Disordered Mechanical Stress and Tissue Engineering Therapies in Intervertebral Disc Degeneration
Abstract
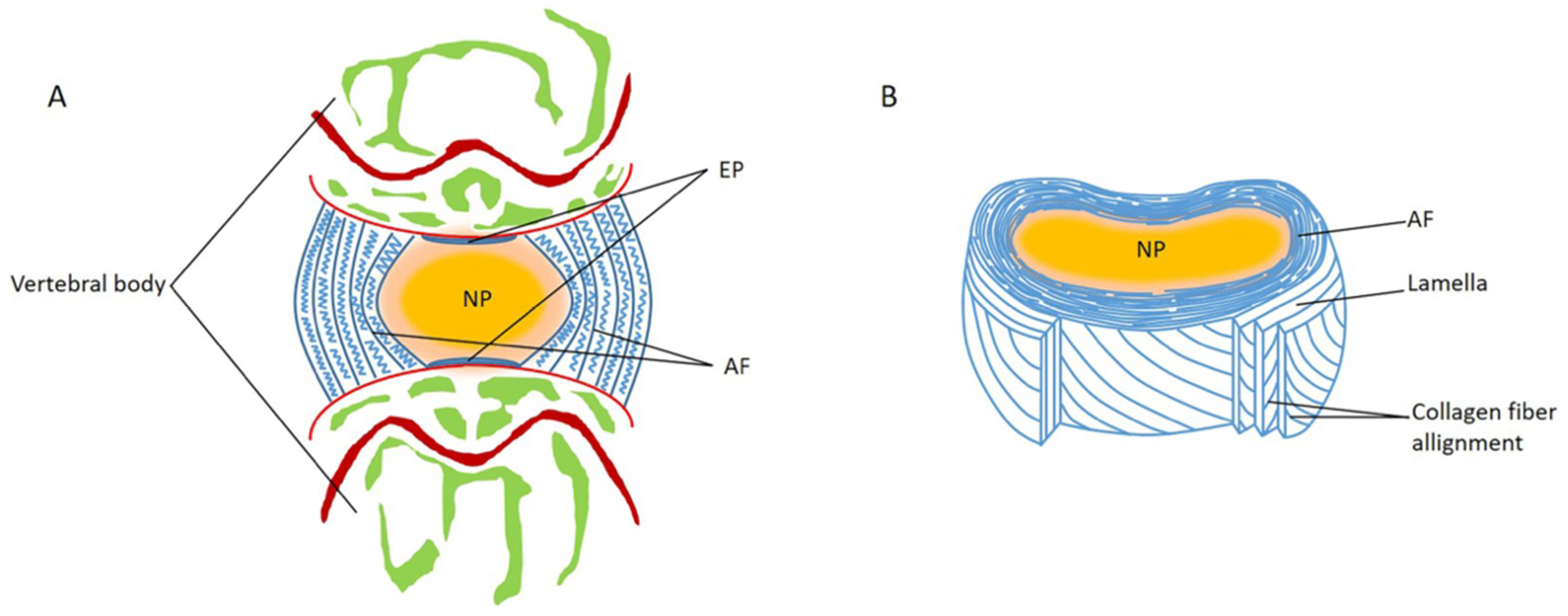
1. Introduction
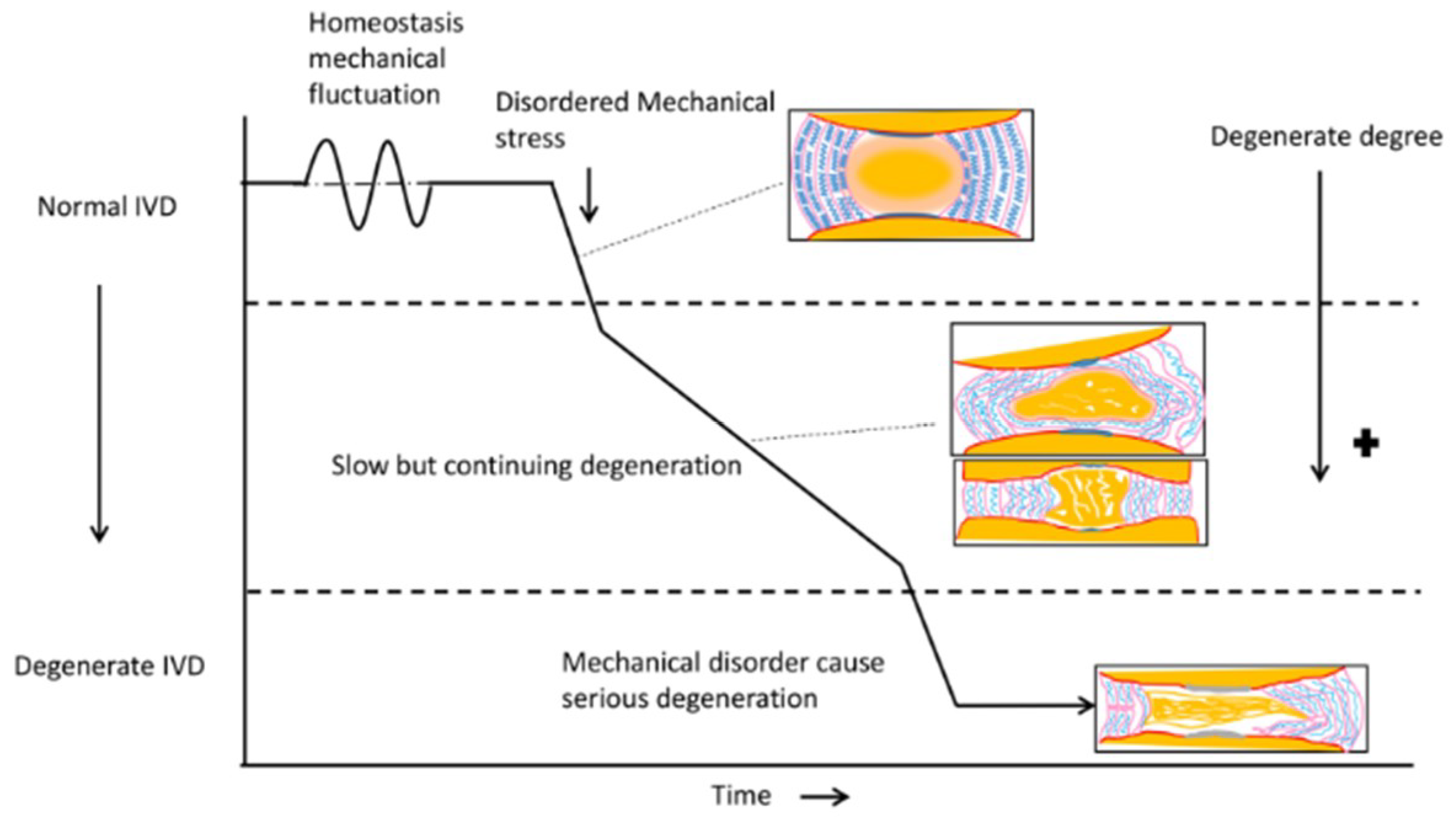
2. Disordered Mechanical Stress Leads to NP Degeneration
3. Disordered Mechanical Stress Leads to AF Degeneration
4. Disordered Mechanical Stress Leads to Angiogenesis
5. Tissue Engineering-Inspired Strategies to Address IDD
5.1. Strategies in IDD Regeneration
5.1.1. NP Regeneration
5.1.2. AF Regeneration
5.1.3. Anti-Angiogenesis
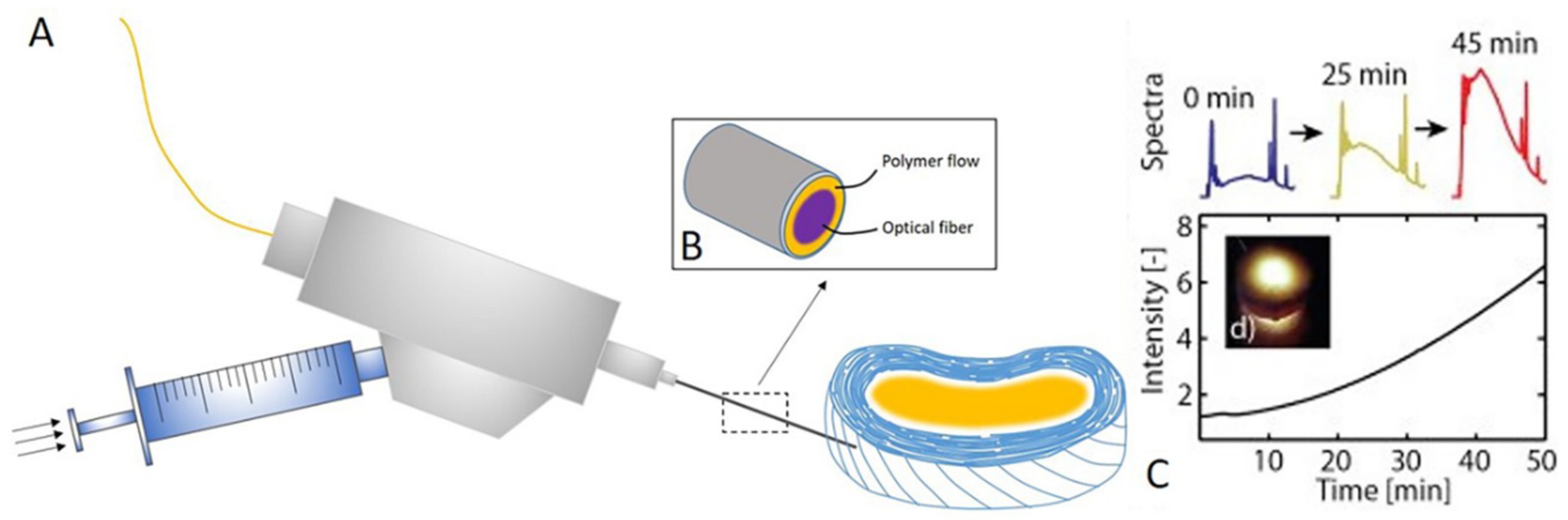
6. Strategies in IVD Displacement
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Guyer, R.D.; Shellock, J.; MacLennan, B.; Hanscom, D.; Knight, R.Q.; McCombe, P.; Jacobs, J.J.; Urban, R.M.; Bradford, D.; Ohnmeiss, D.D. Early failure of metal-on-metal artificial disc prostheses associated with lymphocytic reaction: Diagnosis and treatment experience in four cases. Spine 2011, 36, E492–E497. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.M.; Karppinen, J.; Chan, D.; Ho, D.W.; Song, Y.Q.; Sham, P.; Cheah, K.S.; Leong, J.C.; Luk, K.D. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009, 34, 934–940. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, E.I.T.; Damen, J.; van Meurs, J.B.J.; Ginai, A.Z.; Popham, M.; Hofman, A.; Koes, B.W.; Bierma-Zeinstra, S.M. The Association Between Lumbar Disc Degeneration and Low Back Pain. The Influence of Age, Gender, and Individual Radiographic Features. Spine 2010, 35, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Lambeek, L.C.; van Tulder, M.W.; Swinkels, I.C.; Koppes, L.L.; Anema, J.R.; van Mechelen, W. The trend in total cost of back pain in The Netherlands in the period 2002 to 2007. Spine 2011, 36, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Luoma, K.; Riihimaki, H.; Luukkonen, R.; Raininko, R.; Viikari-Juntura, E.; Lamminen, A. Low back pain in relation to lumbar disc degeneration. Spine 2000, 25, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Scheele, J.; de Schepper, E.I.T.; van Meurs, J.B.J.; Hofman, A.; Koes, B.W.; Luijsterburg, P.A.J.; Bierma-Zeinstra, S.M.A. Association between spinal morning stiffness and lumbar disc degeneration: The Rotterdam Study. Osteoarthr. Cartil. 2012, 20, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Teraguchi, M.; Yoshimura, N.; Hashizume, H.; Muraki, S.; Yamada, H.; Minamide, A.; Oka, H.; Ishimoto, Y.; Nagata, K.; Kagotani, R.; et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: The Wakayama Spine Study. Osteoarthr. Cartil. 2014, 22, 104–110. [Google Scholar] [CrossRef]
- Wang, Y.; Videman, T.; Battie, M.C. ISSLS prize winner: Lumbar vertebral endplate lesions: Associations with disc degeneration and back pain history. Spine 2012, 37, 1490–1496. [Google Scholar] [CrossRef]
- Huang, Y.C.; Urban, J.P.G.; Luk, K.D.K. OPINION Intervertebral disc regeneration: Do nutrients lead the way? Nat. Rev. Rheumatol. 2014, 10, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, F.; Shi, R.; Wang, X.H.; Wu, X.T. Aging and age related stresses: A senescence mechanism of intervertebral disc degeneration. Osteoarthr. Cartil. 2016, 24, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhuang, S.; Mao, Z.; Chen, H. Microendoscopic discectomy for lumbar disc herniation: Surgical technique and outcome in 873 consecutive cases. Spine 2006, 31, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hong, X.; Zhou, B.Y.; Bao, J.P.; Xie, X.H.; Wang, F.; Wu, X.T. Evaluation of transforaminal endoscopic lumbar discectomy in the treatment of lumbar disc herniation. Int. Orthop. 2015, 39, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shi, R.; Cai, F.; Wang, Y.T.; Wu, X.T. Stem Cell Approaches to Intervertebral Disc Regeneration: Obstacles from the Disc Microenvironment. Stem Cells Dev. 2015, 24, 2479–2495. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Andersson, G.B. Stem cell therapy for intervertebral disc regeneration: Obstacles and solutions. Nat. Rev. Rheumatol. 2015, 11, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R.; Muir, H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem. J. 1976, 157, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Hydrogels in acellular and cellular strategies for intervertebral disc regeneration. J. Tissue Eng. Regen. Med. 2013, 7, 85–98. [Google Scholar] [CrossRef]
- Sowa, G.; Vadala, G.; Studer, R.; Kompel, J.; Iucu, C.; Georgescu, H.; Gilbertson, L.; Kang, J. Characterization of intervertebral disc aging: Longitudinal analysis of a rabbit model by magnetic resonance imaging, histology, and gene expression. Spine 2008, 33, 1821–1828. [Google Scholar] [CrossRef]
- Fratzl, P.; Elbaum, R.; Burgert, I. Cellulose fibrils direct plant organ movements. Faraday Discuss. 2008, 139, 275–282. [Google Scholar] [CrossRef]
- Ishihara, H.; Warensjo, K.; Roberts, S.; Urban, J.P. Proteoglycan synthesis in the intervertebral disk nucleus: The role of extracellular osmolality. Am. J. Physiol. 1997, 272, C1499–C1506. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.; Eyre, D.R.; Muir, H. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatol. Rehabilit. 1977, 16, 22–29. [Google Scholar] [CrossRef]
- Melrose, J.; Smith, S.M.; Appleyard, R.C.; Little, C.B. Aggrecan, versican and type VI collagen are components of annular translamellar crossbridges in the intervertebral disc. Eur. Spine J. 2008, 17, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Pezowicz, C.A.; Robertson, P.A.; Broom, N.D. The structural basis of interlamellar cohesion in the intervertebral disc wall. J. Anat. 2006, 208, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Schollmeier, G.; Lahr-Eigen, R.; Lewandrowski, K.U. Observations on fiber-forming collagens in the anulus fibrosus. Spine 2000, 25, 2736–2741. [Google Scholar] [CrossRef] [PubMed]
- Schollum, M.L.; Robertson, P.A.; Broom, N.D. ISSLS prize winner: Microstructure and mechanical disruption of the lumbar disc annulus: Part I: A microscopic investigation of the translamellar bridging network. Spine 2008, 33, 2702–2710. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Fairbank, J.C.; Roberts, S.; Urban, J.P. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine 2005, 30, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tirlapur, U.; Fairbank, J.; Handford, P.; Roberts, S.; Winlove, C.P.; Cui, Z.; Urban, J. Microfibrils, elastin fibres and collagen fibres in the human intervertebral disc and bovine tail disc. J. Anat. 2007, 210, 460–471. [Google Scholar] [CrossRef]
- Roberts, S.; McCall, I.W.; Menage, J.; Haddaway, M.J.; Eisenstein, S.M. Does the thickness of the vertebral subchondral bone reflect the composition of the intervertebral disc? Eur. Spine J. 1997, 6, 385–389. [Google Scholar] [CrossRef]
- Roberts, S.; Menage, J.; Urban, J.P.G. Biochemical and Structural-Properties of the Cartilage Endplate and Its Relation to the Intervertebral-Disk. Spine 1989, 14, 166–174. [Google Scholar] [CrossRef]
- Iatridis, J.C.; MacLean, J.J.; Roughley, P.J.; Alini, M. Effects of mechanical loading on intervertebral disc metabolism in vivo. J. Bone Jt. Surg. Am. 2006, 88 (Suppl. 2), 41–46. [Google Scholar] [CrossRef]
- Wuertz, K.; Godburn, K.; MacLean, J.J.; Barbir, A.; Donnelly, J.S.; Roughley, P.J.; Alini, M.; Iatridis, J.C. In vivo remodeling of intervertebral discs in response to short- and long-term dynamic compression. J. Orthop. Res. 2009, 27, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Farfan, H.F. The torsional injury of the lumbar spine. Spine 1984, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Hutton, W.C. The effect of fatigue on the lumbar intervertebral disc. J. Bone Jt. Surg. Br. Vol. 1983, 65, 199–203. [Google Scholar] [CrossRef]
- Rauck, R.L.; Gargiulo, C.A.; Ruoff, G.E.; Schnitzer, T.J.; Trapp, R.G. Chronic low back pain: New perspectives and treatment guidelines for primary care: Part II. Manag. Care Interface 1998, 11, 71–75. [Google Scholar] [PubMed]
- Nachemson, A.L. Disc pressure measurements. Spine 1981, 6, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Colombier, P.; Camus, A.; Lescaudron, L.; Clouet, J.; Guicheux, J. Intervertebral disc regeneration: A great challenge for tissue engineers. Trends Biotechnol. 2014, 32, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.W.; Au, T.Y.K.; Tam, V.; Cheah, K.S.E.; Chan, D. Coming together is a beginning: The making of an intervertebral disc. Birth Defects Res. Part C 2014, 102, 83–100. [Google Scholar] [CrossRef]
- Hunter, C.J.; Matyas, J.R.; Duncan, N.A. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: A species comparison. J. Anat. 2004, 205, 357–362. [Google Scholar] [CrossRef]
- Risbud, M.V.; Schaer, T.P.; Shapiro, I.M. Toward an Understanding of the Role of Notochordal Cells in the Adult Intervertebral Disc: From Discord to Accord. Dev. Dyn. 2010, 239, 2141–2148. [Google Scholar] [CrossRef]
- Rodrigues-Pinto, R.; Richardson, S.M.; Hoyland, J.A. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. Eur. Spine J. 2014, 23, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Hirai, J.; Matsuda, T. Venous reconstruction using hybrid vascular tissue composed of vascular cells and collagen: Tissue regeneration process. Cell Transpl. 1996, 5, 93–105. [Google Scholar] [CrossRef]
- Klebe, R.J.; Caldwell, H.; Milam, S. Cells Transmit Spatial Information by Orienting Collagen-Fibers. Matrix 1990, 9, 451–458. [Google Scholar] [CrossRef]
- Barocas, V.H.; Tranquillo, R.T. An anisotropic biphasic theory of tissue-equivalent mechanics: The interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. J. Biomech. Eng. 1997, 119, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.L.M.; Vaughn, M.E.; Niklason, L.E. An ultrastructural analysis of collagen in tissue engineered arteries. Ann. Biomed. Eng. 2007, 35, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Chiquet, M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999, 18, 417–426. [Google Scholar] [CrossRef]
- Chiquet, M.; Gelman, L.; Lutz, R.; Maier, S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 911–920. [Google Scholar] [CrossRef]
- Van Vlimmeren, M.A.A.; Driessen-Mol, A.; Oomens, C.W.J.; Baaijens, F.P.T. An In Vitro Model System to Quantify Stress Generation, Compaction, and Retraction in Engineered Heart Valve Tissue. Tissue Eng. Part C-Methods 2011, 17, 983–991. [Google Scholar] [CrossRef]
- Ali, R.; Le Maitre, C.L.; Richardson, S.M.; Hoyland, J.A.; Freemont, A.J. Connective tissue growth factor expression in human intervertebral disc: Implications for angiogenesis in intervertebral disc degeneration. Biotech. Histochem. 2008, 83, 239–245. [Google Scholar] [CrossRef]
- Pufe, T.; Lemke, A.; Kurz, B.; Petersen, W.; Tillmann, B.; Grodzinsky, A.J.; Mentlein, R. Mechanical overload induces VEGF in cartilage discs via hypoxia-inducible factor. Am. J. Pathol. 2004, 164, 185–192. [Google Scholar] [CrossRef]
- Urban, J.P.G.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Setton, L.A.; Chen, J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J. Bone Jt. Surg. Am. 2006, 88A, 52–57. [Google Scholar] [CrossRef]
- Hwang, P.Y.; Chen, J.; Jing, L.; Hoffman, B.D.; Setton, L.A. The Role of Extracellular Matrix Elasticity and Composition in Regulating the Nucleus Pulposus Cell Phenotype in the Intervertebral Disc: A Narrative Review. J. Biomech. Eng. 2014, 136. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.F.; Setton, L.A.; Jarvis, W.; So, S.; Chen, J.; Jing, L.F.; Bullock, R.; Isaacs, R.E.; Brown, C.; Richardson, W.J. Proinflammatory Cytokine Expression Profile in Degenerated and Herniated Human Intervertebral Disc Tissues. Arthritis Rheum. 2010, 62, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chang, H.M.; Cheng, J.C.; Klausen, C.; Leung, P.C.; Yang, X. Transforming growth factor-beta1 increases lysyl oxidase expression by downregulating MIR29A in human granulosa lutein cells. Reproduction 2016, 152, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.J.; Matyas, J.R.; Duncan, N.A. The three-dimensional architecture of the notochordal nucleus pulposus: Novel observations on cell structures in the canine intervertebral disc. J. Anat. 2003, 202, 279–291. [Google Scholar] [CrossRef]
- Hunter, C.J.; Matyas, J.R.; Duncan, N.A. The notochordal cell in the nucleus pulposus: A review in the context of tissue engineering. Tissue Eng. 2003, 9, 667–677. [Google Scholar] [CrossRef]
- Stosiek, P.; Kasper, M.; Karsten, U. Expression of cytokeratin and vimentin in nucleus pulposus cells. Differentiation 1988, 39, 78–81. [Google Scholar] [CrossRef]
- Erwin, W.M.; Ashman, K.; O’Donnel, P.; Inman, R.D. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006, 54, 3859–3867. [Google Scholar] [CrossRef]
- Erwin, W.M.; Islam, D.; Inman, R.D.; Fehlings, M.G.; Tsui, F.W.L. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: Implications for the mechanisms of intervertebral disc degeneration. Arthritis Res. Ther. 2011, 13. [Google Scholar] [CrossRef]
- Guehring, T.; Nerlich, A.; Kroeber, M.; Richter, W.; Omlor, G.W. Sensitivity of notochordal disc cells to mechanical loading: An experimental animal study. Eur. Spine J. 2010, 19, 113–121. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kobayashi, S.; Takeno, K.; Meir, A.; Urban, J.; Baba, H. A phenotypic comparison of proteoglycan production of intervertebral disc cells isolated from rats, rabbits, and bovine tails; which animal model is most suitable to study tissue engineering and biological repair of human disc disorders? Tissue Eng. Part A 2009, 15, 3835–3846. [Google Scholar] [CrossRef]
- Yurube, T.; Hirata, H.; Kakutani, K.; Maeno, K.; Takada, T.; Zhang, Z.Y.; Takayama, K.; Matsushita, T.; Kuroda, R.; Kurosaka, M.; et al. Notochordal cell disappearance and modes of apoptotic cell death in a rat tail static compression-induced disc degeneration model. Arthritis Rec. Ther. 2014, 16. [Google Scholar] [CrossRef]
- Ellis, K.; Hoffman, B.D.; Bagnat, M. The vacuole within: How cellular organization dictates notochord function. Bioarchitecture 2013, 3, 64–68. [Google Scholar] [CrossRef]
- Inoue, H. Three-dimensional observation of collagen framework of intervertebral discs in rats, dogs and humans. Arch. Histol. Jpn. 1973, 36, 39–56. [Google Scholar] [CrossRef][Green Version]
- Roberts, S. Disc morphology in health and disease. Biochem. Soc. Trans. 2002, 30, 864–869. [Google Scholar] [CrossRef]
- Souter, W.A.; Taylor, T.K. Sulphated acid mucopolysaccharide metabolism in the rabbit intervertebral disc. J. Bone Jt. Surg. Br. Vol. 1970, 52, 371–384. [Google Scholar] [CrossRef]
- Gealy, C.; Hayes, A.J.; Buckwell, R.; Young, R.D.; Caterson, B.; Quantock, A.J.; Ralphs, J.R. Actin and Type I Collagen Propeptide Distribution in the Developing Chick Cornea. Investig. Ophth. Vis. Sci. 2009, 50, 1653–1658. [Google Scholar] [CrossRef]
- Canty, E.G.; Starborg, T.; Lu, Y.H.; Humphries, S.M.; Holmes, D.F.; Meadows, R.S.; Huffman, A.; O’Toole, E.T.; Kadler, K.E. Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J. Biol. Chem. 2006, 281, 38592–38598. [Google Scholar] [CrossRef]
- Canty, E.G.; Lu, Y.H.; Meadows, R.S.; Shaw, M.K.; Holmes, D.F.; Kadler, K.E. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J. Cell Biol. 2004, 165, 553–563. [Google Scholar] [CrossRef]
- Sachs, F.; Morris, C.E. Mechanosensitive ion channels in nonspecialized cells. Rev. Physiol. Biochem. Pharmacol. 1998, 132, 1–77. [Google Scholar] [CrossRef]
- Banes, A.J.; Tsuzaki, M.; Yamamoto, J.; Fischer, T.; Brigman, B.; Brown, T.; Miller, L. Mechanoreception at the cellular level: The detection, interpretation, and diversity of responses to mechanical signals. Biochem. Cell Biol. 1995, 73, 349–365. [Google Scholar] [CrossRef]
- Malek, A.M.; Izumo, S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J. Cell Sci. 1996, 109, 713–726. [Google Scholar]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef]
- Lock, J.G.; Wehrle-Haller, B.; Stromblad, S. Cell-matrix adhesion complexes: Master control machinery of cell migration. Semin. Cancer Biol. 2008, 18, 65–76. [Google Scholar] [CrossRef]
- Berrier, A.L.; Yamada, K.M. Cell-matrix adhesion. J. Cell. Physiol. 2007, 213, 565–573. [Google Scholar] [CrossRef]
- Flynn, B.P.; Bhole, A.P.; Saeidi, N.; Liles, M.; DiMarzio, C.A.; Ruberti, J.W. Mechanical Strain Stabilizes Reconstituted Collagen Fibrils against Enzymatic Degradation by Mammalian Collagenase Matrix Metalloproteinase 8 (MMP-8). PLoS ONE 2010, 5, e12377. [Google Scholar] [CrossRef]
- Birkedalhansen, H.; Moore, W.G.I.; Bodden, M.K.; Windsor, L.J.; Birkedalhansen, B.; Decarlo, A.; Engler, J.A. Matrix Metalloproteinases-a Review. Crit. Rev. Oral Biol. Med. 1993, 4, 197–250. [Google Scholar] [CrossRef]
- Ruberti, J.W.; Hallab, N.J. Strain-controlled enzymatic cleavage of collagen in loaded matrix. Biochem. Bioph. Res. Commun. 2005, 336, 483–489. [Google Scholar] [CrossRef]
- Bhole, A.P.; Flynn, B.P.; Liles, M.; Saeidi, N.; Dimarzio, C.A.; Ruberti, J.W. Mechanical strain enhances survivability of collagen micronetworks in the presence of collagenase: Implications for load-bearing matrix growth and stability. Philos. Trans. R. Soc. A 2009, 367, 3339–3362. [Google Scholar] [CrossRef]
- Miles, C.A.; Ghelashvili, M. Polymer-in-a-box mechanism for the thermal stabilization of collagen molecules in fibers. Biophys. J. 1999, 76, 3243–3252. [Google Scholar] [CrossRef]
- Mirza, S.K.; White, A.A., 3rd. Anatomy of intervertebral disc and pathophysiology of herniated disc disease. J. Clin. Laser Med. Surg. 1995, 13, 131–142. [Google Scholar] [CrossRef]
- Neidlinger-Wilke, C.; Liedert, A.; Wuertz, K.; Buser, Z.; Rinkler, C.; Kafer, W.; Ignatius, A.; Claes, L.; Roberts, S.; Johnson, W.E. Mechanical stimulation alters pleiotrophin and aggrecan expression by human intervertebral disc cells and influences their capacity to stimulate endothelial migration. Spine 2009, 34, 663–669. [Google Scholar] [CrossRef]
- Kim, J.H.; Deasy, B.M.; Seo, H.Y.; Studer, R.K.; Vo, N.V.; Georgescu, H.I.; Sowa, G.A.; Kang, J.D. Differentiation of Intervertebral Notochordal Cells Through Live Automated Cell Imaging System In Vitro. Spine 2009, 34, 2486–2493. [Google Scholar] [CrossRef]
- Ruan, D.K.; Xin, H.; Zhang, C.; Wang, C.; Xu, C.; Li, C.; He, Q. Experimental intervertebral disc regeneration with tissue-engineered composite in a canine model. Tissue Eng. Part A 2010, 16, 2381–2389. [Google Scholar] [CrossRef]
- Buser, Z.; Kuelling, F.; Liu, J.; Liebenberg, E.; Thorne, K.J.; Coughlin, D.; Lotz, J.C. Biological and biomechanical effects of fibrin injection into porcine intervertebral discs. Spine 2011, 36, E1201–E1209. [Google Scholar] [CrossRef]
- Li, C.Q.; Huang, B.; Luo, G.; Zhang, C.Z.; Zhuang, Y.; Zhou, Y. Construction of collagen II/hyaluronate/chondroitin-6-sulfate tri-copolymer scaffold for nucleus pulposus tissue engineering and preliminary analysis of its physico-chemical properties and biocompatibility. J. Mater. Sci. Mater. Med. 2010, 21, 741–751. [Google Scholar] [CrossRef]
- Endres, M.; Abbushi, A.; Thomale, U.W.; Cabraja, M.; Kroppenstedt, S.N.; Morawietz, L.; Casalis, P.A.; Zenclussen, M.L.; Lemke, A.J.; Horn, P.; et al. Intervertebral disc regeneration after implantation of a cell-free bioresorbable implant in a rabbit disc degeneration model. Biomaterials 2010, 31, 5836–5841. [Google Scholar] [CrossRef]
- Woiciechowsky, C.; Abbushi, A.; Zenclussen, M.L.; Casalis, P.; Kruger, J.P.; Freymann, U.; Endres, M.; Kaps, C. Regeneration of nucleus pulposus tissue in an ovine intervertebral disc degeneration model by cell-free resorbable polymer scaffolds. J. Tissue Eng. Regen. Med 2014, 8, 811–820. [Google Scholar] [CrossRef]
- Hu, J.; Chen, B.; Guo, F.; Du, J.; Gu, P.; Lin, X.; Yang, W.; Zhang, H.; Lu, M.; Huang, Y.; et al. Injectable silk fibroin/polyurethane composite hydrogel for nucleus pulposus replacement. J. Mater. Sci. Mater. Med. 2012, 23, 711–722. [Google Scholar] [CrossRef]
- Revell, P.A.; Damien, E.; Di Silvio, L.; Gurav, N.; Longinotti, C.; Ambrosio, L. Tissue engineered intervertebral disc repair in the pig using injectable polymers. J. Mater. Sci. Mater. Med. 2007, 18, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.T.; Milby, A.H.; Chiaro, J.A.; Kim, D.H.; Hebela, N.M.; Smith, L.J.; Elliott, D.M.; Mauck, R.L. Translation of an engineered nanofibrous disc-like angle-ply structure for intervertebral disc replacement in a small animal model. Acta Biomater. 2014, 10, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Chik, T.K.; Ma, X.Y.; Choy, T.H.; Li, Y.Y.; Diao, H.J.; Teng, W.K.; Han, S.J.; Cheung, K.M.; Chan, B.P. Photochemically crosslinked collagen annulus plug: A potential solution solving the leakage problem of cell-based therapies for disc degeneration. Acta Biomater. 2013, 9, 8128–8139. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chu, T.; Dong, S.; Hao, Y.; Ren, X.; Wang, J.; Wang, W.; Li, C.; Zhang, Z.; Zhou, Y. Cells scaffold complex for Intervertebral disc Anulus Fibrosus tissue engineering: In vitro culture and product analysis. Mol. Boil. Rep. 2012, 39, 8581–8594. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Roy, A.K.; Vacanti, C.A.; Kojima, K.; Ueda, M.; Bonassar, L.J. Tissue-engineered composites of anulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine 2004, 29, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Bowles, R.D.; Gebhard, H.H.; Hartl, R.; Bonassar, L.J. Tissue-engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proc. Natl. Acad. Sci. USA 2011, 108, 13106–13111. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, Z.; Xiang, X.; Deng, S.; Liu, K.; Xiao, D.; Deng, L.; Feng, G. The establishment and biological assessment of a whole tissue-engineered intervertebral disc with PBST fibers and a chitosan hydrogel in vitro and in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019. [Google Scholar] [CrossRef]
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Gan, Y.B.; Li, P.; Wang, L.Y.; Mo, X.M.; Song, L.; Xu, Y.; Zhao, C.; Ouyang, B.; Tu, B.; Luo, L.; et al. An interpenetrating network-strengthened and toughened hydrogel that supports cell-based nucleus pulposus regeneration. Biomaterials 2017, 136, 12–28. [Google Scholar] [CrossRef]
- Chen, Y.C.; Su, W.Y.; Yang, S.H.; Gefen, A.; Lin, F.H. In situ forming hydrogels composed of oxidized high molecular weight hyaluronic acid and gelatin for nucleus pulposus regeneration. Acta. Biomater. 2013, 9, 5181–5193. [Google Scholar] [CrossRef]
- Gilchrist, C.L.; Chen, J.; Richardson, W.J.; Loeser, R.F.; Setton, L.A. Functional integrin subunits regulating cell-matrix interactions in the intervertebral disc. J. Orthop. Res. 2007, 25, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jing, L.F.; Gilchrist, C.L.; Richardson, W.J.; Fitch, R.D.; Setton, L.A. Expression of Laminin Isoforms, Receptors, and Binding Proteins Unique to Nucleus Pulposus Cells of Immature Intervertebral Disc. Connect. Tissue Res. 2009, 50, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Nettles, D.L.; Richardson, W.J.; Setton, L.A. Integrin expression in cells of the intervertebral disc. J. Anat. 2004, 204, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.T.; Mancino, R.J.; Bowles, R.D.; Brunger, J.M.; Tainter, D.M.; Chen, Y.T.; Richardson, W.J.; Guilak, F.; Setton, L.A. Injectable laminin-functionalized hydrogel for nucleus pulposus regeneration. Biomaterials 2013, 34, 7381–7388. [Google Scholar] [CrossRef] [PubMed]
- Bridgen, D.T.; Fearing, B.V.; Jing, L.F.; Sanchez-Adams, J.; Cohan, M.C.; Guilak, F.; Chen, J.; Setton, L.A. Regulation of human nucleus pulposus cells by peptide-coupled substrates. Acta. Biomater. 2017, 55, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.T.; Hwang, P.Y.; Jeong, C.G.; Jing, L.F.; Chen, J.; Setton, L.A. Photocrosslinkable laminin-functionalized polyethylene glycol hydrogel for intervertebral disc regeneration. Acta. Biomater. 2014, 10, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Zheng, Q.X.; Wu, Y.C.; Liu, Y.D.; Guo, X.D.; Wu, W.G. Culture of nucleus pulposus cells from intervertebral disc on self-assembling KLD-12 peptide hydrogel scaffold. Mater. Sci. Eng. C 2010, 30, 975–980. [Google Scholar] [CrossRef]
- Wang, B.C.; Wu, Y.C.; Shao, Z.W.; Yang, S.H.; Che, B.; Sun, C.X.; Ma, Z.L.; Zhang, Y.N. Functionalized self-assembling peptide nanofiber hydrogel as a scaffold for rabbit nucleus pulposus cells. J. Biomed. Mater. Res. Part A 2012, 100A, 646–653. [Google Scholar] [CrossRef]
- Tao, H.; Zhang, Y.; Wang, C.F.; Zhang, C.; Wang, X.M.; Wang, D.L.; Bai, X.D.; Wen, T.Y.; Xin, H.K.; Wu, J.H.; et al. Biological evaluation of human degenerated nucleus pulposus cells in functionalized self-assembling peptide nanofiber hydrogel scaffold. Tissue Eng. Part A 2014, 20, 1621–1631. [Google Scholar] [CrossRef]
- Wan, S.; Borland, S.; Richardson, S.M.; Merry, C.L.R.; Saiani, A.; Gough, J.E. Self-assembling peptide hydrogel for intervertebral disc tissue engineering. Acta Biomater. 2016, 46, 29–40. [Google Scholar] [CrossRef]
- Feng, G.J.; Zhang, Z.P.; Dang, M.; Zhang, X.J.; Doleyres, Y.; Song, Y.M.; Chen, D.; Ma, P.X. Injectable nanofibrous spongy microspheres for NR4A1 plasmid DNA transfection to reverse fibrotic degeneration and support disc regeneration. Biomaterials 2017, 131, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, R.; Ito, Y.; Inui, M.; Onizuka, N.; Kayama, T.; Kataoka, K.; Suzuki, H.; Mori, M.; Inagawa, M.; Ichinose, S.; et al. Mohawk promotes the maintenance and regeneration of the outer annulus fibrosus of intervertebral discs. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, B.; Yang, Q.; Li, X.; Ma, X.; Xia, Q.; Zhang, Y.; Zhang, C.; Wu, Y.; Zhang, Y. Comparison of decellularization protocols for preparing a decellularized porcine annulus fibrosus scaffold. PLoS ONE 2014, 9, e86723. [Google Scholar] [CrossRef] [PubMed]
- Bowles, R.D.; Setton, L.A. Biomaterials for intervertebral disc regeneration and repair. Biomaterials 2017, 129, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, N.L.; Sen, S.; Baker, B.M.; Elliott, D.M.; Mauck, R.L. Dynamic culture enhances stem cell infiltration and modulates extracellular matrix production on aligned electrospun nanofibrous scaffolds. Acta Biomater. 2011, 7, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Vadala, G.; Mozetic, P.; Rainer, A.; Centola, M.; Loppini, M.; Trombetta, M.; Denaro, V. Bioactive electrospun scaffold for annulus fibrosus repair and regeneration. Eur. Spine J. 2012, 21 (Suppl. 1), S20–S26. [Google Scholar] [CrossRef]
- Pirvu, T.; Blanquer, S.B.G.; Benneker, L.M.; Grijpma, D.W.; Richards, R.G.; Alini, M.; Eglin, D.; Grad, S.; Li, Z. A combined biomaterial and cellular approach for annulus fibrosus rupture repair. Biomaterials 2015, 42, 11–19. [Google Scholar] [CrossRef]
- Zhu, C.H.; Li, J.; Liu, C.; Zhou, P.H.; Yang, H.L.; Li, B. Modulation of the gene expression of annulus fibrosus-derived stem cells using poly(ether carbonate urethane)urea scaffolds of tunable elasticity. Acta Biomater. 2016, 29, 228–238. [Google Scholar] [CrossRef]
- Fujita, N.; Imai, J.I.; Suzuki, T.; Yamada, M.; Ninomiya, K.; Miyamoto, K.; Iwasaki, R.; Morioka, H.; Matsumoto, M.; Chiba, K.; et al. Vascular endothelial growth factor-A is a survival factor for nucleus pulposus cells in the intervertebral disc. Biochem. Bioph. Res. Commun. 2008, 372, 367–372. [Google Scholar] [CrossRef]
- Salo, J.; Kaigle Holm, A.; Indahl, A.; Mackiewicz, Z.; Sukura, A.; Holm, S.; Jamsen, E.; Konttinen, Y.T. Expression of vascular endothelial growth factor receptors coincide with blood vessel in-growth and reactive bone remodelling in experimental intervertebral disc degeneration. Clin. Exp. Rheumatol. 2008, 26, 1018–1026. [Google Scholar]
- Tolonen, J.; Gronblad, M.; Virri, J.; Seitsalo, S.; Rytomaa, T.; Karaharju, E.O. Platelet-derived growth factor and vascular endothelial growth factor expression in disc herniation tissue: And immunohistochemical study. Eur. Spine J. 1997, 6, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Kinzelmann, C.; Benz, K.; Mollenhauer, J.; Wurst, H.; Schlosshauer, B. Suppression of adverse angiogenesis in an albumin-based hydrogel for articular cartilage and intervertebral disc regeneration. Eur. Cell. Mater. 2010, 20, 24–36; discussion 36–37. [Google Scholar] [CrossRef] [PubMed]
- Silva-Correia, J.; Miranda-Goncalves, V.; Salgado, A.J.; Sousa, N.; Oliveira, J.M.; Reis, R.M.; Reis, R.L. Angiogenic potential of gellan-gum-based hydrogels for application in nucleus pulposus regeneration: In vivo study. Tissue Eng. Part A 2012, 18, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Perugini, V.; Guildford, A.L.; Silva-Correia, J.; Oliveira, J.M.; Meikle, S.T.; Reis, R.L.; Santin, M. Anti-angiogenic potential of VEGF blocker dendron loaded on to gellan gum hydrogels for tissue engineering applications. J. Tissue Eng. Regen. Med. 2018, 12, e669–e678. [Google Scholar] [CrossRef] [PubMed]
- Sivan, S.S.; Roberts, S.; Urban, J.P.G.; Menage, J.; Bramhill, J.; Campbell, D.; Franklin, V.J.; Lydon, F.; Merkher, Y.; Maroudas, A.; et al. Injectable hydrogels with high fixed charge density and swelling. pressure for nucleus pulposus repair: Biomimetic glycosaminoglycan analogues. Acta. Biomater. 2014, 10, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Schmocker, A.; Khoushabi, A.; Frauchiger, D.A.; Gantenbein, B.; Schizas, C.; Moser, C.; Bourban, P.E.; Pioletti, D.P. A photopolymerized composite hydrogel and surgical implanting tool for a nucleus pulposus replacement. Biomaterials 2016, 88, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.D. The PDN prosthetic disc-nucleus device. Eur. Spine J. 2002, 11 (Suppl. 2), S137–S142. [Google Scholar] [CrossRef]
- Allen, M.J.; Schoonmaker, J.E.; Bauer, T.W.; Williams, P.F.; Higham, P.A.; Yuan, H.A. Preclinical evaluation of a poly (vinyl alcohol) hydrogel implant as a replacement for the nucleus pulposus. Spine 2004, 29, 515–523. [Google Scholar] [CrossRef]
- Lewis, N.T.; Hussain, M.A.; Mao, J.J. Investigation of nano-mechanical properties of annulus fibrosus using atomic force microscopy. Micron 2008, 39, 1008–1019. [Google Scholar] [CrossRef]
- Holzapfel, G.A.; Schulze-Bauer, C.A.; Feigl, G.; Regitnig, P. Single lamellar mechanics of the human lumbar anulus fibrosus. Biomech. Model. Mechanobiol. 2005, 3, 125–140. [Google Scholar] [CrossRef]
- Guerin, H.L.; Elliott, D.M. Ouantifying the contributions of structure to annulus fibrosus mechanical function using a nonlinear, anisotropic, hyperelastic model. J. Orthop. Res. 2007, 25, 508–516. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, G.; Sen, S.; Cortes, D.; Elliott, D. Biaxial Mechanics are Inhomogeneous and Altered with Degeneration in the Human Annulus Fibrosus. In Proceedings of the Transactions of the 56rd Annual Meeting of the Orthopaedic Research Society, New Orleans, LA, USA, 6–9 March 2010. [Google Scholar]
- Johannessen, W.; Elliott, D.M. Effects of degeneration on the biphasic material properties of human nucleus pulposus in confined compression. Spine 2005, 30, E724–E729. [Google Scholar] [CrossRef] [PubMed]
- Iatridis, J.C.; Setton, L.A.; Weidenbaum, M.; Mow, V.C. The viscoelastic behavior of the non-degenerate human lumbar nucleus pulposus in shear. J. Biomech. 1997, 30, 1005–1013. [Google Scholar] [CrossRef]
- Nerurkar, N.L.; Elliott, D.M.; Mauck, R.L. Mechanical design criteria for intervertebral disc tissue engineering. J. Biomech. 2010, 43, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lang, G.; Chen, X.; Sacks, H.; Mantzur, C.; Tropp, U.; Mader, K.T.; Smallwood, T.C.; Sammon, C.; Richards, R.G.; et al. Polyurethane scaffold with in situ swelling capacity for nucleus pulposus replacement. Biomaterials 2016, 84, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Grunert, P.; Borde, B.H.; Towne, S.B.; Moriguchi, Y.; Hudson, K.D.; Bonassar, L.J.; Hartl, R. Riboflavin crosslinked high-density collagen gel for the repair of annular defects in intervertebral discs: An in vivo study. Acta. Biomater. 2015, 26, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Long, R.G.; Burki, A.; Zysset, P.; Eglin, D.; Grijpma, D.W.; Blanquer, S.B.G.; Hecht, A.C.; Iatridis, J.C. Mechanical restoration and failure analyses of a hydrogel and scaffold composite strategy for annulus fibrosus repair. Acta Biomater. 2016, 30, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Nesti, L.J.; Li, W.J.; Shanti, R.M.; Jiang, Y.J.; Jackson, W.; Freedman, B.A.; Kuklo, T.R.; Giuliani, J.R.; Tuan, R.S. Intervertebral disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold (HANFS) amalgam. Tissue Eng. Part A 2008, 14, 1527–1537. [Google Scholar] [CrossRef]
- Choy, A.T.; Chan, B.P. A Structurally and Functionally Biomimetic Biphasic Scaffold for Intervertebral Disc Tissue Engineering. PLoS ONE 2015, 10, e0131827. [Google Scholar] [CrossRef]
- Chan, L.K.Y.; Leung, V.Y.L.; Tam, V.; Lu, W.W.; Sze, K.Y.; Cheung, K.M.C. Decellularized bovine intervertebral disc as a natural scaffold for xenogenic cell studies. Acta Biomater. 2013, 9, 5262–5272. [Google Scholar] [CrossRef]
- Vergroesen, P.P.; Kingma, I.; Emanuel, K.S.; Hoogendoorn, R.J.; Welting, T.J.; van Royen, B.J.; van Dieen, J.H.; Smit, T.H. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthr. Cartil. 2015, 23, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Neidlinger-Wilke, C.; Mietsch, A.; Rinkler, C.; Wilke, H.J.; Ignatius, A.; Urban, J. Interactions of environmental conditions and mechanical loads have influence on matrix turnover by nucleus pulposus cells. J. Orthop. Res. 2012, 30, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Carter, D.R.; Schurman, D.J. Pressure and shear differentially alter human articular chondrocyte metabolism: A review. Clin. Orthop. Relat. Res. 2004, 427, S89–S95. [Google Scholar]
- Hwang, D.; Gabai, A.S.; Yu, M.; Yew, A.G.; Hsieh, A.H. Role of load history in intervertebral disc mechanics and intradiscal pressure generation. Biomech. Model. Mechanobiol. 2012, 11, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guan, H.F.; Liu, H.Y.; Zhao, L.B.; Li, L.; Zhang, Y.; Tan, P.; Mi, B.G.; Li, F. Epoxyeicosanoids prevent intervertebral disc degeneration in vitro and in vivo. Oncotarget 2017, 8, 3781–3797. [Google Scholar] [CrossRef]
- O’Halloran, D.M.; Pandit, A.S. Tissue-engineering approach to regenerating the intervertebral disc. Tissue Eng. 2007, 13, 1927–1954. [Google Scholar] [CrossRef]
- Mehrkens, A.; Muller, A.M.; Valderrabano, V.; Scharen, S.; Vavken, P. Tissue engineering approaches to degenerative disc disease-a meta-analysis of controlled animal trials. Osteoarthr. Cartil. 2012, 20, 1316–1325. [Google Scholar] [CrossRef]
- Iatridis, J.C.; Nicoll, S.B.; Michalek, A.J.; Walter, B.A.; Gupta, M.S. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: What needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013, 13, 243–262. [Google Scholar] [CrossRef]
- Walker, M.H.; Anderson, D.G. Molecular basis of intervertebral disc degeneration. Spine J. 2004, 4, 158S–166S. [Google Scholar] [CrossRef]
- Alini, M.; Eisenstein, S.M.; Ito, K.; Little, C.; Kettler, A.A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H.J. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 2008, 17, 2–19. [Google Scholar] [CrossRef]

| Species | Age of Skeletal Maturity | Age at Loss of Notochordal Cells (According to Literature) |
|---|---|---|
| Dog (c) | 12 months | 12 months |
| Dog (n/c) | 12 months | 60 months |
| Rabbit | 10 months | 6 months |
| Pig | 12 months | Unknown |
| Cat | 24 months | Never |
| Ferret | n/d | Never |
| Sheep | 12 months | Unknown |
| Rat | 2 months | 12 months |
| Mouse | 4 months | n/d |
| Human | 20 years | 6–10 years |
| Tissue Engineering Strategies in NP Treatment | |||
|---|---|---|---|
| Materials | Test Species | Test Time | Results |
| PLGA | Dog | 8-week | PLGA with cells significantly maintained the height and the stability of disc [85]. |
| Fibrin | Pig | 12-week | Fibrin significantly inhibited the fibrosis and inflammation of NP and enhanced the synthesis of ECM [86]. |
| Collagen II (CII)/hyaluronate (HyA)/chondroitin-6-sulfate (6-CS) | Rabbit | 84-day | The CII/HyA-CS scaffolds have a highly porous structure, high water-binding capacity, and significantly improved mechanical stability. This scaffolds also showed satisfactory biocompatibility [87]. |
| PGA-hyaluronan | Rabbit/Sheep | 12 month/6 month | Enhanced repair tissue formation and MRI intensity [88,89] |
| Silk fibroin (SK) /polyurethane (PU) composite | Pig | NA | SK/PU is an injectable hydrogel with minimally invasive treatment, suitable physical-mechanical properties, and visible CT and T2-weight MRI [90]. |
| Modified hyaluronic acid gels | Pig | 6-week | Both HYAFF® 120 and HYADD 3® treatment supported an NP-like region forming and prevented IVD narrowing, fibrous tissue replacement, and bony end-plates disruption [91]. |
| Tissue engineering strategies in NP treatment in AF treatment | |||
| Electrospun PCL | Rat | 4-week | PCL can mimic the hierarchical organization of the native AF and achieve functional partly with native tissue [92]. |
| Photochemically crosslinked collagen in shape of needle | Rabbit | 1 month | Materials can sustain the physiologically relevant loadings, prevent leakage, and reduce osteophyte formation [93]. |
| Collagen-fibrin gel scaffolds | Rabbit cells in vitro | 4 months | Collagen-fibrin gel significantly delayed the fibrous tissue infiltration. GAG and hydroxyproline content increase over four months [94]. |
| Tissue engineering strategies in the whole IVD | |||
| AF-polyglycolic acid and polylactic acid NP-alginate | Mice | 12-week | The engineered disc maintained the gross morphology and the AF was rich type I collagen but NP contained type II collagen [95]. |
| AF-contracted collagen, NP-alginate | Rat | 6 months | Tissue-engineered IVD maintained disc space height, produced de novo extracellular matrix, and integrated into the spine, yielding an intact motion segment with dynamic mechanical properties similar to that of native IVD [96]. |
| AF- poly (butylene succinate-co-terephthalate) copolyester (PBST), NP-chitosan hydrogel | Rabbit | 4-week | The whole TE-IVD stimulated the natural structure of IVD and retained the height of IVD after four weeks of implant [97]. |
| Tissue Scale | Benchmark | Testing Methods | Mechanical Value |
|---|---|---|---|
| AF (Sub-lamella) | E | Nanoindentation | 0.6–1.2 MPa |
| AF (Single Lamella) | E (f = 0°) | Uniaxial tension | 80–120 MPa |
| E (f = 90°) | 0.22 MPa | ||
| AF (Multiple Lamellae) | Eθ (toe/linear) | Uniaxial tension | 2.5/18–45 MPa |
| Axial fixed E (toe/linear) | Biaxial tension | 9.8/27.2 MPa | |
| NP | P swell | Confined compression | 0.138 MPa |
| ǀG *ǀ | Torsional shear | 7.4–19.8 kPa |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Liu, W.; Xia, T.; Yang, L. Disordered Mechanical Stress and Tissue Engineering Therapies in Intervertebral Disc Degeneration. Polymers 2019, 11, 1151. https://doi.org/10.3390/polym11071151
Zhao R, Liu W, Xia T, Yang L. Disordered Mechanical Stress and Tissue Engineering Therapies in Intervertebral Disc Degeneration. Polymers. 2019; 11(7):1151. https://doi.org/10.3390/polym11071151
Chicago/Turabian StyleZhao, Runze, Wanqian Liu, Tingting Xia, and Li Yang. 2019. "Disordered Mechanical Stress and Tissue Engineering Therapies in Intervertebral Disc Degeneration" Polymers 11, no. 7: 1151. https://doi.org/10.3390/polym11071151
APA StyleZhao, R., Liu, W., Xia, T., & Yang, L. (2019). Disordered Mechanical Stress and Tissue Engineering Therapies in Intervertebral Disc Degeneration. Polymers, 11(7), 1151. https://doi.org/10.3390/polym11071151





