Preparation, Stabilization and Carbonization of a Novel Polyacrylonitrile-Based Carbon Fiber Precursor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
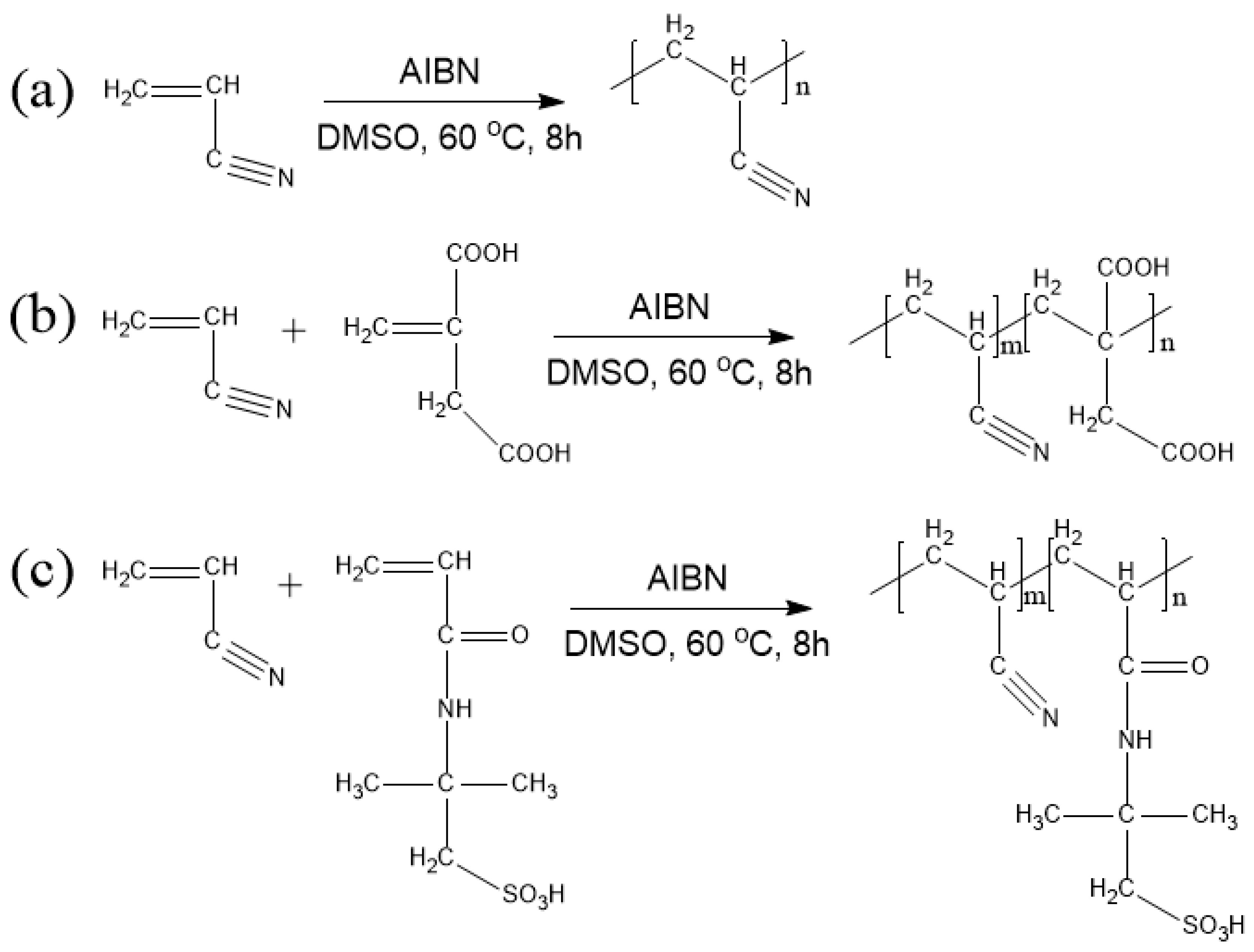
2.2. Preparation of PAN Homopolymer and PAN Copolymers
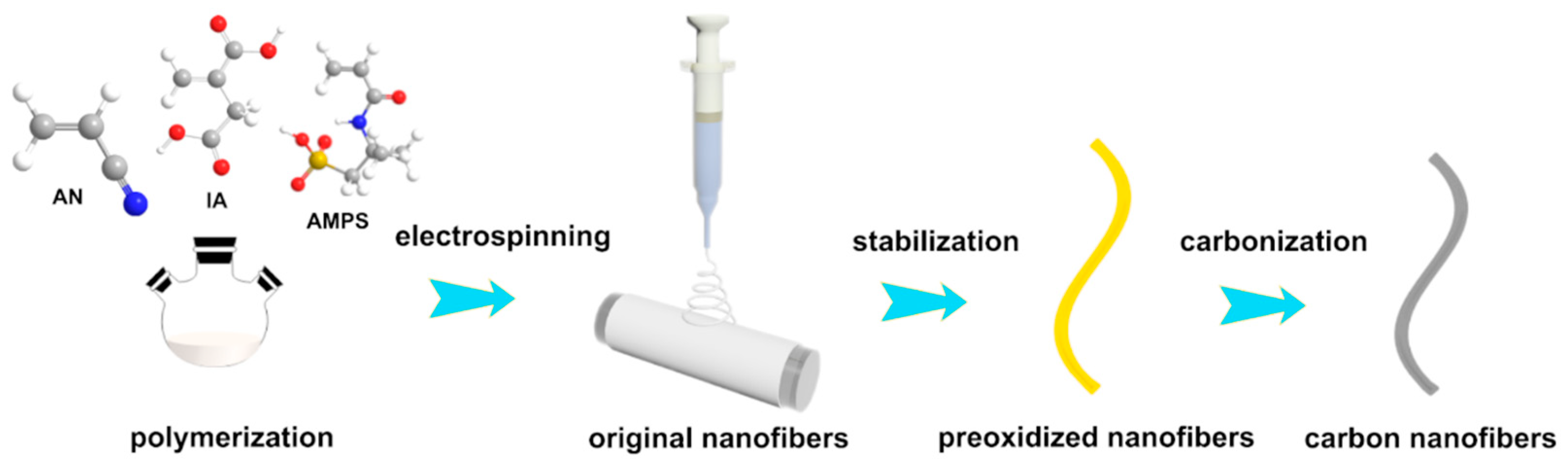
2.3. Preparation of PAN Homopolymer and PAN Copolymers Nanofibers
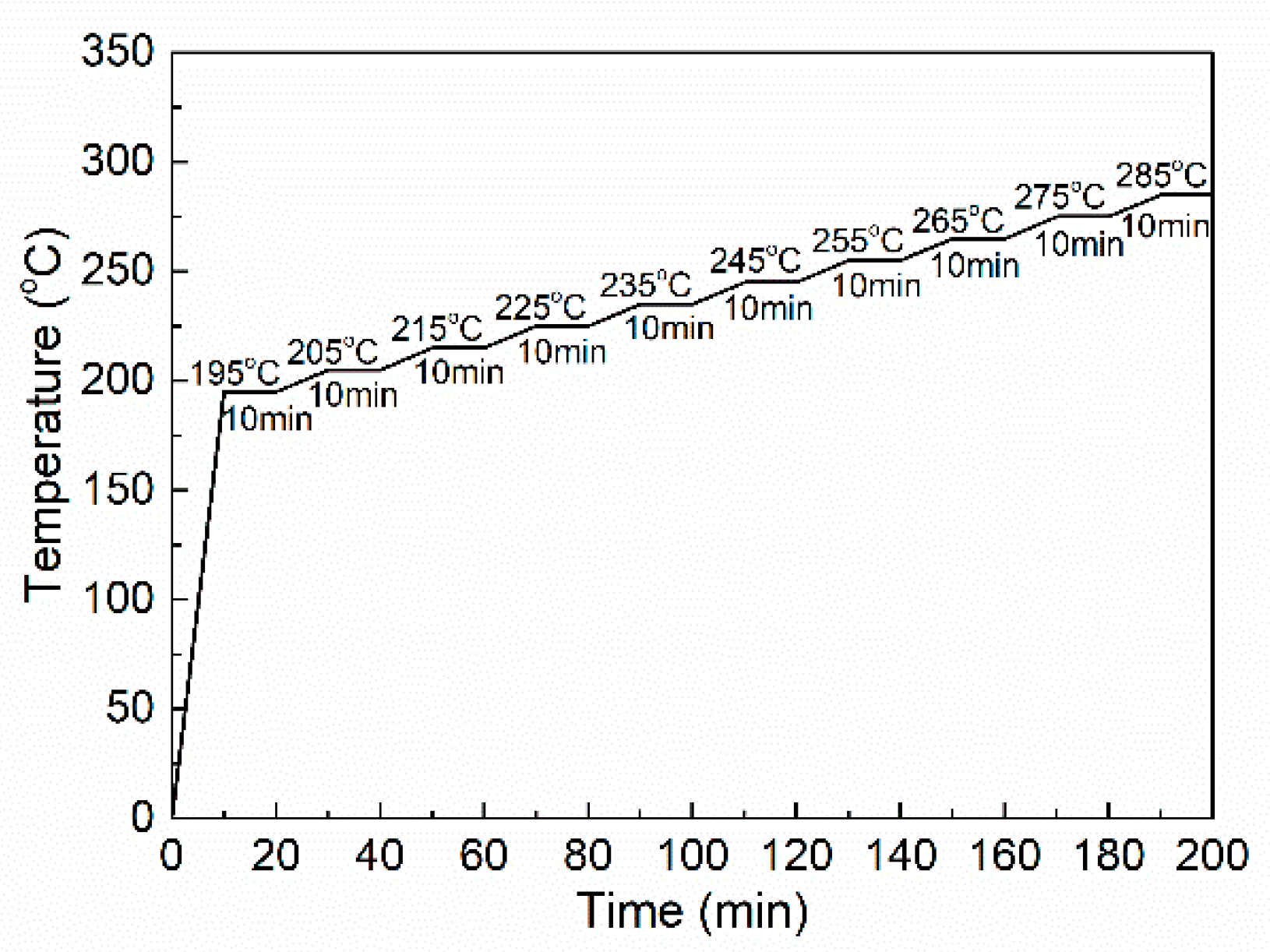
2.4. Thermal Oxidative Stabilization and Carbonization
2.5. Characterization
3. Results and Discussion
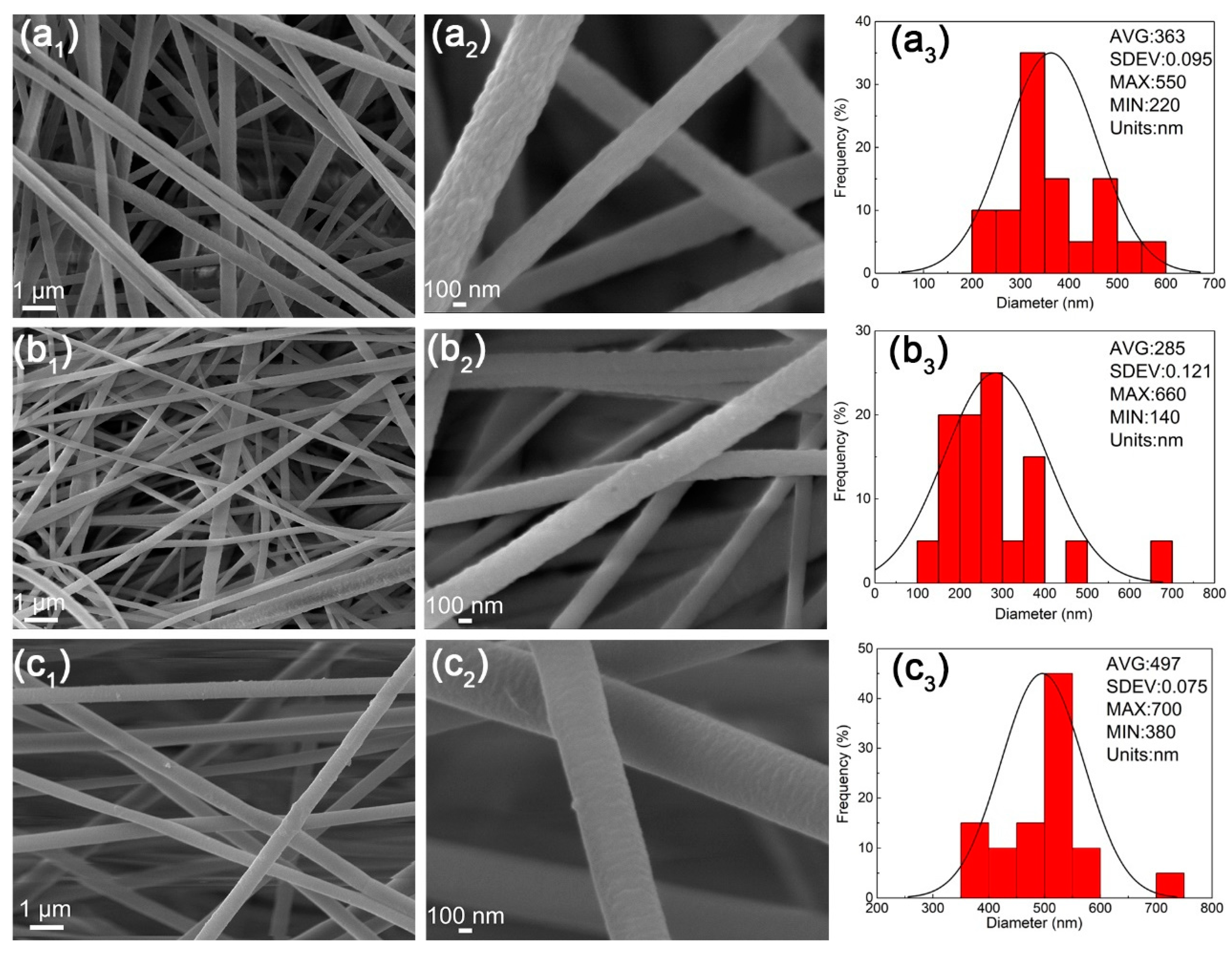
3.1. The Preparation of PAN-Based Nanofibers
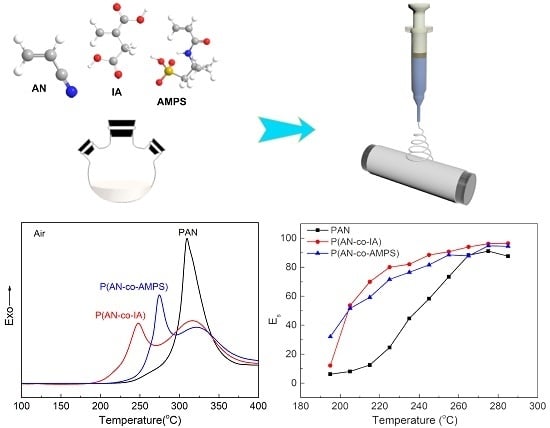
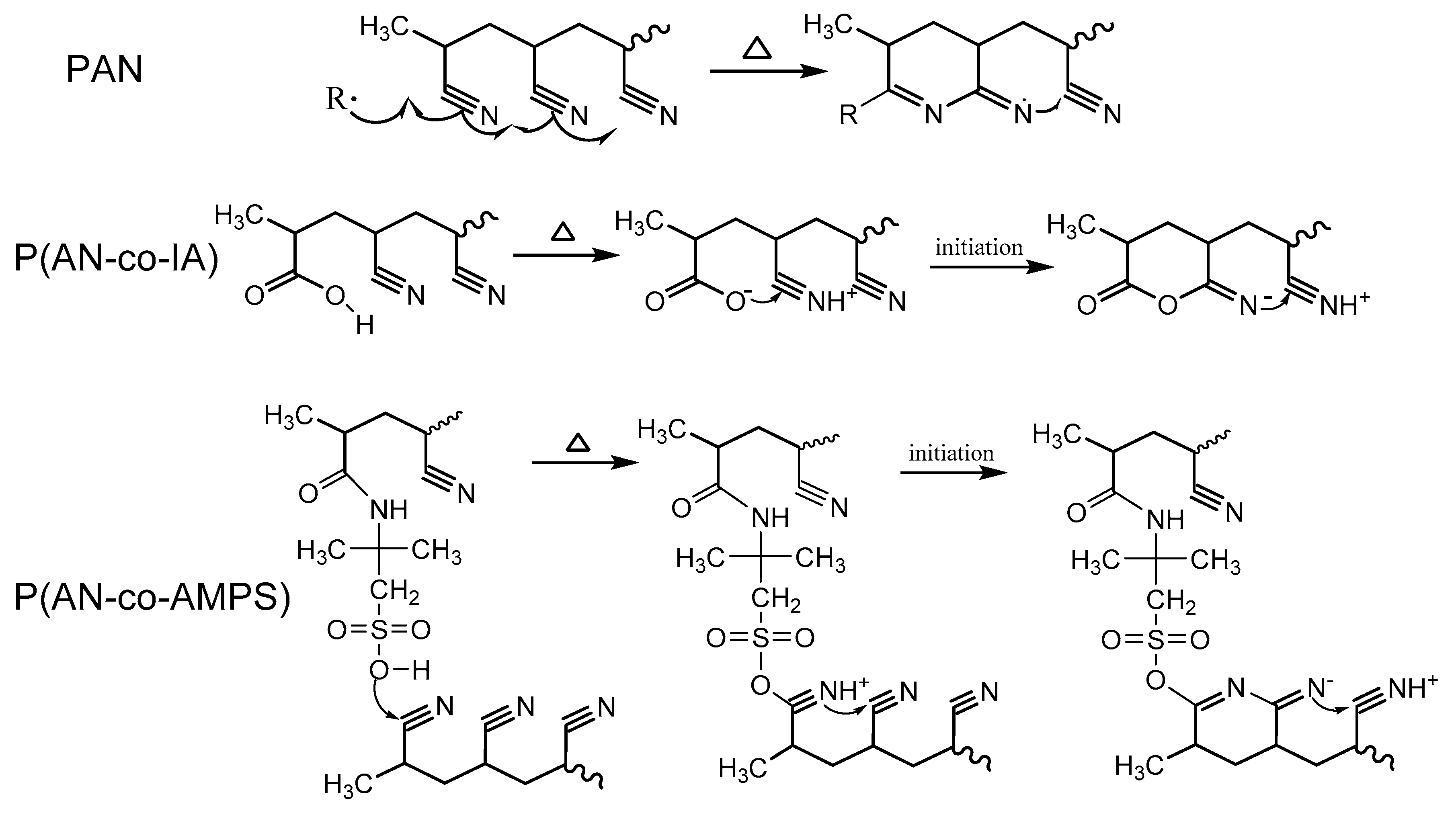
3.2. Thermal Stabilization Studies by DSC
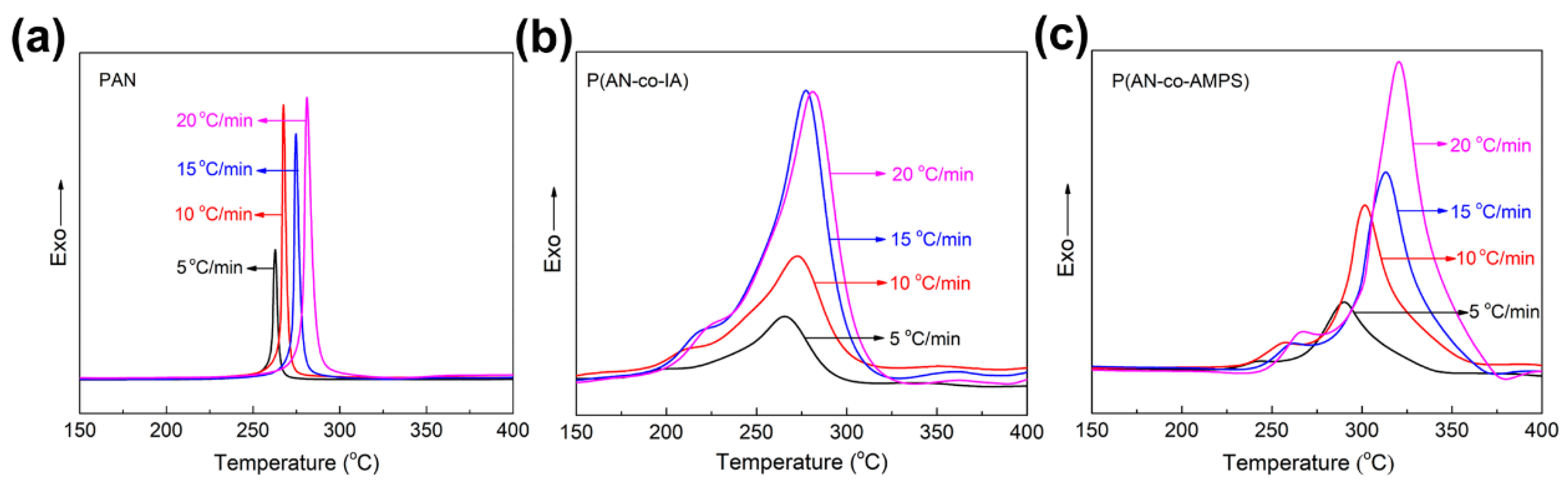
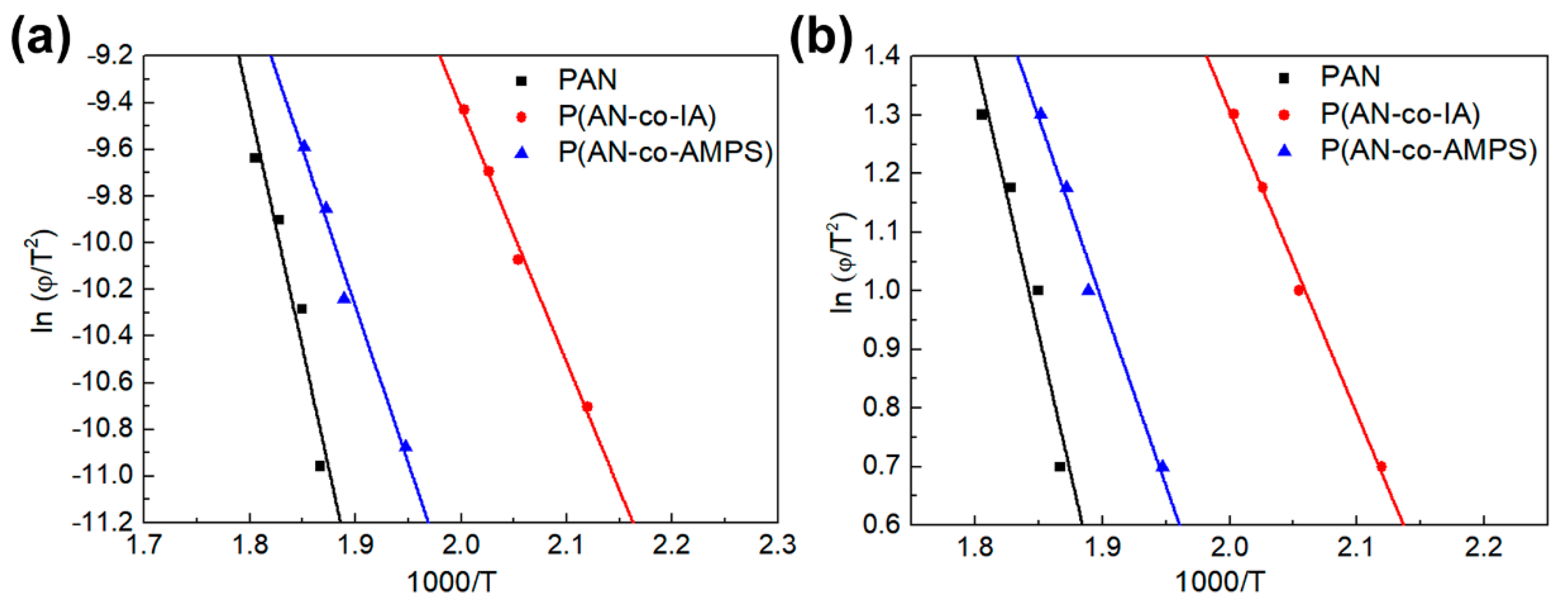
3.3. Evaluation of Activation Energy of Cyclization for PAN and PAN Copolymers Nanofibers
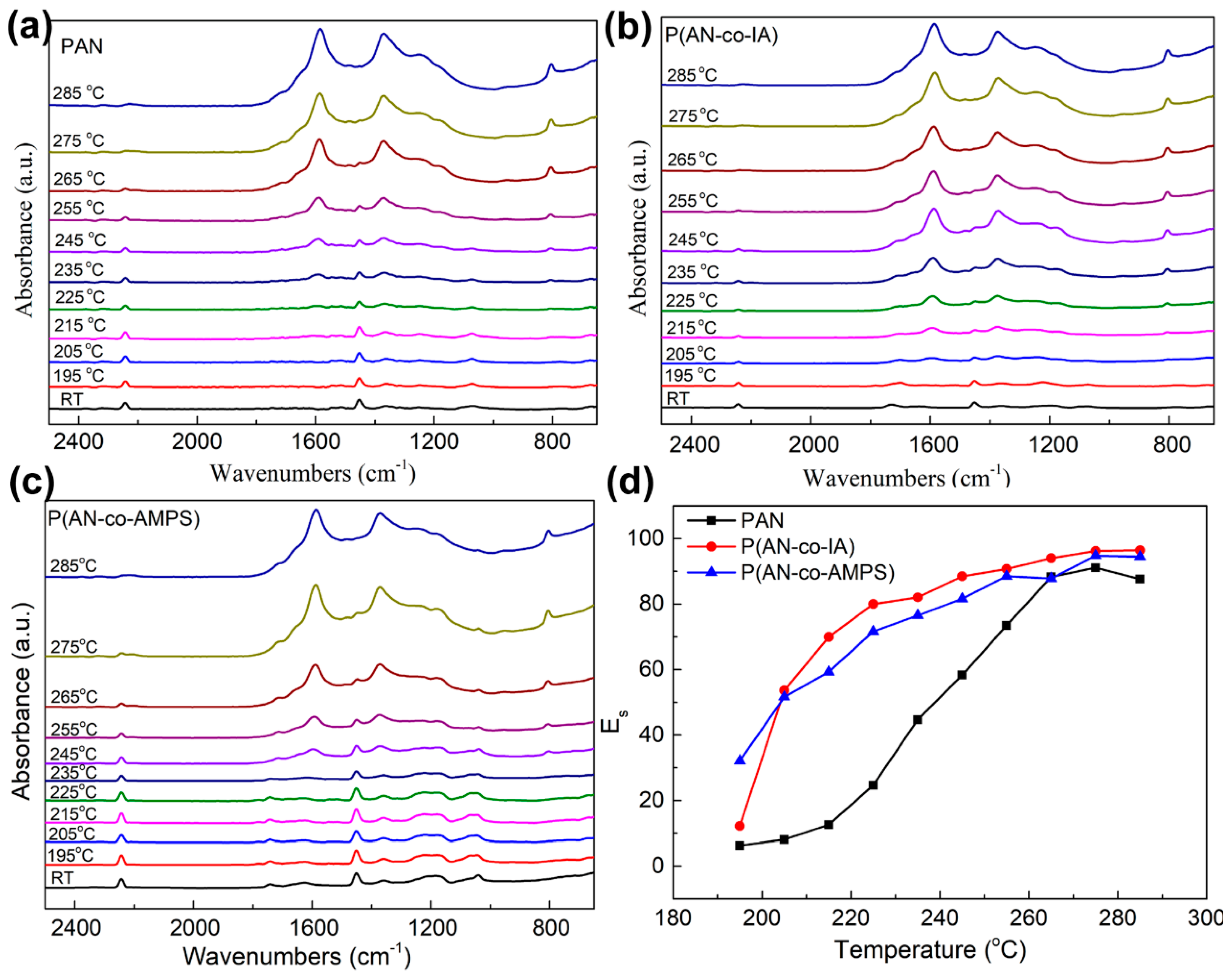
3.4. Structural Evolution during TOS Process by FTIR
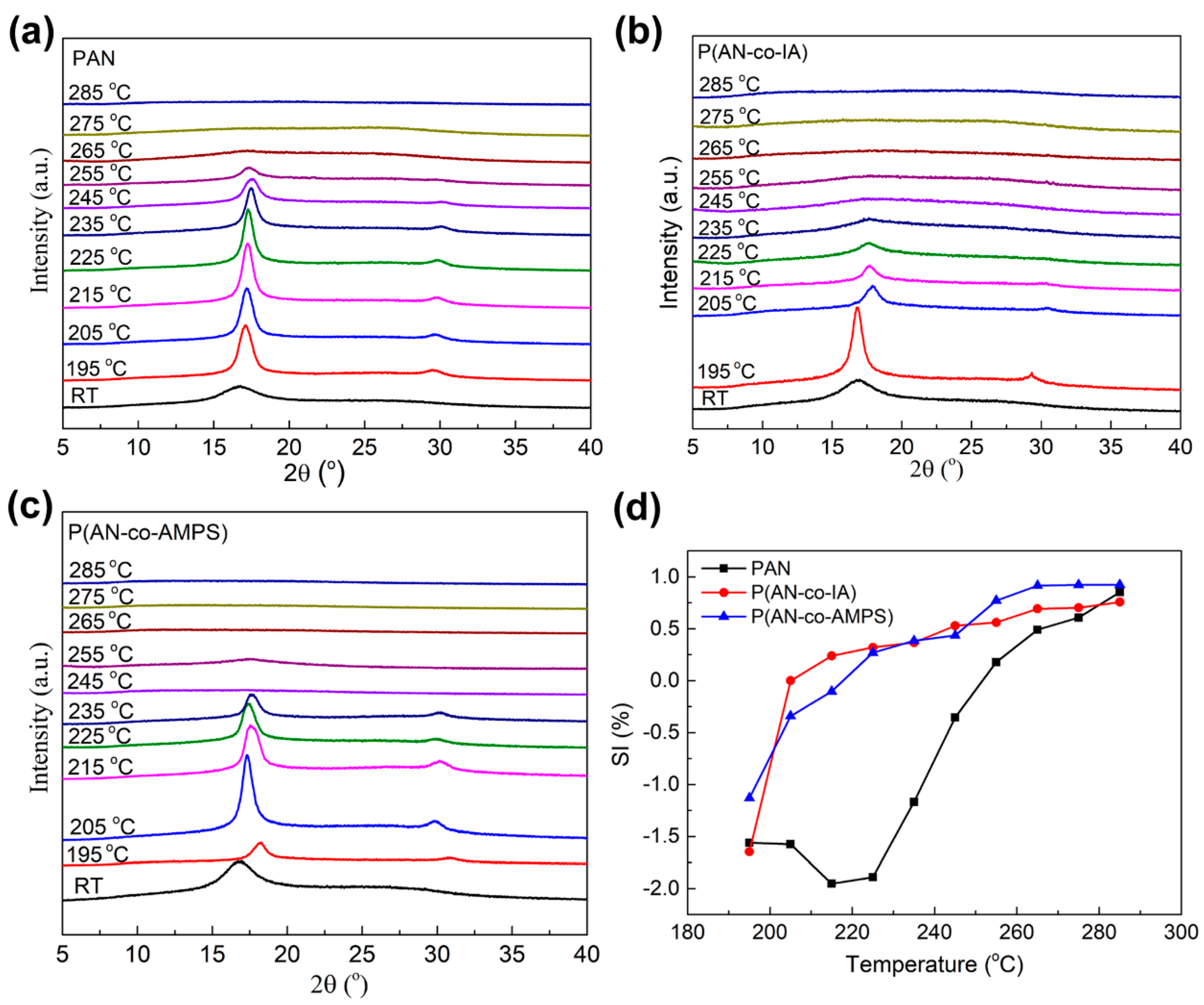
3.5. Structural Evolution during TOS Process by XRD
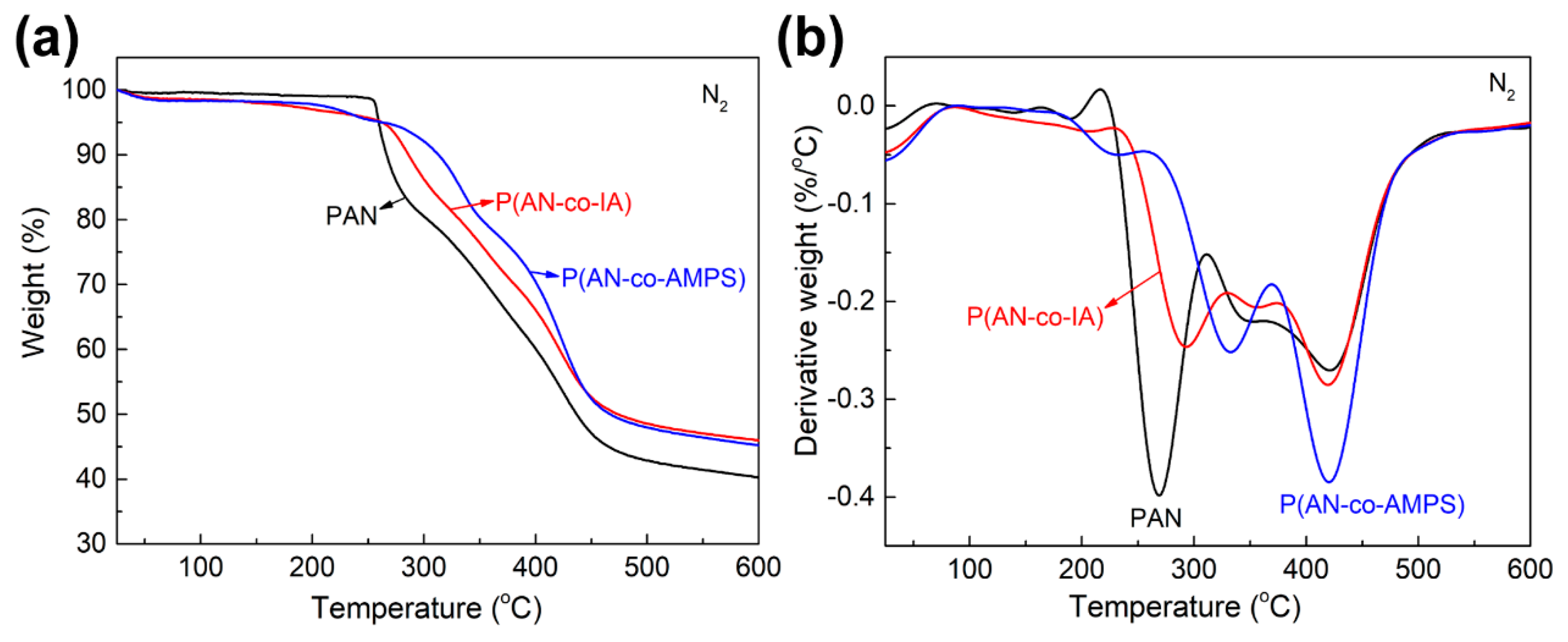
3.6. Thermal Behaviors Studies by TGA
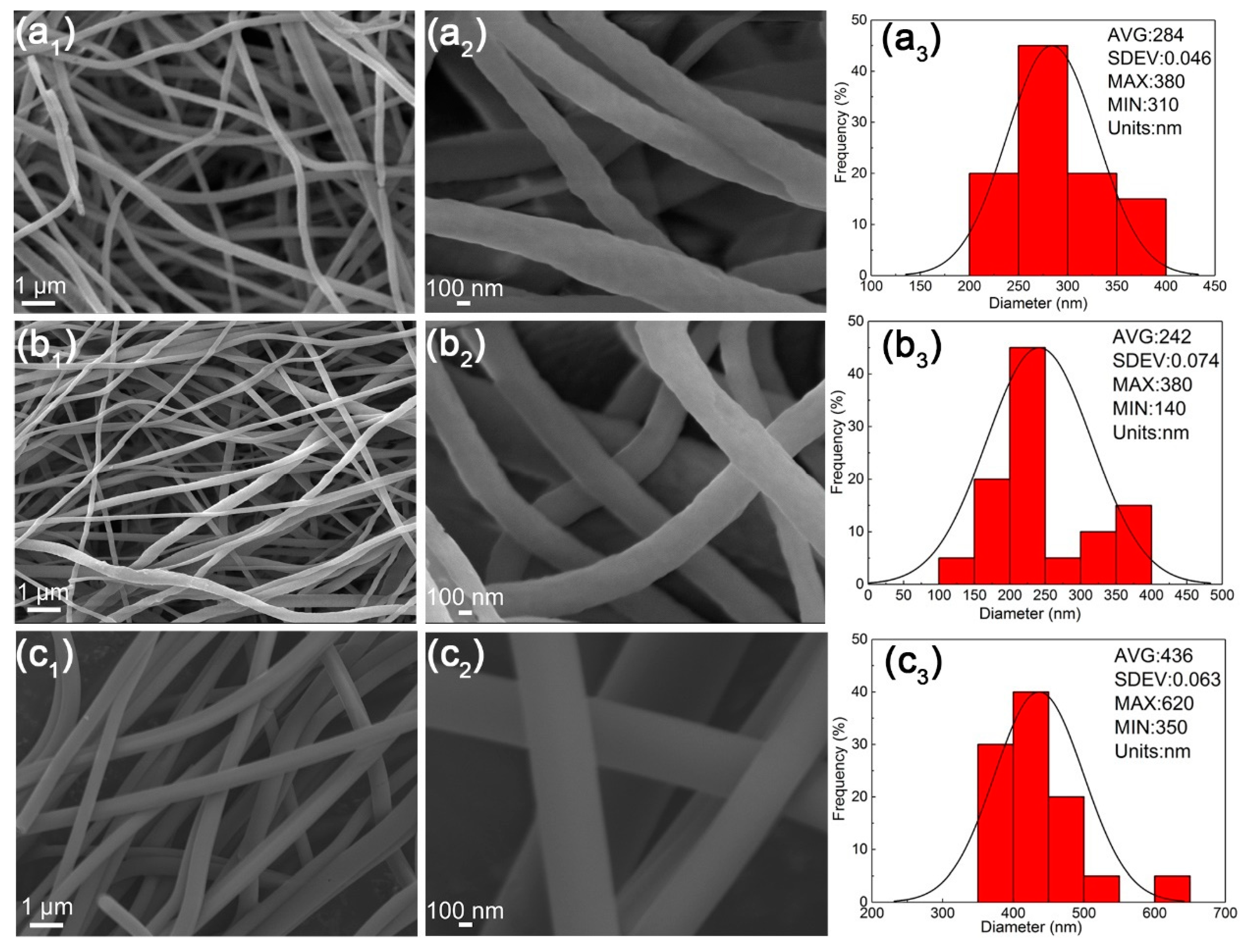
3.7. The Morphologies of Nanofibers after TOS Process
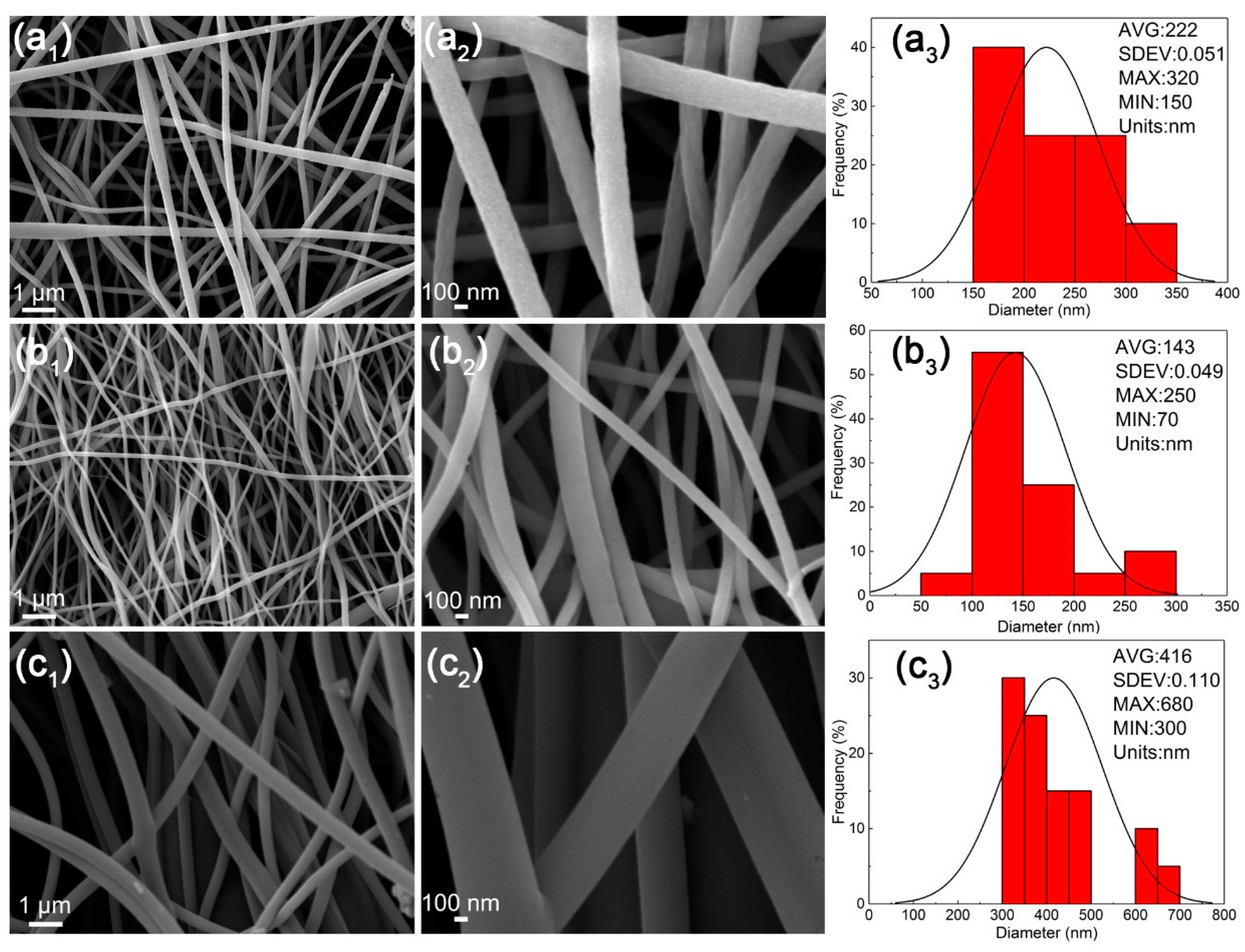
3.8. The Morphologies of Nanofibers after Carbonization
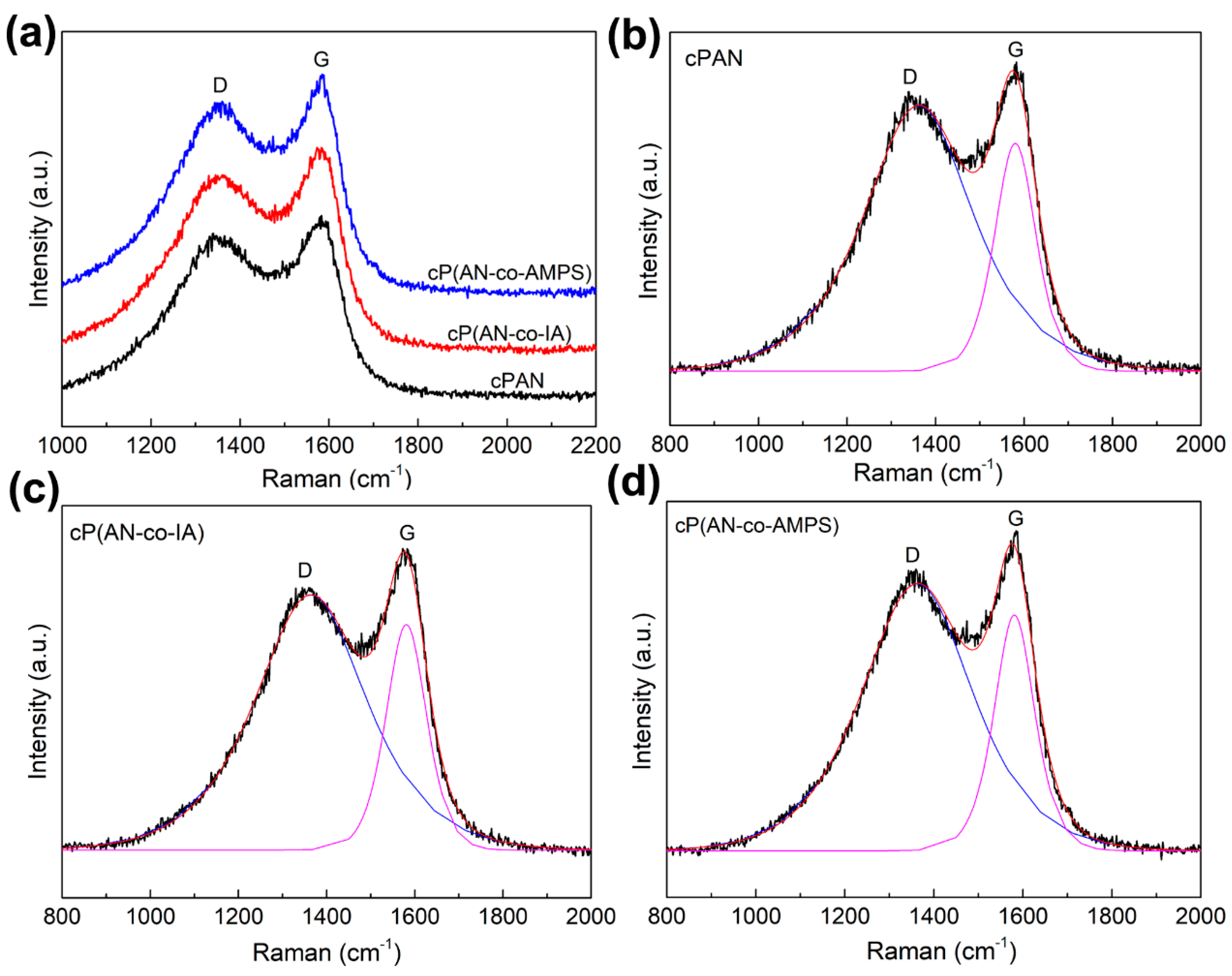
3.9. The Carbon Structure of Nanofibers after Carbonization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Sporl, J.M.; Buchmeiser, M.R. Carbon fibers: Precursor systems, processing, structure, and properties. Angew. Chem. Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.G.; Newcomb, B.A.; Gulgunje, P.V.; Liu, Y.D.; Gupta, K.K.; Kamath, M.G.; Lyons, K.M.; Ghoshal, S.; Pramanik, C.; Giannuzzi, L.; et al. High strength and high modulus carbon fibers. Carbon 2015, 93, 81–87. [Google Scholar] [CrossRef]
- Chukov, D.; Nematulloev, S.; Zadorozhnyy, M.; Tcherdyntsev, V.; Stepashkin, A.; Zherebtsov, D. Structure, Mechanical and Thermal Properties of Polyphenylene Sulfide and Polysulfone Impregnated Carbon Fiber Composites. Polymers 2019, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Kil, H.S.; Lee, S. Fabrication of low-cost carbon fibers using economical precursors and advanced processing technologies. Carbon 2019, 142, 610–649. [Google Scholar] [CrossRef]
- Newcomb, B.A. Processing, structure, and properties of carbon fibers. Compos. Part A Appl. Sci. Manuf. 2016, 91, 262–282. [Google Scholar] [CrossRef]
- Ashley Morris, E.; Weisenberger, M.C.; Abdallah, M.G.; Vautard, F.; Grappe, H.; Ozcan, S.; Paulauskas, F.L.; Eberle, C.; Jackson, D.; Mecham, S.J.; et al. High-performance carbon fibers from very high molecular weight polyacrylonitrile precursors. Carbon 2016, 101, 245–252. [Google Scholar] [CrossRef]
- Xia, K.; Ouyang, Q.; Chen, Y.; Wang, X.; Qian, X.; Wang, L. Preparation and characterization of lignosulfonate-acrylonitrile copolymer as a novel carbon fiber precursor. ACS Sustain. Chem. Eng. 2016, 4, 159–168. [Google Scholar] [CrossRef]
- Tajaddod, N.; Li, H.; Minus, M.L. Low-temperature graphitic formation promoted by confined interphase structures in polyacrylonitrile/carbon nanotube materials. Polymer 2018, 137, 346–357. [Google Scholar] [CrossRef]
- Liu, J.; Xia, S.; Shen, Z.; Xu, L.; Zhang, L.; Peng, J. Study on the oxidative stabilization of polyacrylonitrile fibers by microwave heating. Polym. Degrad. Stab. 2018, 150, 86–91. [Google Scholar] [CrossRef]
- Hao, J.; Liu, Y.; Lu, C. Effect of acrylonitrile sequence distribution on the thermal stabilization reactions and carbon yields of poly (acrylonitrile-co-methyl acrylate). Polym. Degrad. Stab. 2018, 147, 89–96. [Google Scholar] [CrossRef]
- Ngoc, U.N.T.; Hong, S.C. Structural evolution of poly (acrylonitrile-co-itaconic acid) during thermal oxidative stabilization for carbon materials. Macromolecules 2013, 46, 5882–5889. [Google Scholar]
- Liu, H.C.; Chien, A.T.; Newcomb, B.A.; Bakhtiary Davijani, A.A.; Kumar, S. Stabilization kinetics of gel spun polyacrylonitrile/lignin blend fiber. Carbon 2016, 101, 382–389. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Wang, M.; Pang, W.; Ge, X. Structural identification of polyacrylonitrile during thermal treatment by selective 13C labeling and solid-state 13C NMR spectroscopy. Macromolecules 2014, 47, 3901–3908. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, X.; Wang, X.; Huang, J.; Huang, X.; Chen, Y. Simultaneous DSC/TG analysis on the thermal behavior of PAN polymers prepared by aqueous free-radical polymerization. Polym. Degrad. Stab. 2016, 130, 320–327. [Google Scholar] [CrossRef]
- Bajaj, P.; Sen, K.; Hajir Bahrami, S. Solution polymerization of acrylonitrile with vinyl acids in dimethylformamide. J. Appl. Polym. Sci. 1996, 59, 1539–1550. [Google Scholar] [CrossRef]
- Bajaj, P.; Paliwal, D.K.; Gupta, A.K. Acrylonitrile-acrylic acids copolymers. I. Synthesis and characterization. J. Appl. Polym. Sci. 1993, 49, 823–833. [Google Scholar] [CrossRef]
- Lei, D.; Devarayan, K.; Li, X.D.; Choi, W.K.; Seo, M.K.; Kim, B.S. Effects of comonomer with the carboxylic group on stabilization of high molecular weight polyacrylonitrile nanofibrous copolymers. Carbon Lett. 2014, 15, 290–294. [Google Scholar] [CrossRef]
- Tsai, J.S.; Lin, C.H. The effect of the distribution of composition among chains on the properties of polyacrylonitrile precursor for carbon fiber. J. Mater. Sci. 1991, 26, 3996–4000. [Google Scholar] [CrossRef]
- Chen, H.; Pan, Y.; Hou, S.; Shao, Z.; Hong, Y.; Ju, A. Poly (acrylonitrile-co-2-methylenesuccinamic acid) as a potential carbon fiber precursor: Preparation and stabilization. RSC Adv. 2017, 7, 54142–54152. [Google Scholar] [CrossRef]
- Cho, D.W.; Hong, S.C. Synergistic effect of comonomers on the thermal oxidative stabilization of polyacrylonitrile copolymers for carbon materials. Polym. Degrad. Stab. 2019, 161, 191–197. [Google Scholar] [CrossRef]
- Devasia, R.; Nair, C.R.; Sivadasan, P.; Ninan, K.N. High char-yielding poly [acrylonitrile-co- (itaconic acid)-co- (methyl acrylate)]: Synthesis and properties. Polym. Int. 2005, 54, 1110–1118. [Google Scholar] [CrossRef]
- Liu, D.; Ouyang, Q.; Jiang, X.; Ma, H.; Chen, Y.; He, L. Thermal properties and thermal stabilization of lignosulfonate-acrylonitrile-itaconic acid terpolymer for preparation of carbon fiber. Polym. Degrad. Stab. 2018, 150, 57–66. [Google Scholar] [CrossRef]
- Liu, H.C.; Luo, Q.; Zhang, S.; Shi, L.; Yang, J.; Liu, R.; Wang, M.; Zhu, C.; Xu, J. New comonomer for polyacrylonitrile-based carbon fiber: Density functional theory study and experimental analysis. Polymer 2018, 153, 369–377. [Google Scholar] [CrossRef]
- Yadav, M.; Rhee, K.Y. Superabsorbent nanocomposite (alginate-g-PAMPS/MMT): Synthesis, characterization and swelling behavior. Carbohydr. Polym. 2012, 90, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Ju, A.; Guang, S.; Xu, H. Effect of comonomer structure on the stabilization and spinnability of polyacrylonitrile copolymers. Carbon 2013, 54, 323–335. [Google Scholar] [CrossRef]
- Fitzer, E.; Frohs, W.; Heine, M. Optimization of stabilization and carbonization treatment of PAN fibers and structural characterization of the resulting carbon fibers. Carbon 1986, 24, 387–395. [Google Scholar] [CrossRef]
- Ju, A.; Guang, S.; Xu, H. Molecular design and stabilization mechanism of acrylonitrile biopolymer. J. Appl. Polym. Sci. 2013, 129, 3255–3264. [Google Scholar] [CrossRef]
- Li, P.R.; Jia, W.D.; Liu, B.J. Study on the thermal behaviour of copolymers of acrylonitrile with halogenated carboxylic acids. Macromol. Mater. Eng. 1992, 199, 65–74. [Google Scholar]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ozawa, T. New method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Liu, B.J.; Deng, Y.J.; Ma, J.Y.; Cao, C.L.; Wang, J.; Ao, Y.; Zhang, H. The suitable itaconic acid content in polyacrylonitrile copolymers used for PAN-based carbon fibers. J. Appl. Polym. Sci. 2016, 133, 43919–43929. [Google Scholar] [CrossRef]
- Spyridon, S.; Johannis, S. Thermomechanical behaviour of poly [acryloni-trile-co-(methyl acrylate)] fibers oxidatively treated at temperatures up to 180 °C. Polym. Int. 2005, 54, 1474–1483. [Google Scholar]
- Yoo, S.H.; Park, S.; Park, Y.; Lee, D.; Joh, H.I.; Shin, I.; Lee, S. Facile method to fabricate carbon fibers from textile-grade polyacrylonitrile fibers based on electron-beam irradiation and its effect on the subsequent thermal stabilization process. Carbon 2017, 118, 106–113. [Google Scholar] [CrossRef]
- Gaur, S.S.; Dhar, P.; Sonowal, A.; Sharma, A.; Kumar, A.; Katiyar, V. Thermo-mechanically stable sustainable polymer based solid electrolyte membranes for direct methanol fuel cell applications. J. Membr. Sci. 2017, 526, 348–354. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile. Polym. Degrad. Stab. 2008, 93, 1415–1421. [Google Scholar] [CrossRef]
- Kakida, H.; Tashiro, K.; Kobayashi, M. Mechanism and kinetics of stabilization reaction of polyacrylonitrile and related copolymers I. Relationship between isothermal DSC thermogram and FT/IR spectral. Polym. J. 1996, 28, 30–34. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, B.; Sun, L.; Zhang, H. Study on the thermal oxidative stabilization reactions and the formed structures in polyacrylonitrile during thermal treatment. Polym. Degrad. Stab. 2017, 140, 104–113. [Google Scholar] [CrossRef]
- Ghorpade, R.V.; Cho, D.W.; Hong, S.C. Effect of controlled tacticity of polyacrylonitrile (co)polymers on their thermal oxidative stabilization behaviors and the properties of resulting carbon films. Carbon 2017, 121, 502–511. [Google Scholar] [CrossRef]
- Shen, T.; Li, C.; Haley, B.; Desai, S.; Strachan, A. Crystalline and pseudo-crystalline phases of polyacrylonitrile from molecular dynamics: Implications for carbon fiber precursors. Polymer 2018, 155, 13–26. [Google Scholar] [CrossRef]
- Yu, M.J.; Bai, Y.J.; Wang, C.G.; Xu, Y.; Guo, P.Z. A new method for the evaluation of stabilization index of polyacrylonitrile fibers. Mater. Lett. 2007, 61, 2292–2294. [Google Scholar] [CrossRef]
- Bell, J.P.; Dumbleton, J.H. Influence of spinning dope additives and spin bath temperature on the structure and physical properties of acrylic fibers. Text. Res. J. 1971, 41, 196–203. [Google Scholar] [CrossRef]
- Bajaj, P.; Sreekumar, T.V.; Sen, K. Thermal behavior of acrylonitrile copolymers having methacrylic and itaconic acid comonomers. Polymer 2001, 42, 1707–1718. [Google Scholar] [CrossRef]
- Arbab, S.; Zeinolebadi, A. Quantitative analysis of the effects of comonomers and heating conditions on the stabilization reactions of polyacrylonitrile fibers as carbon fiber precursors. Polym. Degrad. Stab. 2017, 139, 107–116. [Google Scholar] [CrossRef]
- Zeng, Z.P.; Shao, Z.C.; Xiao, R.; Lu, Y.G. Structure evolution mechanism of poly (acrylonitrile/itaconic acid/acrylamide) during thermal oxidative stabilization process. Chin. J. Polym. Sci. 2017, 35, 1020–1034. [Google Scholar] [CrossRef]
- Wu, S.; Gao, A.; Wang, Y.; Xu, L. Modification of polyacrylonitrile stabilized fibers via post-thermal treatment in nitrogen prior to carbonization and its effect on the structure of carbon fibers. J. Mater. Sci. 2018, 53, 8627–8638. [Google Scholar] [CrossRef]
- Bahrami, S.H.; Bajaj, P.; Sen, K. Thermal behavior of acrylonitrile carboxylic acid copolymers. J. Appl. Polym. Sci. 2003, 88, 685–698. [Google Scholar] [CrossRef]
- Hao, J.; Li, W.; Suo, X.; Wei, H.; Lu, C.; Liu, Y. Highly isotactic (>60%) polyacrylonitrile-based carbon fiber: Precursor synthesis, fiber spinning, stabilization and carbonization. Polymer 2018, 157, 139–150. [Google Scholar] [CrossRef]
- Qian, X.; Zhi, J.; Chen, L.; Zhong, J.; Wang, X.; Zhang, Y.; Song, S. Evolution of microstructure and electrical property in the conversion of high strength carbon fiber to high modulus and ultrahigh modulus carbon fiber. Compos. Part A 2018, 112, 111–118. [Google Scholar] [CrossRef]
- Wang, Y.; Serrano, S.; Santiago-Avilés, J.J. Raman characterization of carbon nanofibers prepared using electrospinning. Synth. Met. 2003, 138, 423–427. [Google Scholar] [CrossRef]
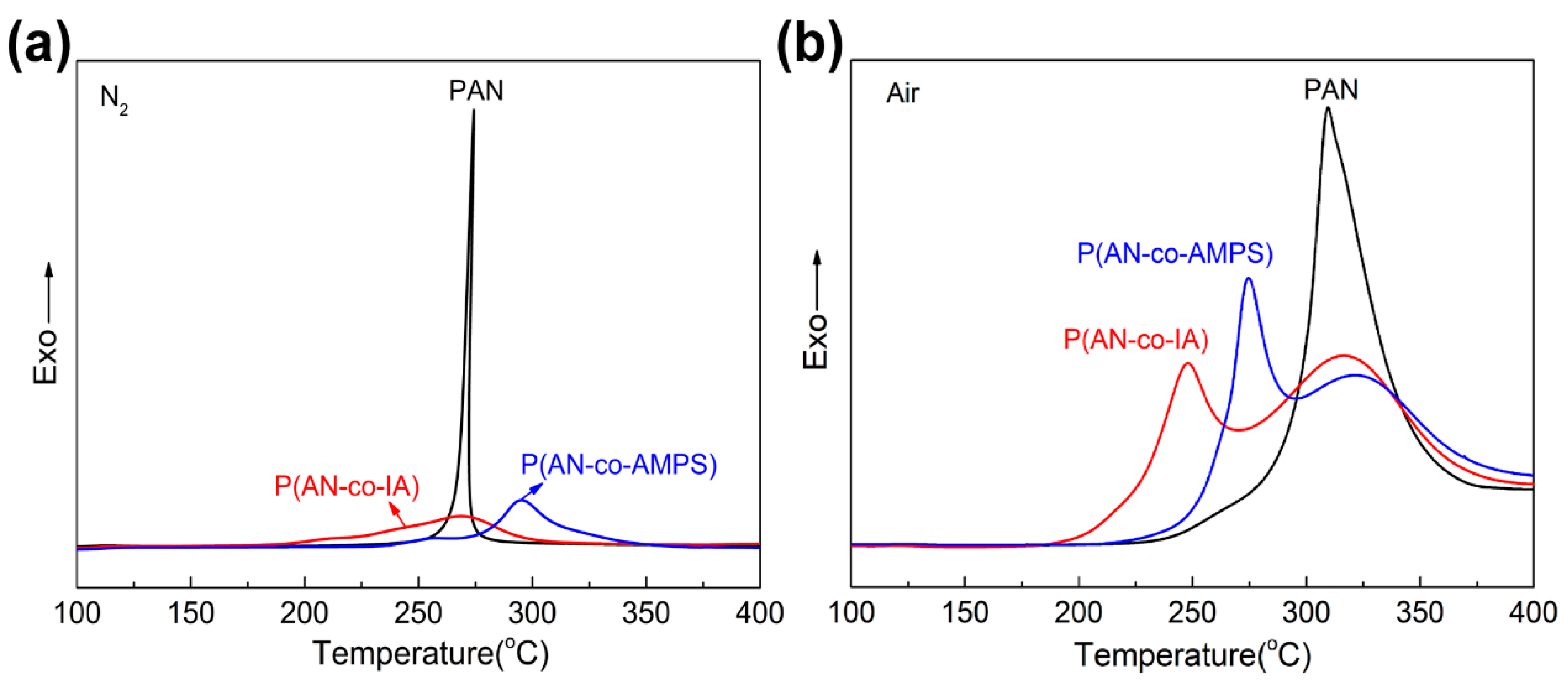
| Polymer Codes | Ti (°C) | Tp1 (°C) | Tp2 (°C) | Tf (°C) | ΔT (°C) | ΔH (J/g) | ΔH/ΔT [J/(g °C)] | |
|---|---|---|---|---|---|---|---|---|
| PAN | N2 | 268.4 | 274.3 | 279.5 | 11.1 | 534.1 | 48.1 | |
| P(AN-co-IA) | 217.4 | 212.1 | 268.8 | 300.6 | 83.2 | 610.0 | 7.3 | |
| P(AN-co-AMPS) | 240.5 | 257.6 | 295.2 | 318.9 | 78.4 | 641.0 | 8.2 | |
| PAN | Air | 297.1 | 309.3 | 345.2 | 48.1 | 4988.8 | 103.7 | |
| P(AN-co-IA) | 222.6 | 247.6 | 316.6 | 400.9 | 178.3 | 5110.7 | 28.7 | |
| P(AN-co-AMPS) | 252.9 | 274.5 | 321.4 | 401.0 | 148.1 | 3987.5 | 26.9 |
| Polymer Codes | Kissinger (kcal/mol) | Ozawa (kcal/mol) | ||
|---|---|---|---|---|
| Peak1 | Peak2 | Peak1 | Peak2 | |
| PAN | 41.2 | 40.4 | ||
| P(AN-co-IA) | 21.7 | 46.9 | 22.1 | 46.0 |
| P(AN-co-AMPS) | 26.6 | 27.5 | 26.8 | 27.8 |
| Codes | ID/IG | Laa (nm) | XGb (%) | D Peak FWHM (cm−1) | G Peak FWHM (cm−1) |
|---|---|---|---|---|---|
| cPAN | 1.166 | 3.77 | 46.168 | 296.5 | 115.2 |
| cP(AN-co-IA) | 1.132 | 3.89 | 46.905 | 283.4 | 111.6 |
| cP(AN-co-AMPS) | 1.133 | 3.88 | 46.889 | 290.6 | 109.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, S.; Yang, J.; Ji, M.; Yu, J.; Wang, M.; Chai, X.; Yang, B.; Zhu, C.; Xu, J. Preparation, Stabilization and Carbonization of a Novel Polyacrylonitrile-Based Carbon Fiber Precursor. Polymers 2019, 11, 1150. https://doi.org/10.3390/polym11071150
Liu H, Zhang S, Yang J, Ji M, Yu J, Wang M, Chai X, Yang B, Zhu C, Xu J. Preparation, Stabilization and Carbonization of a Novel Polyacrylonitrile-Based Carbon Fiber Precursor. Polymers. 2019; 11(7):1150. https://doi.org/10.3390/polym11071150
Chicago/Turabian StyleLiu, Huichao, Shuo Zhang, Jinglong Yang, Muwei Ji, Jiali Yu, Mingliang Wang, Xiaoyan Chai, Bo Yang, Caizhen Zhu, and Jian Xu. 2019. "Preparation, Stabilization and Carbonization of a Novel Polyacrylonitrile-Based Carbon Fiber Precursor" Polymers 11, no. 7: 1150. https://doi.org/10.3390/polym11071150
APA StyleLiu, H., Zhang, S., Yang, J., Ji, M., Yu, J., Wang, M., Chai, X., Yang, B., Zhu, C., & Xu, J. (2019). Preparation, Stabilization and Carbonization of a Novel Polyacrylonitrile-Based Carbon Fiber Precursor. Polymers, 11(7), 1150. https://doi.org/10.3390/polym11071150