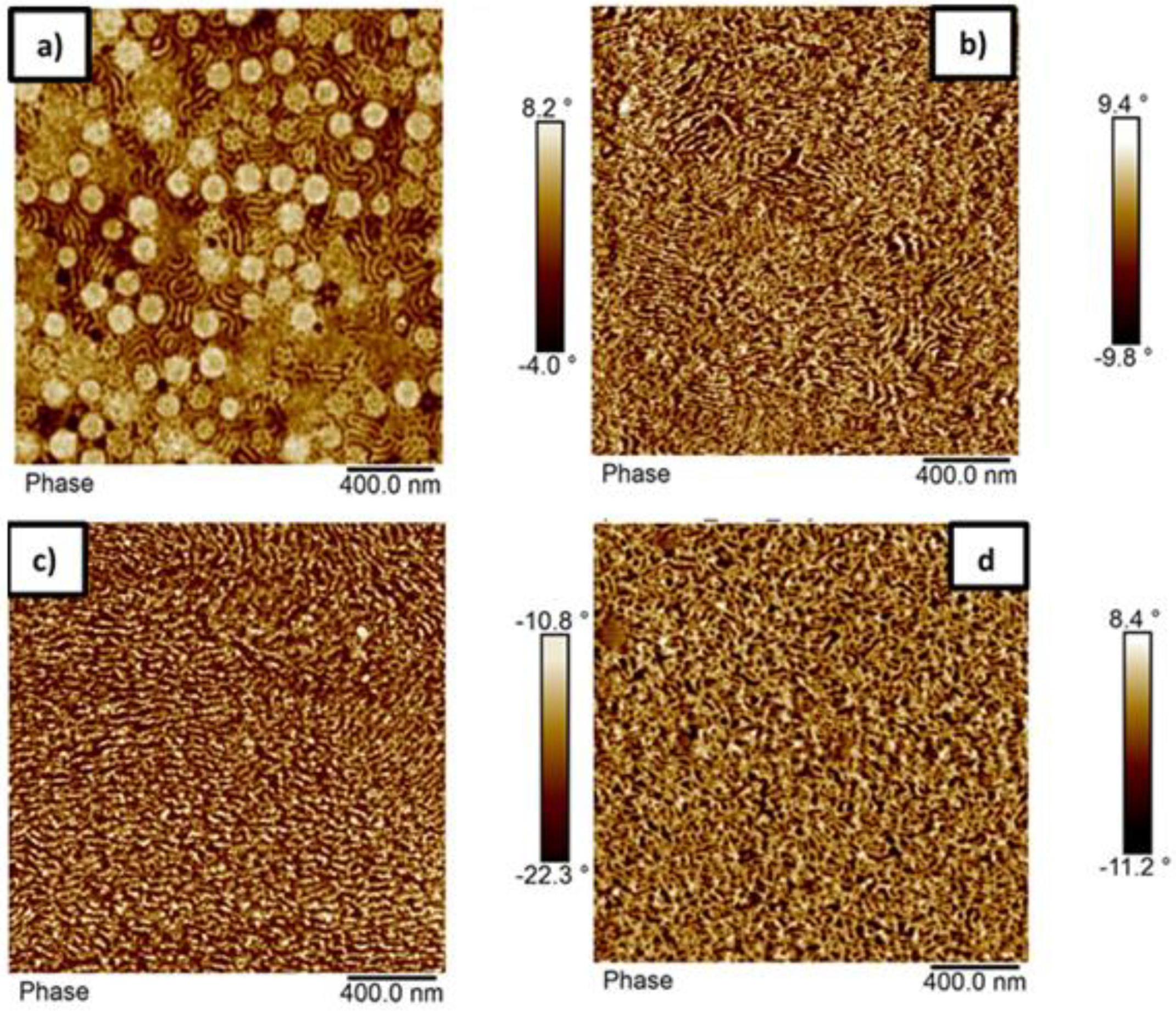
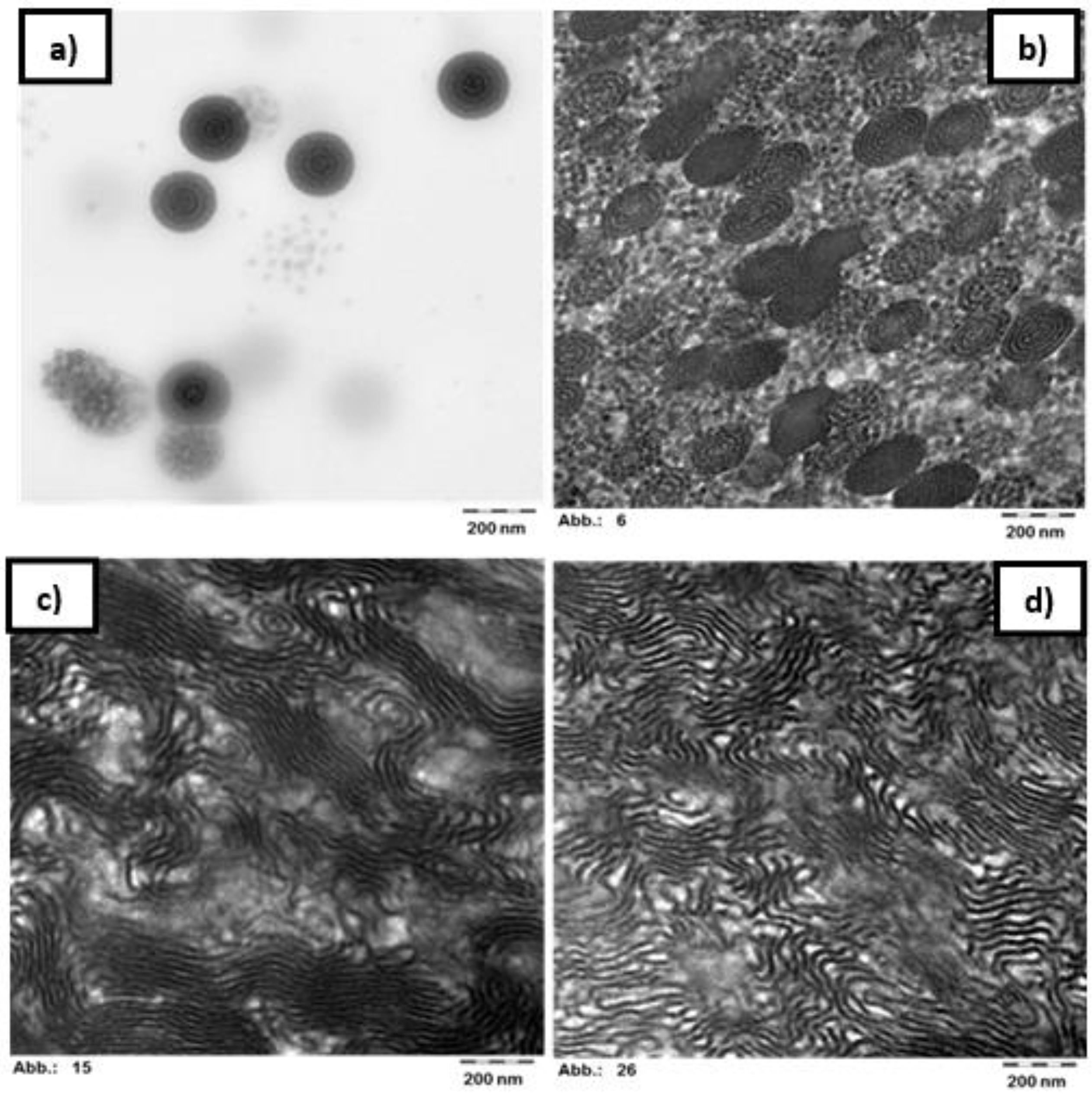
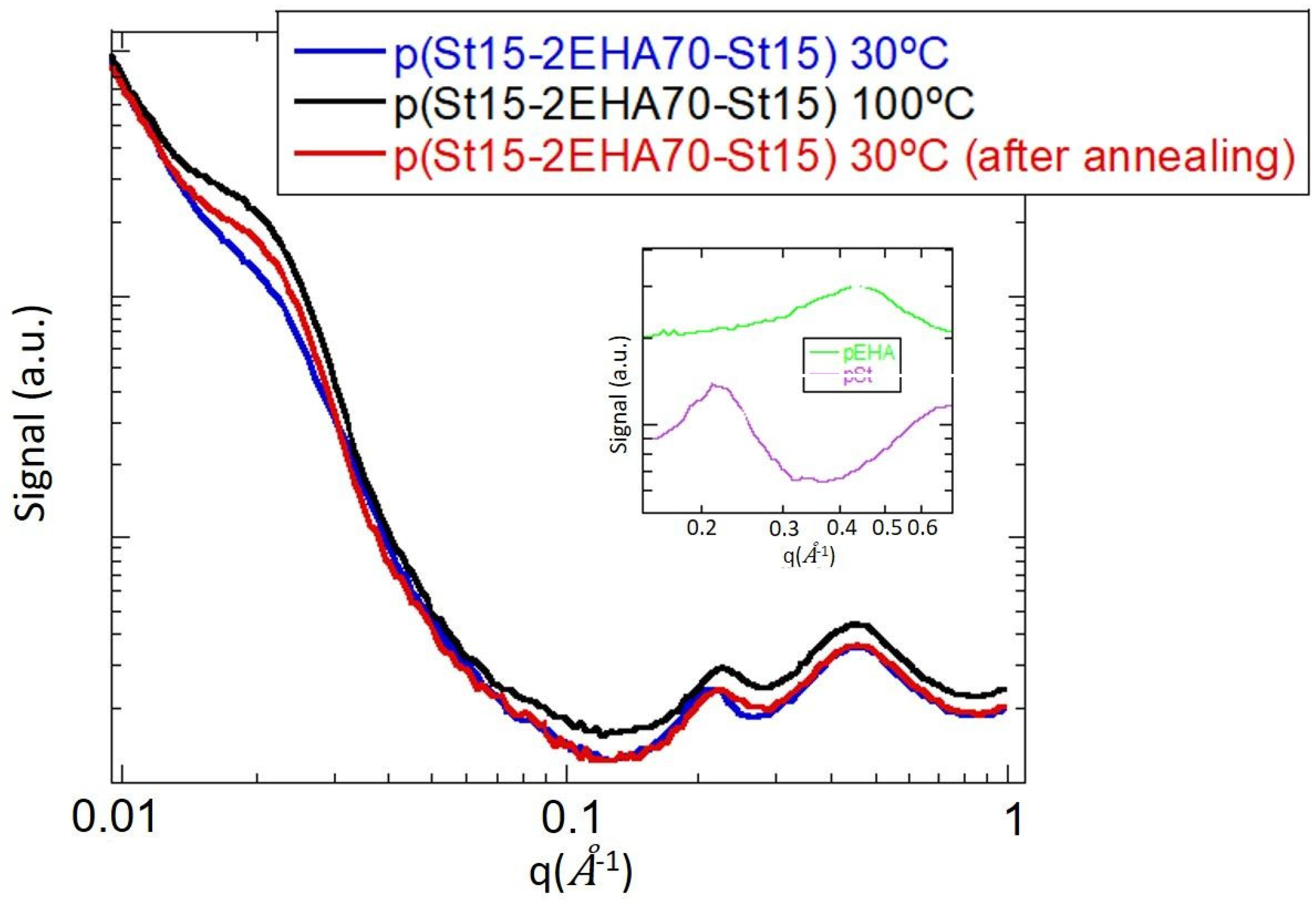
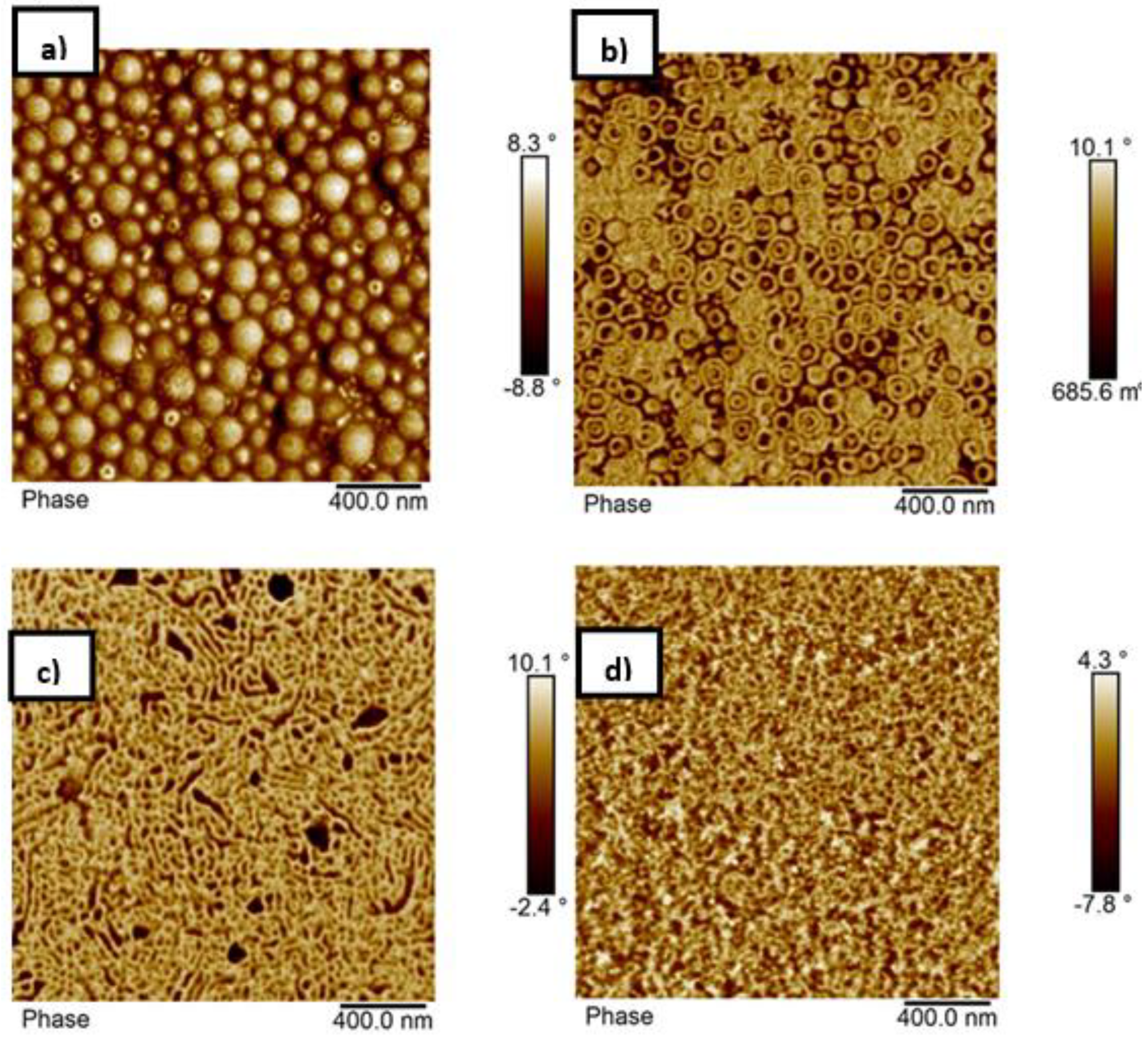
3.4. Morphology of the Block Copolymers
3.5. Viscoelastic Properties of the ABA Hard-Soft-Hard Block Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-Mediated Polymerization: The Pivotal Role of the kd Value of the Initiating Alkoxyamine and the Importance of the Experimental Conditions. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Ruzette, A.V.; Leibler, L. Block copolymers in tomorrow’s plastics. Nat. Mater 2005, 4, 19–31. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, R.K.; Hawker, C.J.; Wooley, K.L. Cross-linked block copolymer micelles: Functional nanostructures of great potential and versatility. Chem. Soc. Rev. 2006, 35, 1068–1083. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Takekoh, R.; Nakatsuru, R.; Okubo, M. Effect of stabilizer on formation of “onionlike” multilayered polystyrene-block-poly(methyl methacrylate) particles. Langmuir 2007, 23, 5978–5983. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Yoon, J.; Thomas, E.L. Enabling nanotechnology with self assembled block copolymer patterns. Polymer 2003, 44, 6725–6760. [Google Scholar] [CrossRef]
- Bang, J.; Kim, S.H.; Drockenmuller, E.; Misner, M.J.; Russell, T.P.; Hawker, C.J. Defect-free nanoporous thin films from ABC triblock copolymers. J. Am. Chem. Soc. 2006, 128, 7622–7629. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block Copolymer Thermodynamics: Theory and Experiment. Annu. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef]
- Okubo, M.; Takekoh, R.; Saito, N. Effect of graft polymer on the formation of micron-sized, monodisperse, “onion-like” alternately multilayered poly(methyl methacrylate)/polystyrene composite particles by reconstruction of morphology with the solvent-absorbing/releasing method. Colloid Polym. Sci. 2003, 281, 945–950. [Google Scholar] [CrossRef]
- Okubo, M.; Saito, N.; Takekoh, R.; Kobayashi, H. Morphology of polystyrene/polystyrene-block-poly(methyl methacrylate)/poly(methyl methacrylate) composite particles. Polymer 2005, 46, 1151–1156. [Google Scholar] [CrossRef]
- Kitayama, Y.; Kishida, K.; Minami, H.; Okubo, M. Preparation of poly(n-butyl acrylate)-b-polystyrene particles by emulsifier-free, organotellurium-mediated living radical emulsion polymerization. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1991–1996. [Google Scholar] [CrossRef]
- Nicolas, J.; Ruzette, A.V.; Farcet, C.; Gérard, P.; Magnet, S.; Charleux, B. Nanostructured latex particles synthesized by nitroxide-mediated controlled/living free-radical polymerization in emulsion. Polymer 2007, 48, 7029–7040. [Google Scholar] [CrossRef]
- Bowes, A.; Mcleary, J.B.; Sanderson, R.D. AB and ABA Type Butyl Acrylate and Styrene Block Copolymers via RAFT-Mediated Miniemulsion Polymerization. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 588–604. [Google Scholar] [CrossRef]
- Butte, A.; Storti, G.; Morbidelli, M. Miniemulsion Living Free Radical Polymerization of Styrene. Macromolecules 2001, 34, 5885–5896. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X. Reversible addition-fragmentation transfer (RAFT) copolymerization of methyl methacrylate and styrene in miniemulsion. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 6248–6258. [Google Scholar] [CrossRef]
- Wei, R.; Luo, Y.; Li, Z. Synthesis of structured nanoparticles of styrene/butadiene block copolymers via RAFT seeded emulsion polymerization. Polymer 2010, 51, 3879–3886. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Zhan, X.; Chen, F.; Rao, G.; Xiong, J. Preparation, kinetics and microstructures of well-defined PS-b-PS/Bd diblock copolymers via RAFT miniemulsion polymerization. J. Polym. Res. 2013, 20, 1–13. [Google Scholar] [CrossRef]
- Wei, R.; Luo, Y.; Zeng, W.; Wang, F.; Xu, S. Styrene–Butadiene–Styrene Triblock Copolymer Latex via Reversible Addition–Fragmentation Chain Transfer Miniemulsion Polymerization. Ind. Eng. Chem. Res. 2012, 51, 15530–15535. [Google Scholar] [CrossRef]
- Froimowicz, P.; Van Heukelum, B.; Scholten, C.; Greiner, K.; Araujo, O.; Landfester, K. Highly symmetric poly(styrene)-block-poly(butadiene-stat-styrene)-block- poly(styrene) copolymer prepared in a non-stop one-pot RAFT polymerization in miniemulsion. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 883–889. [Google Scholar] [CrossRef]
- Yang, L.; Han, Q.; Song, Q.; Li, H.; Zhao, Q.; Shen, Y.; Luo, Y. Control over ABA-type triblock copolymer latex morphology in RAFT miniemulsion polymerization and mechanical properties of the latex films. Colloid Polym. Sci. 2017, 295, 891–902. [Google Scholar] [CrossRef]
- Siljanovska Petreska, G.; Auschra, C.; Paulis, M. Confinement driven crystallization of ABA crystalline-soft-crystalline block copolymers synthesized via RAFT mediated miniemulsion polymerization. Polymer 2018, 158, 327–337. [Google Scholar] [CrossRef]
- De Brouwer, H.; Tsavalas, J.G.; Schork, F.J. Living Radical Polymerization in Miniemulsion Using Reversible Addition−Fragmentation Chain Transfer. Macromolecules 2000, 33, 9239–9246. [Google Scholar] [CrossRef]
- Pérez-Martínez, B.T.; Farías-Cepeda, L.; Ovando-Medina, V.M.; Asua, J.M.; Rosales-Marines, L.; Tomovska, R. Miniemulsion copolymerization of (meth)acrylates in the presence of functionalized multiwalled carbon nanotubes for reinforced coating applications. Beilstein J. Nanotechnol. 2017, 8, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Agirre, A.; Nase, J.; Degrandi, E.; Creton, C.; Asua, J.M. Improving adhesion of acrylic waterborne PSAs to low surface energy materials: Introduction of stearyl acrylate. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5030–5039. [Google Scholar] [CrossRef]
- Koiry, B.P.; Singha, N.K. Copper mediated controlled radical copolymerization of styrene and 2-ethylhexyl acrylate and determination of their reactivity ratios. Front. Chem. 2014, 2, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Plessis, C.; Arzamendi, G.; Leiza, J.R.; Schoonbrood, H.A.S.; Charmot, D.; Asua, J.M. Seeded Semibatch Emulsion Polymerization of n -Butyl Acrylate. Kinetics and Structural Properties. Macromolecules 2000, 33, 5041–5047. [Google Scholar] [CrossRef]
- Ballard, N.; Asua, J.M. Progress in Polymer Science Radical polymerization of acrylic monomers: An overview. Prog. Polym. Sci. 2018, 79, 40–60. [Google Scholar] [CrossRef]
- Rieger, J. The glass transition temperature results of a round robin test. J. Therm. Anal. 1996, 46, 965–972. [Google Scholar] [CrossRef]
- Mehravar, E.; Leiza, J.R.; Asua, J.M. Performance of latexes containing nano-sized crystalline domains formed by comb-like polymers. Polymer 2016, 96, 121–129. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Lee, J.; Wang, T.; Shin, K.; Cho, J. High-Pressure Neutron Scattering and Random-Phase Approximation Analysis of a Molten Baroplastic Diblock Copolymer. Polymer 2019, 175, 265–271. [Google Scholar] [CrossRef]
- Wang, S.; Robertson, M.L. Thermodynamic Interactions between Polystyrene and Long-Chain Poly(n-Alkyl Acrylates) Derived from Plant Oils. ACS Appl. Mater. Interf. 2015, 7, 12109–12118. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Bates, F.S.; Fredrickson, G.H. Block copolymers-designer soft materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Matsen, M.W.; Thompson, R.B. Equilibrium behavior of symmetric ABA triblock copolymer melts. J. Chem. Phys. 1999, 111, 7139–7146. [Google Scholar] [CrossRef]
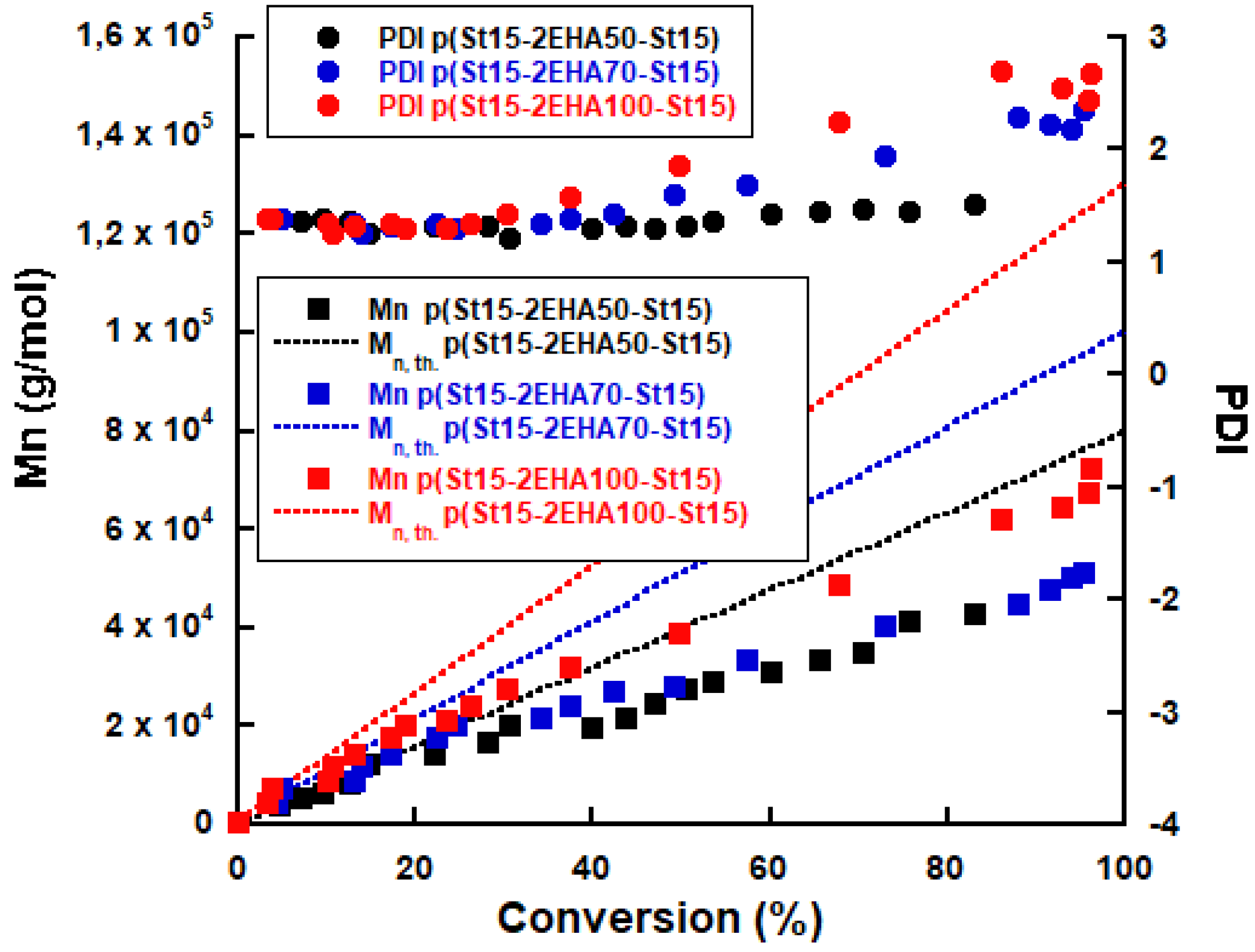



| Reactions | Conversion (%) | Mn,GPC (g/mol) | PDI | dd (nm) | dp (nm) | Conversion (%) | dp (nm) | Mn,GPC (g/mol) | PDI |
|---|---|---|---|---|---|---|---|---|---|
| pSt Homopolymers | p(St-2EHA-St) Block Copolymers | ||||||||
| p(St25-2EHA50-St25) | 81.2 | 39,119 | 1.34 | 119 | 159 | 84.0 | 177 | 73,854 | 1.45 |
| p(St15-2EHA50-St15) | 81.8 | 20,204 | 1.22 | 116 | 154 | 83.1 | 187 | 42,877 | 1.52 |
| p(St15-2EHA70-St15) | 82.0 | 20,044 | 1.29 | 130 | 161 | 95.2 | 187 | 51,046 | 2.36 |
| p(St15-2EHA100-St15) | 82.0 | 20,044 | 1.29 | 130 | 161 | 96.3 | 207 | 72,203 | 2.6 |
| Material Code | Tg1 (°C) | Tg2 (°C) |
|---|---|---|
| DSC analysis of the samples dried at 23 °C | ||
| pSt30 | 58 | - |
| pSt50 | 54 | - |
| p(St25-2EHA50-St25) | −43 | 58 |
| p(St15-2EHA50-St15) | −58 | 55 |
| p(St15-2EHA70-St15) | −60 | 60 |
| p(St15-2EHA100- St15) | −60 | 63 |
| Material Code | Tonset (°C) | Tmax (°C) |
|---|---|---|
| TGA analysis performed in nitrogen atmosphere | ||
| DBTTC | 239.9 | 288.4 |
| p(St25-2EHA50-St25) | 372.7 | 413.8 |
| p(St15-2EHA50-St15) | 365.2 | 402.7 |
| p(St15-2EHA70-St15) | 364.0 | 397.7 |
| p(St15-2EHA100-St15) | 361.4 | 396.6 |
| Sample | Mole Fraction | Weight Fraction | Theoretical Morphology [34] | ||
|---|---|---|---|---|---|
| % St | %2EHA+SA | %St+SA | %2EHA | ||
| p(St25-2EHA50-St25) | 61.0 | 39.0 | 40.6 | 59.4 | L/G |
| p(St15-2EHA50-St15) | 55.3 | 44.7 | 35.7 | 64.3 | G/C |
| p(St15-2EHA70-St15) | 35.6 | 64.4 | 20.8 | 79.2 | S/C |
| p(St15-2EHA100-St15) | 33.2 | 66.8 | 19.2 | 80.8 | S |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siljanovska Petreska, G.; Arbe, A.; Auschra, C.; Paulis, M. Mechanical and Morphological Properties of Waterborne ABA Hard-Soft-Hard Block Copolymers Synthesized by Means of RAFT Miniemulsion Polymerization. Polymers 2019, 11, 1259. https://doi.org/10.3390/polym11081259
Siljanovska Petreska G, Arbe A, Auschra C, Paulis M. Mechanical and Morphological Properties of Waterborne ABA Hard-Soft-Hard Block Copolymers Synthesized by Means of RAFT Miniemulsion Polymerization. Polymers. 2019; 11(8):1259. https://doi.org/10.3390/polym11081259
Chicago/Turabian StyleSiljanovska Petreska, Gordana, Arantxa Arbe, Clemens Auschra, and Maria Paulis. 2019. "Mechanical and Morphological Properties of Waterborne ABA Hard-Soft-Hard Block Copolymers Synthesized by Means of RAFT Miniemulsion Polymerization" Polymers 11, no. 8: 1259. https://doi.org/10.3390/polym11081259
APA StyleSiljanovska Petreska, G., Arbe, A., Auschra, C., & Paulis, M. (2019). Mechanical and Morphological Properties of Waterborne ABA Hard-Soft-Hard Block Copolymers Synthesized by Means of RAFT Miniemulsion Polymerization. Polymers, 11(8), 1259. https://doi.org/10.3390/polym11081259