The Effect of Degradation of Soda Lignin Using Pd/SO42−/ZrO2 as a Catalyst: Improved Reactivity and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalysts Preparation
2.3. Degradation Reaction
2.4. Catalysts Characterization
2.5. Products Characterization
2.6. Antioxidant Activity Evaluation
3. Results
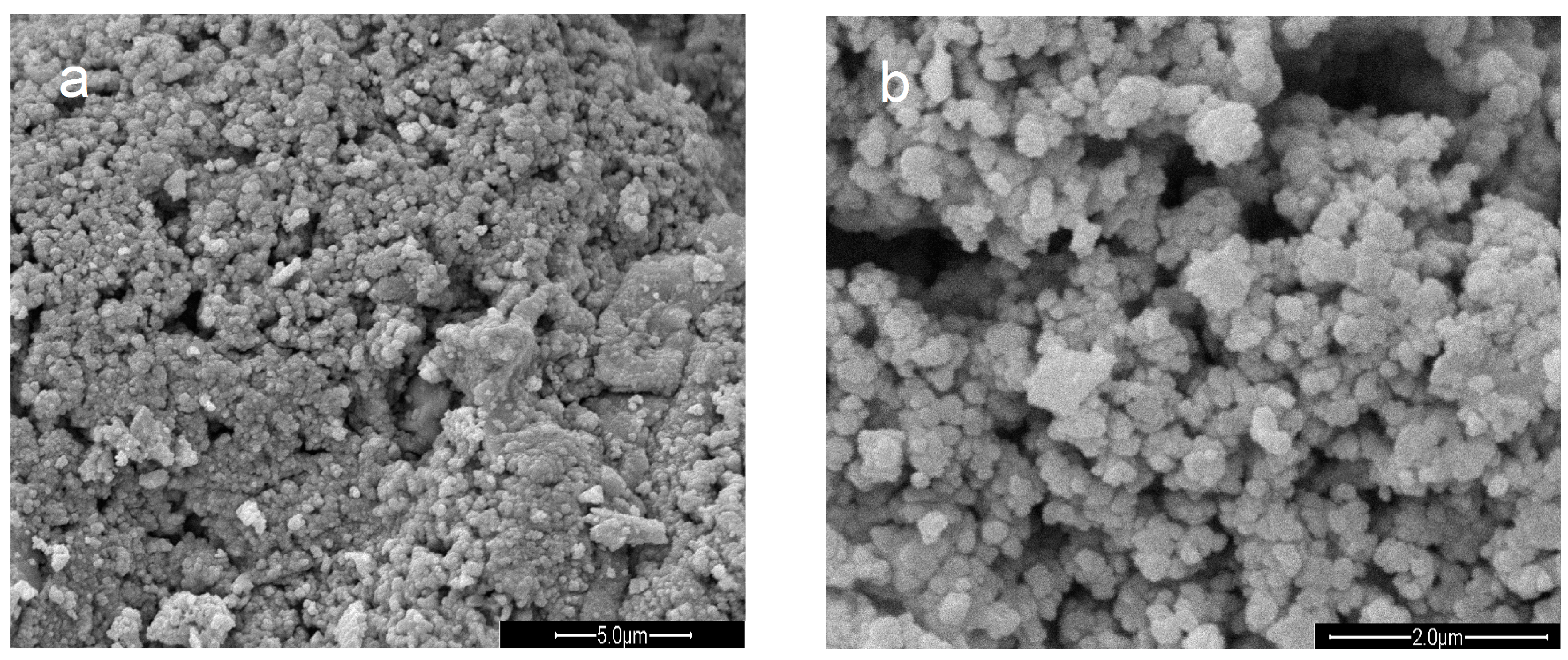
3.1. Characterization of the Catalysts
3.2. Characterization of the Products
3.2.1. Reaction Conditions
3.2.2. FT-IR Spectra
3.2.3. NMR Spectroscopy
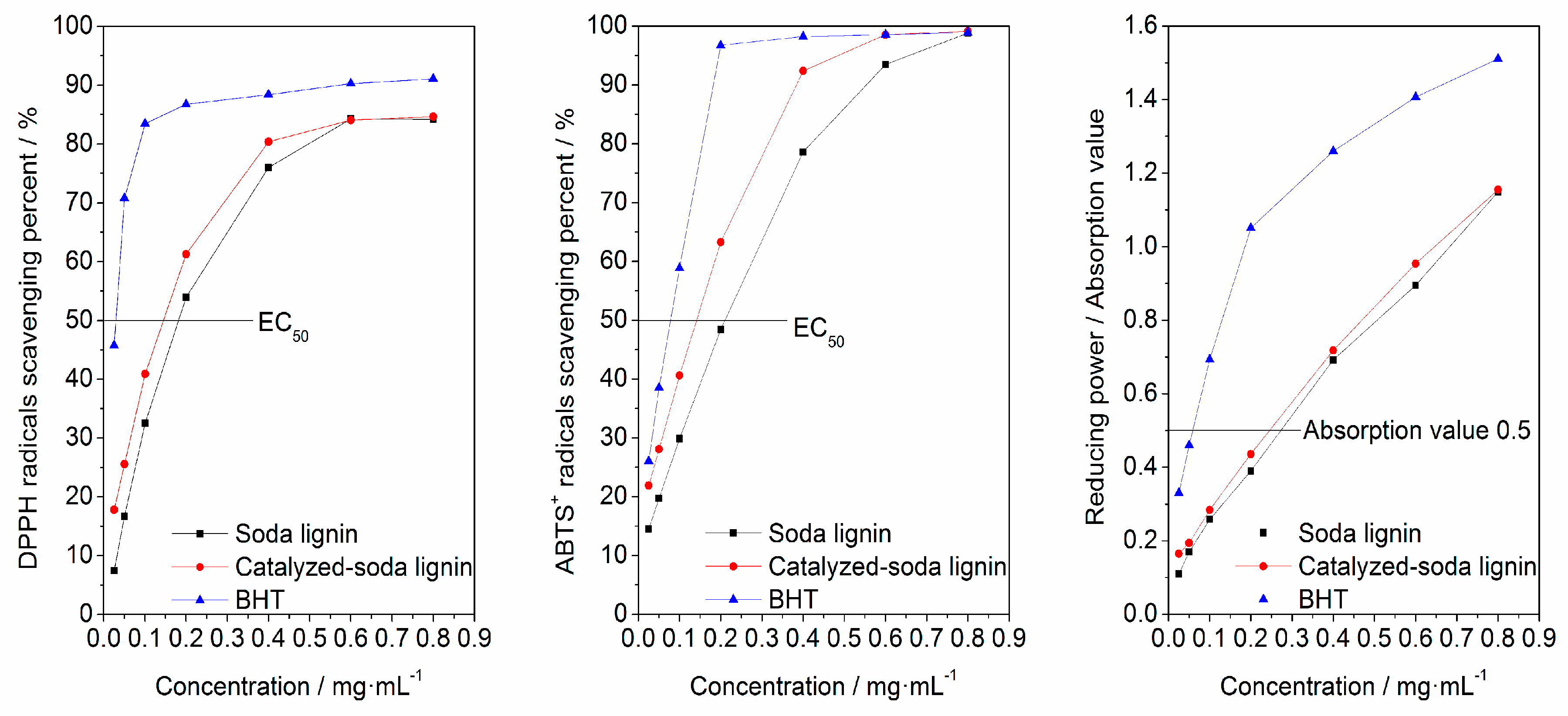
3.3. Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shu, R.Y.; Zhang, Q.; Ma, L.L.; Xu, Y.; Chen, P.R.; Wang, G.C.; Wang, T.J. Insight into the solvent, temperature and time effects on the hydrogenolysis of hydrolyzed lignin. Bioresour. Technol. 2016, 221, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, P.F.; Liu, N.N.; Shen, D.K. Lignin depolymerization to aromatic monomers and oligomers in isopropanol assisted by microwave heating. Polym. Degrad. Stabil. 2017, 135, 54–60. [Google Scholar] [CrossRef]
- Subbotina, E.; Galkin, M.V.; Samec, J.S.M. Pd/C-Catalyzed hydrogenolysis of dibenzodioxocin lignin model compounds using silanes and water as hydrogen source. ACS Sustain. Chem. Eng. 2017, 5, 3726–3731. [Google Scholar] [CrossRef]
- Laurichesse, S.; Averous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Guvenatam, B.; Kursun, O.; Heeres, E.H.J.; Pidko, E.A.; Hensen, E.J.M. Hydrodeoxygenation of mono- and dimeric lignin model compounds on noble metal catalysts. Catal. Today. 2014, 233, 83–91. [Google Scholar] [CrossRef]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pdmetal catalysts for cross-couplings and related reactions in the 21st century: A critical review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef] [PubMed]
- To, C.T.; Chan, K.S. Catalytic carbon-carbon sigma-bond hydrogenolysis. Tetrahedron Lett. 2016, 57, 4664–4669. [Google Scholar] [CrossRef]
- Zhu, G.D.; Ouyang, X.P.; Jiang, L.F.; Zhu, Y.; Jin, D.X.; Pang, Y.X.; Qiu, X.Q. Effect of functional groups on hydrogenolysis of lignin model compounds. Fuel Process. Technol. 2016, 154, 132–138. [Google Scholar] [CrossRef]
- Pu, W.; Ren, S.X.; Ma, Y.L.; Fang, G.Z. Antioxidant activity of hydrogen-reduced alkali lignin prepared using Palladium/Carbon (Pd/C) as catalyst. Food Sci. 2013, 34, 6–10. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.M.; Fang, G.Z. The catalytic effects of two-step pretreatment actived carbon loaded Pd by HNO3-H2O2 on alkali lignin. Chem. Ind. For. Prod. 2014, 34, 13–20. [Google Scholar] [CrossRef]
- Mo, H.B.; Chen, X.P.; Liao, X.Y.; Zhou, T. Sustainable synthesis of 5-hydroxymethylfurfural from waste cotton stalk catalyzed by solid superacid-SO42−/ZrO2. J. Cent. South Univ. 2017, 24, 1745–1753. [Google Scholar] [CrossRef]
- Zhang, S.M.; Liu, L.; Ma, Y.L.; Fang, G.Z. Antioxidant activity of hydrogen-reduced alkali lignin prepared using SO42−/ZrO2 as catalyst. J. Funct. Mater. 2014, 45, 71–75. [Google Scholar] [CrossRef]
- Zhang, S.M.; Zhang, Y.; Liu, L.; Fang, G.Z. Antioxidant activity of organosolv lignin degraded using SO42−/ZrO2 as catalyst. BioResources 2015, 10, 6819–6829. [Google Scholar] [CrossRef]
- Zhang, S.M.; Su, L.; Liu, L.; Fang, G.Z. Degradation on hydrogenolysis of soda lignin using CuO/SO42−/ZrO2 as catalyst. Ind. Crop. Prod. 2015, 77, 451–457. [Google Scholar] [CrossRef]
- Lai, Y.Z.; Funaoka, M. The distribution of phenolic hydroxyl groups in hardwood lignins. J. Wood Chem. Technol. 1993, 13, 43–57. [Google Scholar] [CrossRef]
- Lu, Q.; Zhu, M.H.; Zu, Y.G.; Liu, W.J.; Yang, L.; Zhang, Y.; Zhao, X.H.; Zhang, X.N.; Zhang, X.N.; Li, W.G. Comparative antioxidant activity of nanoscale lignin prepared by a supercritical antisolvent (SAS) process with non-nanoscale lignin. Food Chem. 2012, 135, 63–67. [Google Scholar] [CrossRef]
- Wang, X.L.; Ren, K.W.; Pan, X.M.; Lin, R.; Ma, J.X. Influence of solid acid catalysts on steam reforming of dimethyl ether for hydrogen production. Chin. J. Catal. 2009, 30, 297–304. [Google Scholar] [CrossRef]
- Song, Y.Q.; Tian, J.; Ye, Y.R.; Jin, Y.Q.; Zhou, X.L.; Wang, J.A.; Xu, L.Y. Effects of calcination temperature and water-washing treatment on n-hexane hydroisomerization behavior of Pt-promoted sulfated zirconia based catalysts. Catal. Today 2013, 212, 108–114. [Google Scholar] [CrossRef]
- Tengco, J.M.M.; Lugo-José, Y.K.; Monnier, J.R.; Regalbuto, J.R. Chemisorption-XRD particle size discrepancy of carbon supportedpalladium: Carbon decoration of Pd? Catal. Today 2015, 246, 9–14. [Google Scholar] [CrossRef]
- Li, Z.S.; Ji, S.; Polletb, B.G.; Shen, P.K. A Co3W3C promoted Pd catalyst exhibiting competitive performance over Pt/C catalysts towards the oxygen reduction reaction. Chem. Commun. 2015, 50, 566–568. [Google Scholar] [CrossRef]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–27. [Google Scholar] [CrossRef]
- Jahan, M.S.; Chowdhury, D.A.N.; Islam, M.K. Atmospheric formic acid pulping and TCF bleaching of dhaincha (Sesbaniaaculeata), kash (Saccharumspontaneum) and banana stem (Musa Cavendish). Ind. Crop. Prod. 2007, 26, 324–331. [Google Scholar] [CrossRef]
- Zhang, L.; Gellerstedt, G. Quantitative 2D HSQC NMR determination of polymer structures by selecting suitable internal standard references. Magn. Reson. Chem. 2007, 45, 37–45. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Recent advances in characterization of lignin polymer by solution-state nuclear magnetic resonance (NMR) methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef]
- Wen, J.L.; Xue, B.L.; Xu, F.; Sun, R.C. Unveiling the structural heterogeneity of bamboo lignin by in situ HSQC NMR technique. BioEnergy Res. 2012, 5, 886–903. [Google Scholar] [CrossRef]
- Mousavioun, P.; Doherty, W.O.S. Chemical and thermal properties of fractionated bagasse soda lignin. Ind. Crop. Prod. 2010, 31, 52–58. [Google Scholar] [CrossRef]
- Ge, Y.Y.; Wei, Q.; Li, Z.L. Preparation and evaluation of the free radical scavenging activities of nanoscale lignin biomaterials. BioResources 2014, 9, 6699–6706. [Google Scholar] [CrossRef]
- Pinheiro, F.G.C.; Soares, A.K.L.; Santaella, S.T.; Silva, L.M.A.E.; Canuto, K.M.; Caceres, C.A.; Rosa, M.D.; Feitosa, J.P.D.; Leitao, R.C. Optimization of the acetosolv extraction of lignin from sugarcane bagasse for phenolic resin production. Ind. Crop. Prod. 2017, 96, 80–90. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yuan, Z.S.; Mahmood, N.; Huang, S.H.; Xu, C. Sustainable bio-phenol-hydroxymethylfurfural resins using phenolated de-polymerized hydrolysis lignin and their application in bio-composites. Ind. Crop. Prod. 2016, 79, 84–90. [Google Scholar] [CrossRef]
- Su, L.; Zhang, S.M.; Jiang, G.Q.; Pang, J.Y.; Wang, D.; Shi, J.Y.; Fang, G.Z. Layer-by-layer self-assembly of a lignin–poly(vinyl alcohol) based polyelectrolyte with a conductivity method. J. Appl. Polym. Sci. 2017, 134, 44416. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, D.; Bei, Y.; Ren, S.X.; Fang, G.Z. Flocculation performance of trimethyl quaternary ammonium salt of lignin-sodium alginate polyampholyte. BioResources 2013, 8, 3544–3555. [Google Scholar] [CrossRef]
- Zhang, S.M.; Liu, L.; Fang, G.Z.; Yan, N.; Ren, S.X.; Ma, Y.L. Hydrogenolysis and activation of soda lignin using [BMIM]Cl as a catalyst and solvent. Polymers 2017, 9, 279. [Google Scholar] [CrossRef]
- Miles-Barrett, D.M.; Neal, A.R.; Hand, C.; Montgomery, J.R.D.; Panovic, I.; Ojo, O.S.; Lancefield, C.S.; Cordes, D.B.; Slawin, A.M.Z.; Lebl, T.; et al. The synthesis and analysis of lignin-bound Hibbert ketone structures in technical lignins. Org. Biomol. Chem. 2016, 14, 10023–10030. [Google Scholar] [CrossRef]
- Jia, S.Y.; Cox, B.J.; Guo, X.W.; Zhang, Z.C.; Ekerdt, J.G. Cleaving the beta-O-4 bonds of lignin model compounds in an acidic ionic liquid, 1-H-3-Methylimidazolium chloride: An optional strategy for the degradation of lignin. ChemSusChem 2010, 3, 1078–1084. [Google Scholar] [CrossRef]
- Mu, W.; Ben, H.X.; Du, X.T.; Zhang, X.D.; Hu, F.; Liu, W.; Ragauskas, A.J.; Deng, Y.L. Noble metal catalyzed aqueous phase hydrogenation and hydrodeoxygenation of lignin-derived pyrolysis oil and related model compounds. Bioresour. Technol. 2014, 173, 6–10. [Google Scholar] [CrossRef]
- Barta, K.; Matson, T.D.; Fettig, M.L.; Scott, S.L.; Iretskii, A.V.; Ford, P.C. Catalytic disassembly of an organosolv lignin via hydrogen transfer from supercritical methanol. Green Chem. 2010, 12, 1640–1647. [Google Scholar] [CrossRef]
- Zhang, J.G.; Teo, J.; Chen, X.; Asakura, H.; Tanaka, T.; Teramura, K.; Yan, N. A series of NiM (M= Ru, Rh, and Pd) bimetallic catalysts for effective lignin hydrogenolysis in water. ACS Catal. 2014, 4, 1574–1583. [Google Scholar] [CrossRef]

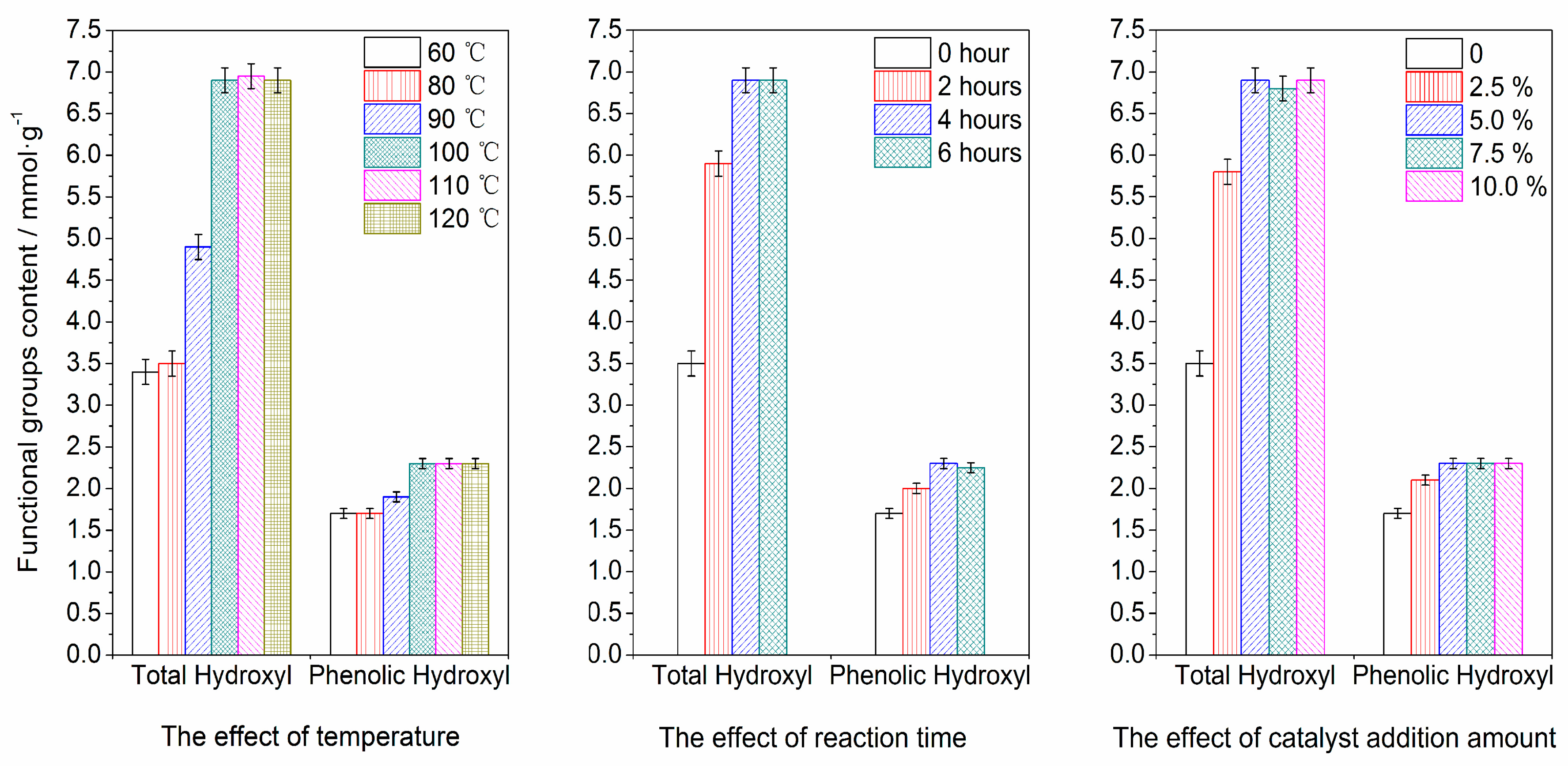



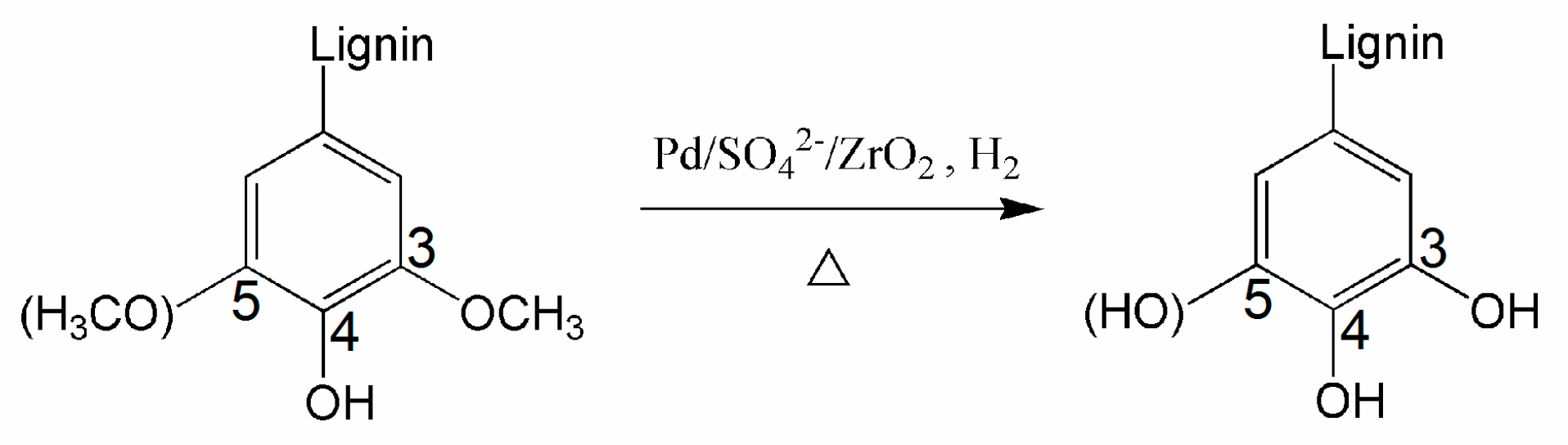
| Sample | Catalyst | Solution | Functional Groups Value (mmol·g−1) | Mw (g·mol−1) | Mn (g·mol−1) | Mw/Mn | Yield (wt.%) | |
|---|---|---|---|---|---|---|---|---|
| Total Hydroxyl (±0.15) | Phenol Hydroxyl (±0.06) | |||||||
| L0 | — | — | 3.50 | 1.70 | 8200 | 3700 | 2.22 | — |
| L9 | None | Dioxane-water | 3.50 | 1.70 | 8200 | 3700 | 2.22 | 99.99 |
| L4 | Pd/SZ | Dioxane-water | 6.90 | 2.30 | 4900 | 2400 | 2.04 | 82.33 |
| L13 | None | NaOH | 3.50 | 1.70 | 8000 | 3600 | 2.22 | 97.50 |
| L14 | Pd/SZ | NaOH | 6.80 | 1.90 | 5200 | 2400 | 2.17 | 79.24 |
| L15 | None | [BMIM]Cl | 5.30 | 1.90 | 6900 | 3000 | 2.30 | 80.00 |
| L16 | Pd/SZ | [BMIM]Cl | 6.90 | 2.20 | 5100 | 2300 | 2.22 | 81.03 |
| L17 | None | DMSO | 3.50 | 1.70 | 8400 | 3700 | 2.27 | 96.00 |
| L18 | Pd/SZ | DMSO | 7.00 | 2.20 | 5400 | 2700 | 2.00 | 74.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Fang, G.; Chen, H.; Lang, Q. The Effect of Degradation of Soda Lignin Using Pd/SO42−/ZrO2 as a Catalyst: Improved Reactivity and Antioxidant Activity. Polymers 2019, 11, 1218. https://doi.org/10.3390/polym11071218
Zhang S, Fang G, Chen H, Lang Q. The Effect of Degradation of Soda Lignin Using Pd/SO42−/ZrO2 as a Catalyst: Improved Reactivity and Antioxidant Activity. Polymers. 2019; 11(7):1218. https://doi.org/10.3390/polym11071218
Chicago/Turabian StyleZhang, Shengming, Guizhen Fang, Haitao Chen, and Qian Lang. 2019. "The Effect of Degradation of Soda Lignin Using Pd/SO42−/ZrO2 as a Catalyst: Improved Reactivity and Antioxidant Activity" Polymers 11, no. 7: 1218. https://doi.org/10.3390/polym11071218
APA StyleZhang, S., Fang, G., Chen, H., & Lang, Q. (2019). The Effect of Degradation of Soda Lignin Using Pd/SO42−/ZrO2 as a Catalyst: Improved Reactivity and Antioxidant Activity. Polymers, 11(7), 1218. https://doi.org/10.3390/polym11071218





