Controlled Shape and Porosity of Polymeric Colloids by Photo-Induced Phase Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dispersed Phase-NOA81-Mixture-Preparation
2.3. Colloidal Formation
2.4. Characterization
2.5. Cross-Sectioning
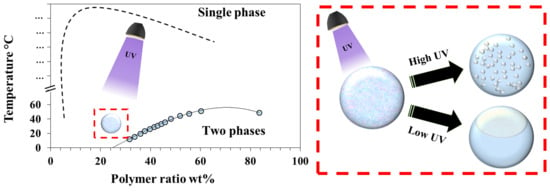
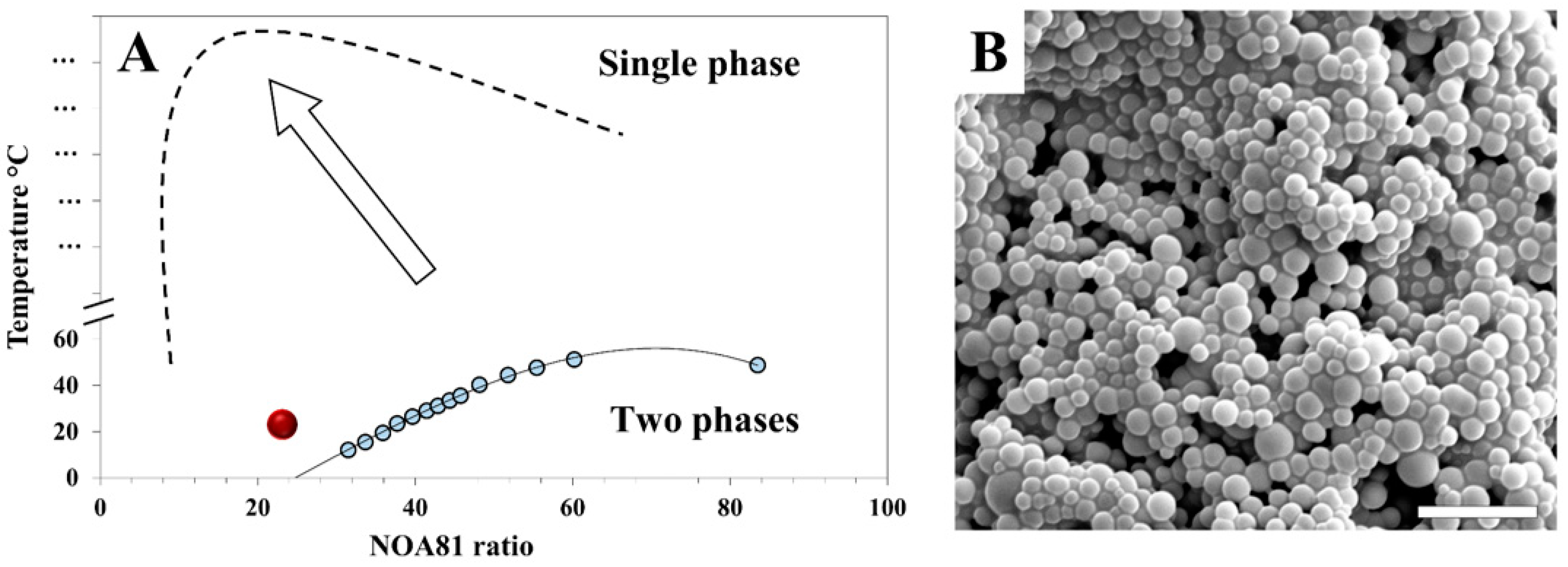
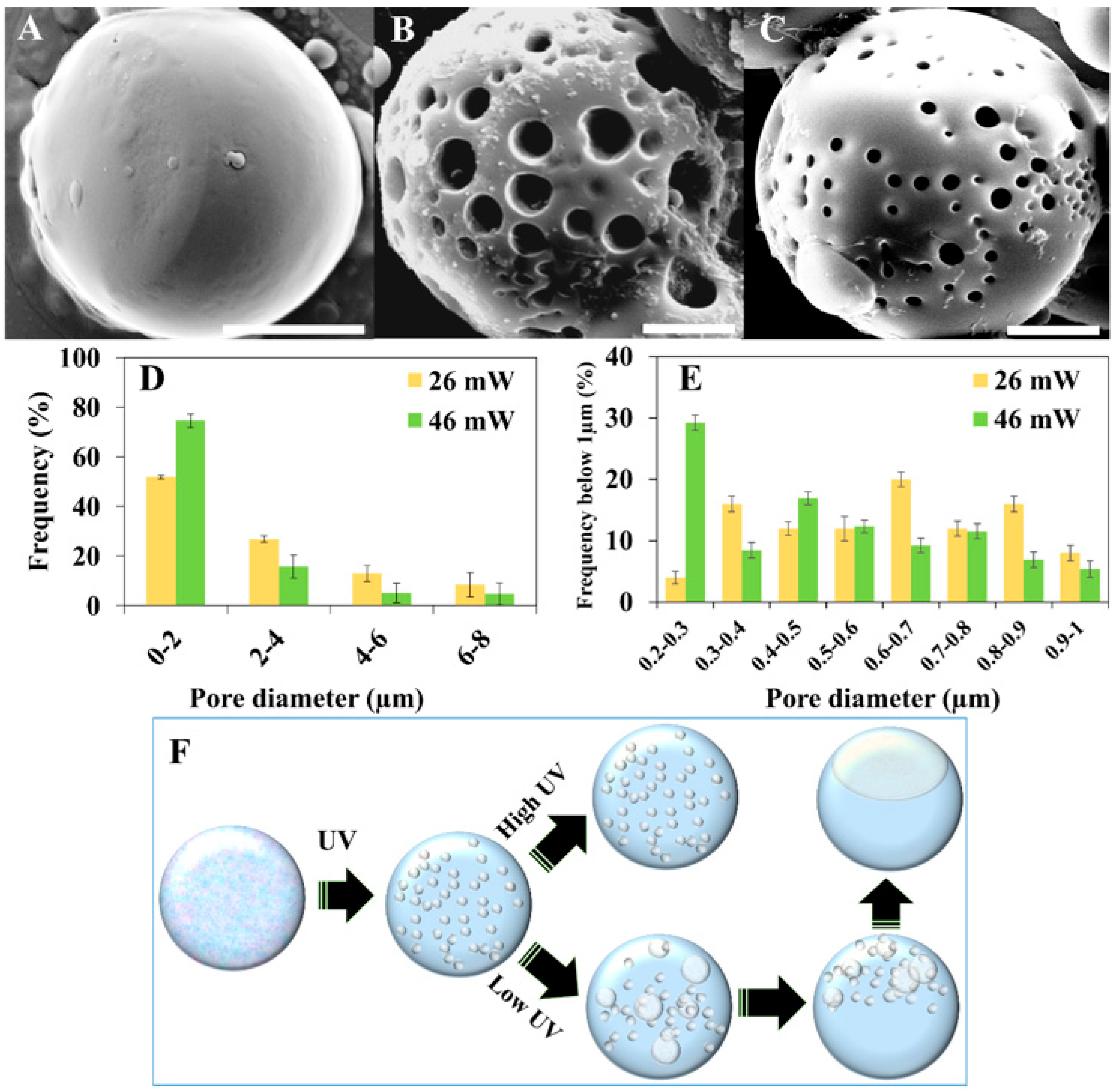
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dugyala, V.R.; Daware, S.V.; Basavaraj, M.G. Shape anisotropic colloids: Synthesis, packing behavior, evaporation driven assembly, and their application in emulsion stabilization. Soft Matter 2013, 9, 6711. [Google Scholar] [CrossRef]
- Sacanna, S.; Rossi, L.; Wouterse, A.; Philipse, A.P. Observation of a shape-dependent density maximum in random packings and glasses of colloidal silica ellipsoids. J. Phys. Condens. Matter 2007, 19, 376108. [Google Scholar] [CrossRef]
- Yunker, P.J.; Still, T.; Lohr, M.A.; Yodh, A.G. Suppression of the coffee-ring effect by shape-dependent capillary interactions. Nature 2011, 476, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952–959. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C.M.; Xia, Y.; Cobley, L.M. Gold Nanocages: Synthesis, Properties, and Applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Jacob, I.; Edri, E.; Lasnoy, E.; Piperno, S.; Shpaisman, H. Influencing colloidal formation with optical traps. Soft Matter 2017, 13, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Ruffner, D.B.; Shpaisman, H.; Grier, D.G. Light-driven three-dimensional rotational motion of dandelion-shaped microparticles. Appl. Phys. Lett. 2013, 102, 071103. [Google Scholar]
- Armon, N.; Greenberg, E.; Layani, M.; Rosen, Y.S.; Magdassi, S.; Shpaisman, H. Continuous Nanoparticle Assembly by a Modulated Photo-Induced Microbubble for Fabrication of Micrometric Conductive Patterns. ACS Appl. Mater. Interfaces 2017, 9, 44214–44221. [Google Scholar] [CrossRef] [PubMed]
- Sazan, H.; Piperno, S.; Layani, M.; Magdassi, S.; Shpaisman, H. Directed assembly of nanoparticles into continuous microstructures by standing surface acoustic waves. J. Colloid Interface Sci. 2019, 536, 701–709. [Google Scholar] [CrossRef]
- Piperno, S.; Sazan, H.; Shpaisman, H. Simultaneous polymerization and patterning: A one step acoustic directed assembly method. Polymer 2019, 173, 20–26. [Google Scholar] [CrossRef]
- Greenberg, E.; Armon, N.; Kapon, O.; Ben-Ishai, M.; Shpaisman, H. Nanostructure and Mechanism of Metal Deposition by a Laser-Induced Photothermal Reaction. Adv. Mater. Interfaces 2019, 1900541. [Google Scholar] [CrossRef]
- Sacanna, S.; Pine, D.J. Shape-anisotropic colloids: Building blocks for complex assemblies. Curr. Opin. Colloid Interface Sci. 2011, 16, 96–105. [Google Scholar] [CrossRef]
- Sacanna, S.; Irvine, W.T.M.; Chaikin, P.M.; Pine, D.J. Lock and key colloids. Nature 2010, 464, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Sacanna, S.; Irvine, W.T.M.; Chaikin, P.M.; Pine, D.J.; Philipse, A.P. Cubic crystals from cubic colloids. Soft Matter 2011, 7, 4139–4142. [Google Scholar] [CrossRef]
- Shelke, Y.; Sabapathy, M.; Mani, E. Staggered Linear Assembly of Spherical-Cap Colloids. Langmuir 2017, 33, 6760–6768. [Google Scholar] [CrossRef] [PubMed]
- Gokmen, M.T.; Du Prez, F.E. Porous polymer particles—A comprehensive guide to synthesis, characterization, functionalization and applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef]
- Rao, T.P.; Praveen, R.S.; Daniel, S. Styrene-Divinyl Benzene Copolymers: Synthesis, Characterization, and Their Role in Inorganic Trace Analysis. Crit. Rev. Anal. Chem. 2004, 34, 177–193. [Google Scholar] [CrossRef]
- Berek, D. Size exclusion chromatography—A blessing and a curse of science and technology of synthetic polymers. J. Sep. Sci. 2010, 33, 315–335. [Google Scholar] [CrossRef]
- Lu, J.; Toy, P.H. Organic Polymer Supports for Synthesis and for Reagent and Catalyst Immobilization. Chem. Rev. 2009, 109, 815–838. [Google Scholar] [CrossRef]
- Lackner, K. Capture of carbon dioxide from ambient air. Eur. Phys. J. Spéc. Top. 2009, 176, 93–106. [Google Scholar] [CrossRef]
- Li, M.; Katsouras, I.; Piliego, C.; Glasser, G.; Lieberwirth, I.; Blom, P.W.M.; De Leeuw, D.M. Controlling the microstructure of poly(vinylidene-fluoride) (PVDF) thin films for microelectronics. J. Mater. Chem. C 2013, 1, 7695. [Google Scholar] [CrossRef]
- Elbert, D.L. Liquid–liquid two-phase systems for the production of porous hydrogels and hydrogel microspheres for biomedical applications: A tutorial review. Acta Biomater. 2011, 7, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Kaity, S.; Maiti, S.; Pal, D.; Ghosh, A.; Banerjee, S.; Ghosh, A.; Ghosh, A.K. Microsponges: A novel strategy for drug delivery system. J. Adv. Pharm. Technol. Res. 2010, 1, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.C.; Hosoya, K.; Svec, F.; Fréchet, J.M.J. Polymeric porogens used in the preparation of novel monodispersed macroporous polymeric separation media for high-performance liquid chromatography. Anal. Chem. 1992, 64, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Wen, W.; Sheng, P. Microfluidic Fabrication of Porous Polymer Microspheres: Dual Reactions in Single Droplets. Langmuir 2009, 25, 7072–7077. [Google Scholar] [CrossRef] [PubMed]
- Dobry, A.; Boyer-Kawenoki, F.; Boyer-Kawenoki, F. Phase separation in polymer solution. J. Polym. Sci. 1947, 2, 90–100. [Google Scholar] [CrossRef]
- Gao, F.; Su, Z.-G.; Wang, P.; Ma, G.-H. Double Emulsion Templated Microcapsules with Single Hollow Cavities and Thickness-Controllable Shells. Langmuir 2009, 25, 3832–3838. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.F.; Brujic, J. Tailoring of High-Order Multiple Emulsions by the Liquid-Liquid Phase Separation of Ternary Mixtures. Angew. Chem. 2014, 126, 11987–11991. [Google Scholar] [CrossRef]
- Fan, J.-B.; Song, Y.; Liu, H.; Lu, Z.; Zhang, F.; Liu, H.; Meng, J.; Gu, L.; Wang, S.; Jiang, L. A general strategy to synthesize chemically and topologically anisotropic Janus particles. Sci. Adv. 2017, 3, e1603203. [Google Scholar] [CrossRef]
- Guenthner, A.J.; Hess, D.M.; Cash, J.J. Morphology development in photopolymerization-induced phase separated mixtures of UV-curable thiol-ene adhesive and low molecular weight solvents. Polymer 2008, 49, 5533–5540. [Google Scholar] [CrossRef]
- Theberge, A.B.; Courtois, F.; Schaerli, Y.; Fischlechner, M.; Abell, C.; Hollfelder, F.; Huck, W.T.S. Microdroplets in Microfluidics: An Evolving Platform for Discoveries in Chemistry and Biology. Angew. Chem. Int. Ed. 2010, 49, 5846–5868. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Abate, A.R.; Lee, D.; Terentjev, E.M.; Weitz, D.A. Microfluidic Assembly of Magnetic Hydrogel Particles with Uniformly Anisotropic Structure. Adv. Mater. 2009, 21, 3201–3204. [Google Scholar] [CrossRef]
- Hashimoto, M.; Garstecki, P.; Whitesides, G.M. Synthesis of Composite Emulsions and Complex Foams with the use of Microfluidic Flow-Focusing Devices. Small 2007, 3, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Köster, S.; Angilè, F.E.; Duan, H.; Agresti, J.J.; Wintner, A.; Schmitz, C.; Rowat, A.C.; Merten, C.A.; Pisignano, D.; Griffiths, A.D.; et al. Drop-based microfluidic devices for encapsulation of single cells. Lab Chip 2008, 8, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Agresti, J.J.; Antipov, E.; Abate, A.R.; Ahn, K.; Rowat, A.C.; Baret, J.-C.; Marquez, M.; Klibanov, A.M.; Griffiths, A.D.; Weitz, D.A. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc. Natl. Acad. Sci. USA 2010, 107, 6550. [Google Scholar] [CrossRef] [PubMed]
- Holtze, C.; Rowat, A.C.; Agresti, J.J.; Hutchison, J.B.; Angilè, F.E.; Schmitz, C.H.J.; Köster, S.; Duan, H.; Humphry, K.J.; Scanga, R.A.; et al. Biocompatible surfactants for water-in-fluorocarbon emulsions. Lab Chip 2008, 8, 1632. [Google Scholar] [CrossRef]
- Ildefonso, M.; Candoni, N.; Veesler, S. A Cheap, Easy Microfluidic Crystallization Device Ensuring Universal Solvent Compatibility. Org. Process. Res. Dev. 2012, 16, 556–560. [Google Scholar] [CrossRef]
- Wang, C.; Shpaisman, H.; Hollingsworth, A.D.; Grier, D.G. Celebrating Soft Matter’s 10th Anniversary: Monitoring colloidal growth with holographic microscopy. Soft Matter 2015, 11, 1062–1066. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadad, E.; Edri, E.; Shpaisman, H. Controlled Shape and Porosity of Polymeric Colloids by Photo-Induced Phase Separation. Polymers 2019, 11, 1225. https://doi.org/10.3390/polym11071225
Hadad E, Edri E, Shpaisman H. Controlled Shape and Porosity of Polymeric Colloids by Photo-Induced Phase Separation. Polymers. 2019; 11(7):1225. https://doi.org/10.3390/polym11071225
Chicago/Turabian StyleHadad, Elad, Eitan Edri, and Hagay Shpaisman. 2019. "Controlled Shape and Porosity of Polymeric Colloids by Photo-Induced Phase Separation" Polymers 11, no. 7: 1225. https://doi.org/10.3390/polym11071225
APA StyleHadad, E., Edri, E., & Shpaisman, H. (2019). Controlled Shape and Porosity of Polymeric Colloids by Photo-Induced Phase Separation. Polymers, 11(7), 1225. https://doi.org/10.3390/polym11071225