Identification and Quantification of Cell Gas Evolution in Rigid Polyurethane Foams by Novel GCMS Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Foam Characterization
2.3. Measurement of Foam Thermal Conductivity
2.4. Measurement of Foam Cell Gas
2.4.1. Sampling
2.4.2. Chromatographic Analysis
2.4.3. Determination of Cell Gas Content
2.4.4. Prediction of Thermal Conductivity of the Gas Phase of a PU Foam
3. Results and Discussion
3.1. Morphological Characterization
3.2. RPU Foam Cell Gas Composition, Calculated Thermal Conductivity of the Gas Phase and Measured Foam Thermal Conductivity
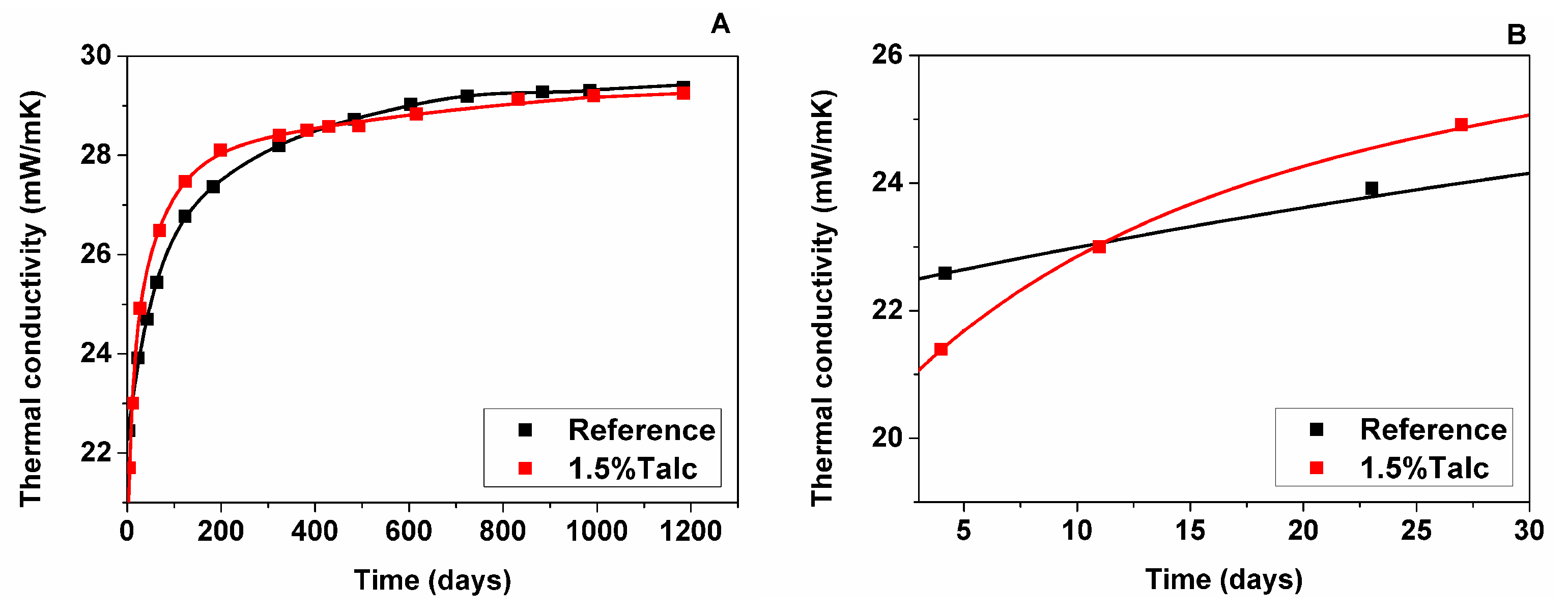
3.2.1. Measured Foam Thermal Conductivity
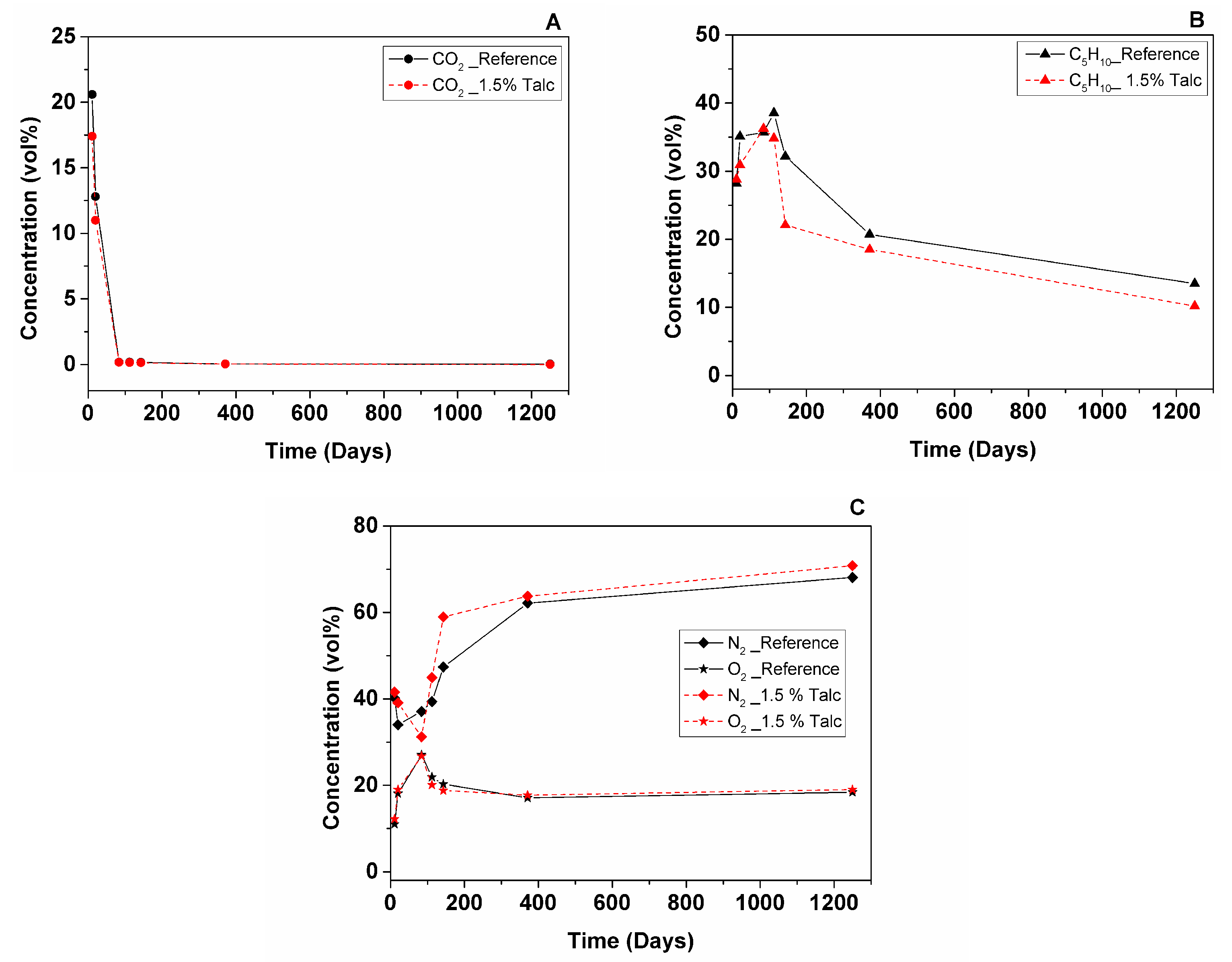
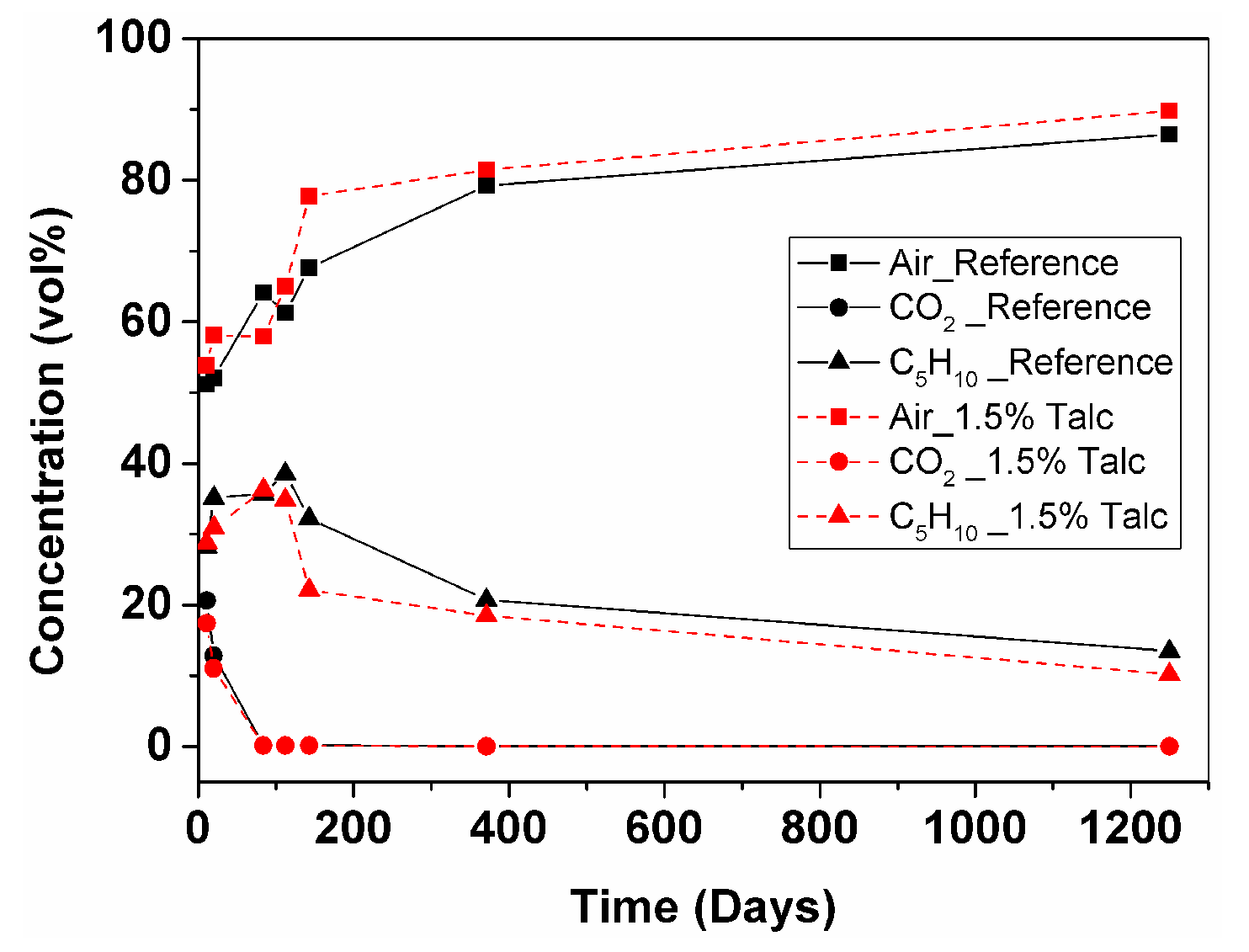
3.2.2. Foam Cell Gas Composition
4. Conclusions
- Considering that the diffusion of gases is one of the mechanisms for deterioration of foam thermal insulation, the measurement of foam thermal conductivity itself does not show the situation with gas composition in every individual foam. Therefore, the GCMS gas content method has shown its benefit as an excellent instrument for the determination of foam cell gas composition and the diffusion of insulation cell gases out of the foam and diffusion of air into the foam cells.
- The presented study was done on two foam types, a reference and one modified with 1.5% talc that have already been described in previous articles, but the gas content method applied in this work made the research of talc incorporation into the foam structure much more comprehensive.
- The sample with talc presented a decrease in the cell size of around 50%, which reduced the radiative contribution to the total thermal conductivity at the initial time of foam manufacturing and, thus, promoted an enhancement of the thermal conductivity. However, that initial thermal improvement shown by foam with 1.5% talc was lost, which has been explained by a higher gas diffusion and, thus, an increase in the thermal conductivity of the cell gas mixture with time.
- GCMS measurement of reference and 1.5% talc modified RPU foams in 10 × 8 × 2.5 cm geometry showed that CO2 leaves the foam after 2.5 months (from 21% to 0.03% for the reference foam and from 17% to 0.03% for the foam with 1.5% talc). C5H10 deviates during 3.5 months, which could be explained by liquid cyclopentane that was probably not evaporated completely during foam manufacturing (from 28% up to 39% for the reference foam and from 29% up to 35% for the foam with 1.5% talc), then it starts to leave the foam and after 3.5 years its content is 13% for the reference and 10% for the foam with talc. Air diffuses inside the cells faster until the one-year point (from 51% up to 79% for the reference and from 54% up to 81% for the foam with talc) and then more slowly until the 3.5-year point (reaching 86% for the reference and 90% for the foam with talc).
- The study of gas mixture content with time could help in the development of the manufacturing of foam with desirable gas compositions and to control the initial thermal conductivity of the foam and, correspondingly, the long-term value.
Author Contributions
Funding
Conflicts of Interest
References
- Szycher, M. Szycher’s Handbook of Polyurethanes, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Choi, S.W.; Jung, J.M.; Yoo, H.M.; Kim, S.H.; Lee, W.I. Analysis of thermal properties and heat transfer mechanisms for polyurethane foams blown with water. J. Therm. Anal. Calorim. 2018, 132, 1253–1262. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, W.-Z.; Li, Y.-M.; Tao, W.-Q. Experimental study of the thermal conductivity of polyurethane foams. Appl. Therm. Eng. 2017, 115, 528–538. [Google Scholar] [CrossRef]
- Santiago-Calvo, M.; Tirado-Mediavilla, J.; Rauhe, J.C.; Jensen, L.R.; Ruiz-Herrero, J.L.; Villafañe, F.; Rodríguez-Pérez, M. Ángel Evaluation of the thermal conductivity and mechanical properties of water blown polyurethane rigid foams reinforced with carbon nanofibers. Eur. Polym. J. 2018, 108, 98–106. [Google Scholar] [CrossRef]
- Glicksman, L. Low Density Cellular Plastics: Physical Basis of Behaviour; Springer Netherlands: London, UK, 1994. [Google Scholar]
- Hasanzadeh, R.; Azdast, T.; Doniavi, A.; Lee, R.E. Multi-objective optimization of heat transfer mechanisms of microcellular polymeric foams from thermal-insulation point of view. Therm. Sci. Eng. Prog. 2019, 9, 21–29. [Google Scholar] [CrossRef]
- Ostrogorsky, A.G.; Glicksman, L.R.; Reitz, D.W. Aging of polyurethane foams. Int. J. Heat Mass Transf. 1986, 29, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.-J.; Yamaguchi, M.; Ohmori, T. Thermal conductivity of polyurethane foams from room temperature to 20 K. Cryog. 1997, 37, 305–312. [Google Scholar] [CrossRef]
- Eaves, D. Handbook of Polymer Foams; Rapra Technology Limited: Shawbury, UK, 2004. [Google Scholar]
- Estravís, S.; Tirado-Mediavilla, J.; Santiago-Calvo, M.; Ruiz-Herrero, J.L.; Villafañe, F.; Rodríguez-Pérez, M. Ángel Rigid polyurethane foams with infused nanoclays: Relationship between cellular structure and thermal conductivity. Eur. Polym. J. 2016, 80, 1–15. [Google Scholar] [CrossRef]
- Santiago-Calvo, M.; Tirado-Mediavilla, J.; Ruiz-Herrero, J.L.; Villafañe, F.; Rodríguez-Pérez, M.Á. Long-term thermal conductivity of cyclopentane-water blown rigid polyurethane foams reinforced with different types of fillers. Polym. Int. 2019. Submitted. [Google Scholar]
- ASTM D1622-08: Standard Test Method for Apparent Density of Rigid Cellular Plastics. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/D1622-08.htm (accessed on 17 July 2019).
- ASTM D6226-10: Standard Test Method for Open Cell Content of Rigid Cellular Plastics. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/D6226-10.htm (accessed on 17 July 2019).
- Pinto, J.; Solórzano, E.; Rodriguez-Perez, M.A.; De Saja, J.A. Characterization of the cellular structure based on user-interactive image analysis procedures. J. Cell. Plast. 2013, 49, 555–575. [Google Scholar] [CrossRef]
- UNE-EN 12667:2002. Thermal performance of building materials and products. Determination of thermal resistance by means of guarded hot plate and heat flow meter methods. Products of high and medium thermal resistance. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0027459 (accessed on 17 July 2019).
- NIST: National Institute of Standards and Technology. Available online: https://www.nist.gov/ (accessed on 17 July 2019).
- Vassiliou, C.-M.; Assael, M.J.; Huber, M.L.; Perkins, R.A. Reference Correlations of the Thermal Conductivity of Cyclopentane, iso-Pentane, and n-Pentane. J. Phys. Chem. Ref. Data 2015, 44, 33102. [Google Scholar] [CrossRef] [Green Version]
- Leung, S.N.; Wong, A.; Wang, L.C.; Park, C.B. Mechanism of extensional stress-induced cell formation in polymeric foaming processes with the presence of nucleating agents. J. Supercrit. Fluids 2012, 63, 187–198. [Google Scholar] [CrossRef]
- Santiago-Calvo, M.; Tirado-Mediavilla, J.; Ruiz-Herrero, J.L.; Rodríguez-Pérez, M.; Ángel Villafañe, F. The effects of functional nanofillers on the reaction kinetics, microstructure, thermal and mechanical properties of water blown rigid polyurethane foams. Polymers 2018, 150, 138–149. [Google Scholar] [CrossRef]
- Olsson, M.E.; Jarfelt, U.; Fröling, M.; Mangs, S.; Ramnas, O. Diffusion of Cyclopentane in Polyurethane Foam at Different Temperatures and Implications for District Heating Pipes. J. Cell. Plast. 2002, 38, 177–188. [Google Scholar] [CrossRef]

| Foam Index | Density (Kg/m3) | OC (%) | Φ3D (µm) | AR | Foaming Reaction Temperature (°C) |
|---|---|---|---|---|---|
| Reference | 31.2 ± 1.7 | 8.1 ± 1.9 | 608 ± 68 | 1.11 ± 0.29 | 105.9 |
| 1.5%Talc | 35.6 ± 1.2 | 9.5 ± 3.1 | 307 ± 98 | 1.27 ± 0.27 | 121.9 |
| Sample, Index/Storage Time | N2, vol% | O2, vol% | CO2, vol% | C5H10, vol% | λgas calcul, mW·m−1·K−1 |
|---|---|---|---|---|---|
| Reference | |||||
| 12 days | 40.20 | 11.00 | 20.60 | 28.20 | 19.34 |
| 21 days | 34.00 | 18.10 | 12.80 | 35.10 | 19.48 |
| 85 days (2.5 months) | 37.11 | 27.02 | 0.17 | 35.70 | 21.39 |
| 113 days (3.5 months) | 39.39 | 21.89 | 0.17 | 38.54 | 20.95 |
| 144 days (4.5 months) | 47.37 | 20.28 | 0.16 | 32.19 | 21.81 |
| 372 days (1 year) | 62.16 | 17.08 | 0.038 | 20.72 | 23.26 |
| 1250 days (3.5 years) | 68.13 | 18.35 | 0.032 | 13.48 | 24.11 |
| 1.5% Talc | |||||
| 12 days | 41.60 | 12.20 | 17.40 | 28.80 | 19.70 |
| 21 days | 39.10 | 19.00 | 11.00 | 30.90 | 20.35 |
| 85 days (2.5 months) | 31.23 | 26.73 | 0.16 | 36.25 | 21.03 |
| 113 days (3.5 months) | 44.94 | 20.09 | 0.14 | 34.83 | 21.46 |
| 144 days (4.5 months) | 58.96 | 18.79 | 0.13 | 22.12 | 23.09 |
| 372 days (1 year) | 63.75 | 17.73 | 0.042 | 18.49 | 23.53 |
| 1250 days (3.5 years) | 70.78 | 19.04 | 0.030 | 10.15 | 24.48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galakhova, A.; Santiago-Calvo, M.; Tirado-Mediavilla, J.; Villafañe, F.; Rodríguez-Pérez, M.Á.; Riess, G. Identification and Quantification of Cell Gas Evolution in Rigid Polyurethane Foams by Novel GCMS Methodology. Polymers 2019, 11, 1192. https://doi.org/10.3390/polym11071192
Galakhova A, Santiago-Calvo M, Tirado-Mediavilla J, Villafañe F, Rodríguez-Pérez MÁ, Riess G. Identification and Quantification of Cell Gas Evolution in Rigid Polyurethane Foams by Novel GCMS Methodology. Polymers. 2019; 11(7):1192. https://doi.org/10.3390/polym11071192
Chicago/Turabian StyleGalakhova, Anastasiia, Mercedes Santiago-Calvo, Josias Tirado-Mediavilla, Fernando Villafañe, Miguel Ángel Rodríguez-Pérez, and Gisbert Riess. 2019. "Identification and Quantification of Cell Gas Evolution in Rigid Polyurethane Foams by Novel GCMS Methodology" Polymers 11, no. 7: 1192. https://doi.org/10.3390/polym11071192
APA StyleGalakhova, A., Santiago-Calvo, M., Tirado-Mediavilla, J., Villafañe, F., Rodríguez-Pérez, M. Á., & Riess, G. (2019). Identification and Quantification of Cell Gas Evolution in Rigid Polyurethane Foams by Novel GCMS Methodology. Polymers, 11(7), 1192. https://doi.org/10.3390/polym11071192








