Melt-Mixed Thermoplastic Nanocomposite Containing Carbon Nanotubes and Titanium Dioxide for Flame Retardancy Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
Synthesis of PP/CNT Nanocomposites
2.3. Characterization
2.3.1. X-Ray Diffraction (XRD)
2.3.2. Fourier Transform Infrared Spectroscopy FTIR (ATR)
2.3.3. Melt Flow Index (MFI)
2.3.4. Thermal Stability (DSC and TGA)
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. Mechanical Properties Analysis
2.3.7. Electrical Resistivity
2.3.8. Calorimetric Cone
3. Results and Discussion
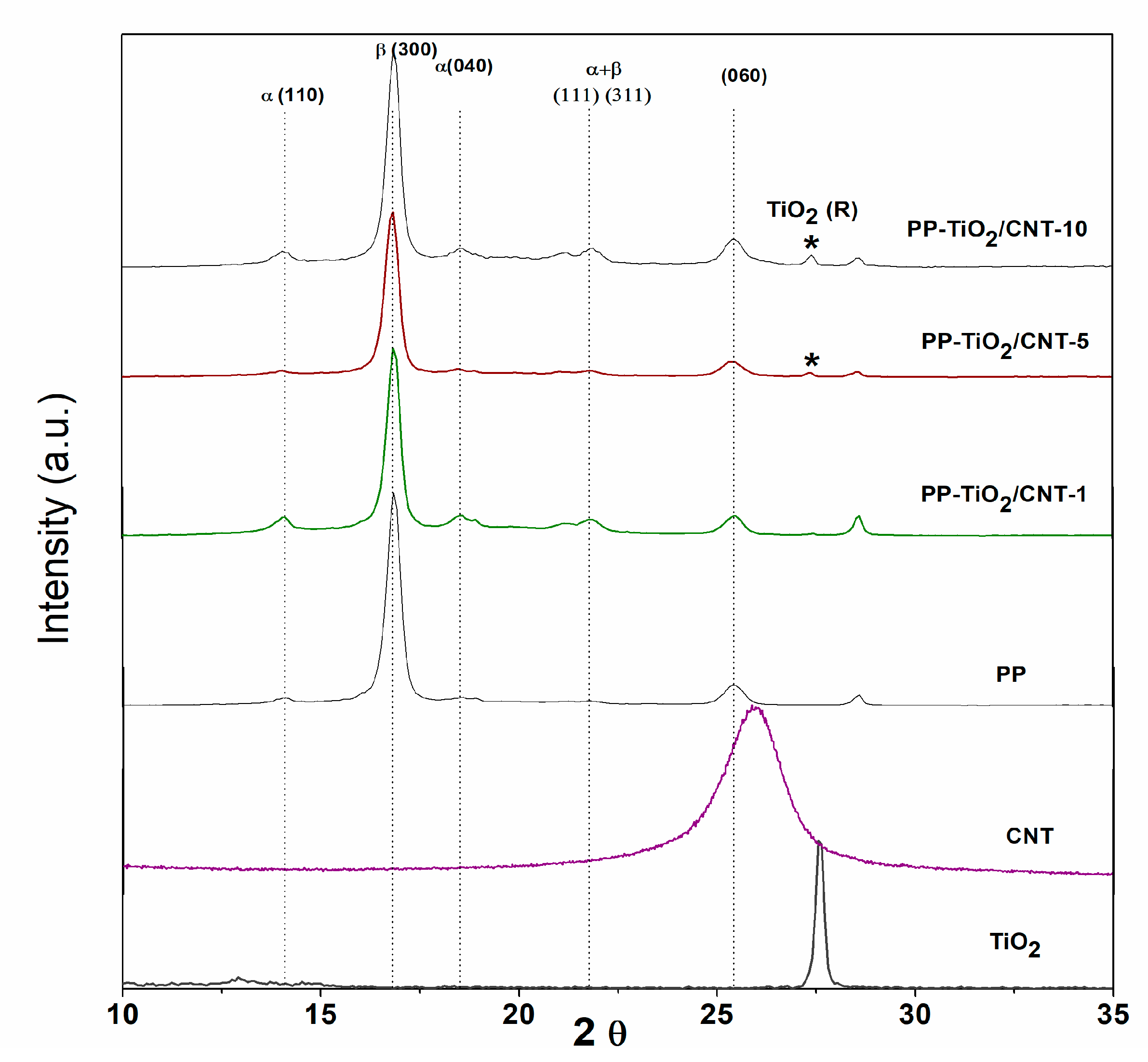
3.1. X-ray Diffraction
3.2. Fourier Transform Infrared FTIR (ATR)
3.3. Evaluation of Melt Flow Index (MFI)
3.4. Thermogravimetric Analysis (TGA)
3.5. Differential Scan Calorimetry (DSC) Analysis
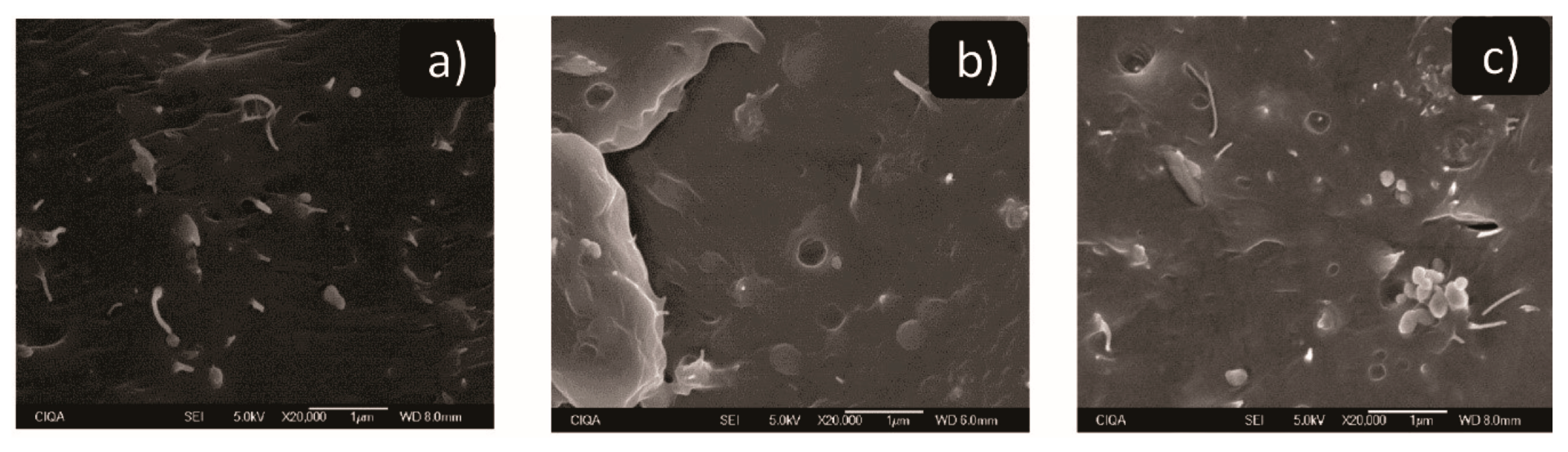
3.6. Electron Scan Microscopy
3.7. Electrical Conductivity
3.8. Mechanical Properties
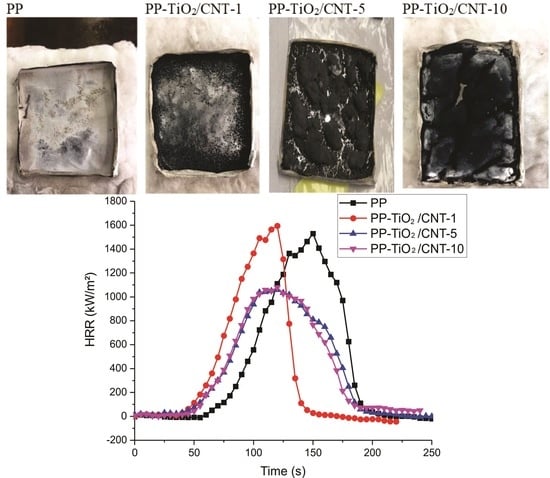
3.9. Calorimetric Cone
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panzino, F.; Piza, A.; Pociello, N.; García, J.J.; Luaces, C.; Pou, J. Estudio multicéntrico sobre factores de riesgo de lesiones en accidentes de automóvil. In Anales de Pediatría; Elsevier Doyma: Amsterdam, The Netherlands, 2009; Volume 71, pp. 25–30. [Google Scholar] [CrossRef]
- Adanur, S. Polymers and Fiber. Wellington Sears Handbook of Industrial Textiles; Routledge: New York, NY, USA, 2017; Volume 2, pp. 12–37. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Thomas, N.L. Zinc compounds as flame retardants and smoke suppressants for rigid PVC. Plast. Rubber Compos. 2003, 32, 413–419. [Google Scholar] [CrossRef]
- Lam, Y.L.; Kan, C.W.; Yuen, C.W.M. Effect of titanium dioxide on the flame-retardant finishing of cotton fabric. J. Appl. Polym. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.; Hofmann, D.; Mülhaupt, R.; Schartel, B. Carbon black, multiwall carbon nanotubes, expanded graphite and functionalized graphene flame retarded polypropylene nanocomposites. Polym. Adv. Technol. 2013, 24, 916–926. [Google Scholar] [CrossRef]
- Mubarak, Y.A.; Abbadi, F.O.; Tobgy, A.H. Effect of iron oxide nanoparticles on the morphological properties of isotactic polypropylene. J. Appl. Polym. Sci. 2010, 115, 3423–3433. [Google Scholar] [CrossRef]
- Hufenbach, W.; Böhm, R.; Thieme, M.; Winkler, A.; Mäder, E.; Rausch, J.; Schade, M. Polypropylene/glass fibre 3D-textile reinforced composites for automotive applications. Mater. Des. 2011, 32, 1468–1476. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Jarusombuti, S.; Fueangvivat, V.; Bauchongkol, P.; White, R.H. Coir fiber reinforced polypropylene composite panel for automotive interior applications. Fibers Polym. 2011, 12, 919. [Google Scholar] [CrossRef]
- Saujanya, C.; Radhakrishnan, S. Structure development and crystallization behaviour of PP/nanoparticulate composite. Polymer 2001, 42, 6723–6731. [Google Scholar] [CrossRef]
- Mishra, S.; Sonawane, S.H.; Singh, R.P.; Bendale, A.; Patil, K. Effect of nano-Mg(OH)2 on the mechanical and flame-Retarding properties of polypropylene composites. J. Appl. Polym. Sci. 2004, 94, 116–122. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Demir, H.; Arkis, E.; Balköse, D.; Ülkü, S. Synergistic effect of natural zeolites on flame retardant additives. Polym. Degrad. Stab. 2005, 89, 478–483. [Google Scholar] [CrossRef]
- Ke, C.H.; Li, J.; Fang, K.; Zhu, K.; Zhu, J.; Yan, Q.; Wang, Y. Synergistic effect between a novel hyperbranched charring agent and ammonium polyphosphate on the flame retardant and anti-dripping properties of polylactide. Polym. Degrad. Stab. 2010, 95, 763–770. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Muhammad, B. Review of Applications of Polymer/Carbon nanotube and Epoxy/CNT Composites. Polym. Plast. Technol. Eng. 2016, 55, 1167–1191. [Google Scholar] [CrossRef]
- Lau, A.K.T.; Hui, D. The revolutionary creation of new advanced materials-carbon nanotube composites. Compos. Part B Eng. 2002, 33, 263–277. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Grulke, E.; Hilding, J.; Groth, K.; Harris, R.; Butler, K.; Douglas, J. Thermal and flammability properties of polypropylene/carbon nanotube nanocomposites. Polymer 2004, 45, 4227–4239. [Google Scholar] [CrossRef]
- Antunes, M.; Mudarra, M.; Velasco, J.I. Broad-band electrical conductivity of carbon nanofibre-reinforced polypropylene foams. Carbon 2011, 49, 708–717. [Google Scholar] [CrossRef]
- Seo, M.K.; Park, S. Electrical resistivity and rheological behaviors of carbon nanotubes-filled polypropylene composites. Chem. Phys. Lett. 2004, 395, 44–48. [Google Scholar] [CrossRef]
- Avalos, F.; Ramos, L.F.; Ramirez, E.; Sanchez, S.; Mendez, J.; Zitzumbo, R. Nucleating effect of carbón nanoparticles and their influence on the thermal and chemical stability of polypropylene. J. Nanomater. 2012, 2012, 104. [Google Scholar] [CrossRef]
- Kurahatti, R.V.; Surendranathan, A.O.; Kori, S.A.; Singh, N.; Kumar, A.R.; Srivastava, S. Defence applications of polymer nanocomposites. Def. Sci. J. 2010, 60, 551–563. [Google Scholar] [CrossRef]
- Aydemir, D.; Uzun, G.; Gumus, H.; Yildiz, S.; Gumus, S.; Bardak, T.; Gunduz, G. Nanocomposites of polypropylene/nano titanium dioxide: Effect of loading rates of nano titanium dioxide. Mater. Sci. 2016, 22, 364–369. [Google Scholar] [CrossRef]
- Esthappan, S.K.; Kuttappan, S.K.; Joseph, R. Thermal and mechanical properties of polypropylene/titanium dioxide nanocomposite fibers. Mater. Des. 2012, 37, 537–542. [Google Scholar] [CrossRef]
- El-dessouky, H.M.; Lawrence, C.A. Nanoparticles dispersion in processing functionalized PP/TiO2 nanocomposite: Distribution and properties. J. Nanopart. Res. 2011, 13, 1115–1124. [Google Scholar] [CrossRef]
- Cassagnau, P.; Legare, V.; Fenouillot, F. Reactive processing of thermoplastic polymer: A review of the fundamental aspect. Int. Polym. Process. 2007, 22, 218–258. [Google Scholar] [CrossRef]
- Chiamori, H.C.; Brown, J.W.; Adhiprakasha, E.V.; Hantsoo, E.T.; Straalsund, J.B.; Melosh, N.A.; Pruitt, B.L. Suspension of nanoparticles in SU-8: Processing and characterization of nanocomposite polymers. Microelectron. J. 2008, 39, 228–236. [Google Scholar] [CrossRef]
- Logakis, E.; Pollatos, E.; Pandis, C.; Peoglos, V.; Zuburtikis, I.; Delides, C.G.; Vatalis, A.; Gjoka, M.; Syskakis, E.; Viras, K.; et al. Structure–property relationships in isotactic polypropylene/multi-walled carbon nanotubes nanocomposites. Compos. Sci. Technol. 2010, 70, 328–335. [Google Scholar] [CrossRef]
- Crossman, R.A. Conductive composites past, present, and future. Polym. Eng. Sci. 1985, 25, 507–513. [Google Scholar] [CrossRef]
- Wang, S.; Abdellah, A.; Shaoyun, G.; Chuanxi, X. Preparation of microporous polypropylene/titanium dioxide composite membranes with enhanced electrolyte uptake capability via melt extruding and stretching. Polymers 2017, 9, 110. [Google Scholar] [CrossRef]
- Bahloul, W.; Walid, B.; Flavien, M.; Veronique, B.L.; Philippe, C. Structural characterisation and antibacterial activity of PP/TiO2 nanocomposites prepared by an insitu sol–gel method. Mater. Chem. Phys. 2012, 134, 399–406. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J. Effect of nucleating agent on the crystallization behavior, crystal form and solar reflectance of polypropylene. Sol. Energy Mater. Sol. Cells 2013, 117, 577–584. [Google Scholar] [CrossRef]
- Zohrevand, A.; Ajji, A.; Mighri, F. Morphology and properties of highly filled iPP/TiO2 nanocomposites. Polym. Eng. Sci. 2014, 54, 874–886. [Google Scholar] [CrossRef]
- Liu, Z.; Jian, Z.; Fang, J.; Xu, X.; Zhu, X.; Wu, S. Low-temperature reverse microemulsion synthesis, characterization, and photocatalytic performance of nanocrystalline titanium dioxide. Int. J. Photoenergy 2011, 2012, 702503. [Google Scholar] [CrossRef]
- Leon, A.; Reuquen, P.; Garin, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P. FTIR and Raman characterization of TiO2 nanoparticles coated with polyethylene glycol as carrier for 2-methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Shim, J.J. Green synthesis and characterization of carbon nanotubes/polyaniline nanocomposites. J. Spectrosc. 2015, 2015, 297804. [Google Scholar] [CrossRef]
- Yildrim, A.; Seckin, T. In situ preparation of polyether amine functionalized MWCNT nanofiller as reinforcing agents. Adv. Mater. Sci. Eng. 2014, 2014, 356920. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Q.; Li, K.; Hu, G.H. Preparation of polypropylene/carbon nanotube composite powder with a solid-state mechanochemical pulverization process. J. Appl. Polym. Sci. 2004, 93, 378–386. [Google Scholar] [CrossRef]
- Qian, Z.; Qian, J.; Lerou, F.; Tanga, P.; Li, D. Antioxidant intercalated hydrocalumite as multifunction nanofiller for Poly (propylene): Synthesis, thermal stability, light stability, and anti-migration property. Polym. Degrad. Stab. 2017, 140, 9–16. [Google Scholar] [CrossRef]
- Esthappan, S.K.; Kuttappan, S.K.; Joseph, R. Effect of titanium dioxide on the thermal ageing of polypropylene. Polym. Degrad. Stab. 2012, 97, 615–620. [Google Scholar] [CrossRef]
- Nurul, M.S.; Mariatti, M. Effect of thermal conductive fillers on the properties of polypropylene composites. J. Thermoplast. Compos. 2013, 26, 627–639. [Google Scholar] [CrossRef]
- Bhagat, N.A.; Shrivastava, N.K.; Suin, S.; Maiti, S.; Khatua, B.B. Development of electrical conductivity in PP/HDPE/MWCNT nanocomposite by melt mixing at very low loading of MWCNT. Polym. Compos. 2013, 34, 787–798. [Google Scholar] [CrossRef]
- Zhou, T.Y.; Tsui, G.C.P.; Liang, J.Z.; Zou, S.Y.; Tangm, C.Y. Thermal properties and thermal stability of PP/MWCNT. Compos. Part B Eng. 2016, 90, 107–114. [Google Scholar] [CrossRef]
- Hapuarachchi, T.D.; Peijs, T.; Bilotti, E. Thermal degradation and flammability behavior of polypropylene/clay/carbon nanotube composite systems. Polym. Adv. Technol. 2013, 24, 331–338. [Google Scholar] [CrossRef]
- Yang, F.; Nelson, G.L. Polymer/silica nanocomposites prepared via extrusion. Polym. Adv. Technol. 2006, 17, 320–326. [Google Scholar] [CrossRef]
- Chu, C.C.; White, K.L.; Liun, P.; Zhangn, X.; Suen, H.J. Electrical conductivity and thermal stability of polypropylene containing well-dispersed multi-walled carbon nanotubes disentangled with exfoliated nanoplatelets. Carbon 2012, 50, 4711–4721. [Google Scholar] [CrossRef]
- Al-Shere, S.Z.; Al-Amshany, Z.M.; Al Sulami, Q.A.; Tashkandi, N.Y.; Hussein, M.A.; El-Shishtawy, R. The preparation of carbon nanofillers and their role on the performance of variable polymer nancomposites. Des. Monomers Polym. 2019, 22, 8–53. [Google Scholar] [CrossRef]
- Vega, J.; Martinez, J.; Trujillo, M.; Arnal, M.; Muller, A.; Bredeau, S.; Dubois, P. Rheology, Processing, Tensile Properties, and Crystallization of Polyethylene/Carbon Nanotube Nanocomposites. Macromolecules 2009, 42, 4719–4727. [Google Scholar] [CrossRef]
- Vega, J.F.; da Silva, Y.; Vicente Alique, E.; Nuńez Ramírez, R.; Trujillo, M.; Arnal, M.L.; Müller, A.J.; Dubois, P.; Martínez Salazar, J. Influence of Chain Branching and Molecular Weight on Melt Rheology and Crystallization of Polyethylene/Carbon Nanotube Nanocomposites. Macromolecules 2014, 47, 5668–5681. [Google Scholar] [CrossRef]
- Trujillo, M.; Arnal, M.; Müller, A.J.; Bredeau, S.; Bonduel, D.; Dubois, P.; Hamley, I.; Castelletto, V. Thermal Fractionation and Isothermal Crystallization of Polyethylene Nanocomposites Prepared by in Situ Polymerization. Macromolecules 2008, 41, 2087–2095. [Google Scholar] [CrossRef]
- Trujillo, M.; Arnal, M.; Müller, A.J.; Laredo, E.; Bredeau, S.; Bonduel, D.; Dubois, P. Thermal and Morphological Characterization of Nanocomposites Prepared by in Situ Polymerization of High- Density Polyethylene on Carbon Nanotubes. Macromolecules 2007, 40, 6268–6276. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, X.; He, Q.; Wei, H.; Long, J.; Guo, J.; Gu, H.; Yu, J.; Liu, J.; Ding, D.; et al. Electrically conductive polypropylene nanocomposites with negative permittivity at low carbón nanotube loading levels. ACS Appl. Mater. Interfaces 2015, 7, 6125–6138. [Google Scholar] [CrossRef]
- Laird, E.D.; Li, C.Y. Structure and Morphology Control in Crystalline Polymer-Carbon Nanotube Nanocomposites. Macromolecules 2013, 46, 2877–2891. [Google Scholar] [CrossRef]
- Grady, B.P.; Pompeo, F.; Shambaugh, R.L.; Resasco, D.E. Nucleation of polypropylene crystallization by single-walled carbon nanotubes. J. Phys. Chem. B 2002, 106, 5852–5858. [Google Scholar] [CrossRef]
- Bhattacharyya, A.R.; Sreekumar, T.V.; Liu, T.; Kumar, S.; Ericson, L.M.; Hauge, R.H.; Smalley, R.E. Crystallization and orientation studies in polypropylene/single wall carbon nanotube composite. Polymer 2003, 44, 2373–2377. [Google Scholar] [CrossRef]
- Xu, D.H.; Wang, Z.G. Role of multi-wall carbon nanotube network in composites to crystallization of isotactic polypropylene matrix. Polymer 2008, 49, 330–338. [Google Scholar] [CrossRef]
- Haggenmueller, R.; Fischer, J.E.; Winey, K.I. Single wall carbon nanotube/polyethylene nanocomposites: Nucleating and templating polyethylene crystallites. Macromolecules 2006, 39, 2964–2971. [Google Scholar] [CrossRef]
- Funck, A.; Kaminsky, W. Polypropylene carbon nanotube composites by in situ polymerization. Compos. Sci. Technol. 2007, 67, 906–915. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Deng, L.; Fang, H.; Zhang, Y.; Wang, Z. More dominant shear flow effect assisted by added carbon nanotubes on crystallization kinetics of isotactic polypropylene in nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 1364–1375. [Google Scholar] [CrossRef]
- Maharramov, A.M.; Ramazanov, M.A.; Ahmadova, A.B.; Hajiyeva, F.V.; Hasanova, U.A. Structure and dielectric properties of nanocomposites based on isotactic polypropylene and titanium nanoparticles. Dig. J. Nanomater. Bios. 2016, 11, 781–786. [Google Scholar]
- Sierra, R.; Pérez, M.; Valdez, J.; Ávila, C.; Jimenez, E.J.; Mata, J.; Soto, E.; Cadenas, G. Synthesis and Thermomechanical Characterization of Nylon 6/Cu Nanocomposites Produced by an Ultrasound-Assisted Extrusion Method. Adv. Mater. Sci. Eng. 2018, 2018, 4792735. [Google Scholar] [CrossRef]
- Pan, Y.; Feng, H.K.; Li, L.; Chan, S.; Zhao, J.; Kay, Y. Effects of hybrid fillers on the electrical conductivity and EMI shielding efficiency of polypropylene/conductive filler composites. Macromol. Res. 2013, 21, 905–910. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, H.K.F.; Li, L.; Chan, S.H.; Zhao, J.; Juay, Y.K. Annealing induced electrical conductivity jump of multi-walled carbon nanotube/polypropylene composites and influence of molecular weight of polypropylene. J. Polym. Sci. Polym. Phys. 2010, 48, 2238–2247. [Google Scholar] [CrossRef]
- Steinmann, W.; Vad, T.; Weise, B.; Wulfhorst, J.; Seide, G.; Gries, T.; Heidelmann, M.; Weirich, T. Extrusion of CNT-modified polymers with low viscosity-influence of crystallization and CNT orientation on the electrical properties. Polym. Polym. Compos. 2013, 21, 473–482. [Google Scholar] [CrossRef]
- Ramazanov, MA.; Hajiyeva, F.V.; Maharramov, A.M. Structure and properties of PP/TiO2 based polymer nanocomposites. Integr. Ferroelectr. 2018, 192, 103–112. [Google Scholar] [CrossRef]
- Rafiee, R. Fabrication of carbon nanotube/polymer nanocomposite. In Carbon Nanotube-Reinforced Polymers: From Nanoscale to Macroscale; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 61–76. [Google Scholar]
- Song, Y.S.; Youn, J.R. Influence of dispersion states of carbon nanotubes on physical properties of epoxy nanocomposites. Carbon 2005, 43, 1378–1385. [Google Scholar] [CrossRef]
- Zhao, Q.; Hu, Y.; Wang, X. Mechanical performance and flame retardancy of polypropylene composites containing zeolite and multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2016, 133, 42875–42879. [Google Scholar] [CrossRef]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Toniazzo, V.; Ruch, D. Intumescent coating of (polyallylamine-polyphosphates) deposited on polyamide fabrics via layer-by-layer technique. Polym Degrad Stab. 2014, 106, 158–164. [Google Scholar] [CrossRef]






| Sample | CNT + TiO2 (wt %) | PP (g) | TiO2/CNT (g) |
|---|---|---|---|
| PP | 0 | 200 | 0 |
| PP-TiO2/CNT-1 | 1 | 198 | 2 |
| PP-TiO2/CNT-5 | 5 | 190 | 10 |
| PP-TiO2/CNT-10 | 10 | 180 | 20 |
| Sample | MFI (g/10 min) |
|---|---|
| PP | 0.76 |
| PP-TiO2/CNT-1 | 0.56 |
| PP-TiO2/CNT-5 | 0.40 |
| PP-TiO2/CNT-10 | 0.24 |
| Sample | T5% (°C) | T50% (°C) | Tmax (°C) | Residue at 550 °C (%) |
|---|---|---|---|---|
| PP | 417 | 454 | 459 | 0 |
| PP-TiO2/CNT-1 | 435 | 460 | 462 | 1.00 |
| PP-TiO2/CNT-5 | 448 | 469 | 472. | 4.92 |
| PP-TiO2/CNT-10 | 454 | 474 | 475 | 8.02 |
| Nanocomposite | Tm (°C) | Enthalpy of Fusion (J/g) | Enthalpy of Crystallization (J/g) | Xc (%) |
|---|---|---|---|---|
| PP | 156.93 | 95.82 | 93.72 | 45.84 |
| PP-TiO2/CNT-1 | 156.16 | 93.45 | 95.35 | 44.71 |
| PP-TiO2/CNT-5 | 157.14 | 91.06 | 92.82 | 43.56 |
| PP-TiO2/CNT-10 | 157.07 | 88.75 | 93.63 | 42.46 |
| Sample | Surface Resistance Ω/sq | Volumetric Resistance Ω cm | Electric Conductivity S/m |
|---|---|---|---|
| PP | 2.35 × 1013 | 1 × 1018 | 1 × 10−18 |
| PP-TiO2/CNT-1 | 6 × 10 16 | 3 × 1017 | 3.0 × 10−18 |
| PP-TiO2/CNT-5 | 3 × 1012 | 5 × 1016 | 2.0 × 10−17 |
| PP-TiO2/CNT-10 | 6.5 × 109 | 7.2 × 109 | 1.4 × 10−10 |
| Sample | Xc | Tensile Strength (MPa) | Nominal Strain at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|
| PP | 56.36 | 23.93 ± 0.12 | 53.37 | 971.57 ± 29.1 |
| PP-TiO2/CNT-1 | 54.97 | 23.93 ± 0.19 | 45.78 | 894.98 ± 27.1 |
| PP-TiO2/CNT-5 | 53.56 | 23.32 ± 0.08 | 33.24 | 961.28 ± 31.0 |
| PP-TiO2/CNT-10 | 52.20 | 22.75 ± 0.37 | 22.28 | 1077.44 ± 26.2 |
| Sample | Peak HRR (kW/m²) | THR (MJ/m²) | Residue (%) |
|---|---|---|---|
| PP | 1529.53 | 66.42 | 0.09 |
| PP-TiO2/CNT-1 | 1593.83 | 65.91 | 34.3 |
| PP-TiO2/CNT-5 | 1058.49 | 43.49 | 88.4 |
| PP-TiO2/CNT-10 | 1079.94 | 44.06 | 91.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabello-Alvarado, C.; Reyes-Rodríguez, P.; Andrade-Guel, M.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Cruz-Delgado, V.J.; Melo-López, L.; Quiñones-Jurado, Z.V.; Ávila-Orta, C.A. Melt-Mixed Thermoplastic Nanocomposite Containing Carbon Nanotubes and Titanium Dioxide for Flame Retardancy Applications. Polymers 2019, 11, 1204. https://doi.org/10.3390/polym11071204
Cabello-Alvarado C, Reyes-Rodríguez P, Andrade-Guel M, Cadenas-Pliego G, Pérez-Alvarez M, Cruz-Delgado VJ, Melo-López L, Quiñones-Jurado ZV, Ávila-Orta CA. Melt-Mixed Thermoplastic Nanocomposite Containing Carbon Nanotubes and Titanium Dioxide for Flame Retardancy Applications. Polymers. 2019; 11(7):1204. https://doi.org/10.3390/polym11071204
Chicago/Turabian StyleCabello-Alvarado, C., P. Reyes-Rodríguez, M. Andrade-Guel, G. Cadenas-Pliego, M. Pérez-Alvarez, V.J. Cruz-Delgado, L. Melo-López, Z.V. Quiñones-Jurado, and C.A. Ávila-Orta. 2019. "Melt-Mixed Thermoplastic Nanocomposite Containing Carbon Nanotubes and Titanium Dioxide for Flame Retardancy Applications" Polymers 11, no. 7: 1204. https://doi.org/10.3390/polym11071204
APA StyleCabello-Alvarado, C., Reyes-Rodríguez, P., Andrade-Guel, M., Cadenas-Pliego, G., Pérez-Alvarez, M., Cruz-Delgado, V. J., Melo-López, L., Quiñones-Jurado, Z. V., & Ávila-Orta, C. A. (2019). Melt-Mixed Thermoplastic Nanocomposite Containing Carbon Nanotubes and Titanium Dioxide for Flame Retardancy Applications. Polymers, 11(7), 1204. https://doi.org/10.3390/polym11071204