Effect of Crosslinking Degree on Sulfonated Poly(aryl ether nitrile)s As Candidates for Proton Exchange Membranes
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. SPEN Synthesis
2.3. Characterization
2.4. Ion Exchange Capacity (IEC)
2.5. Methanol Permeability and Proton Conductivity
2.6. Water Uptake and Swelling Ratio
3. Results and Discussion
3.1. Characterization of SPEN and CSPEN
3.2. The Gel Content of Sulfonated Poly(aryl ether nitrile)
3.3. Thermal Properties
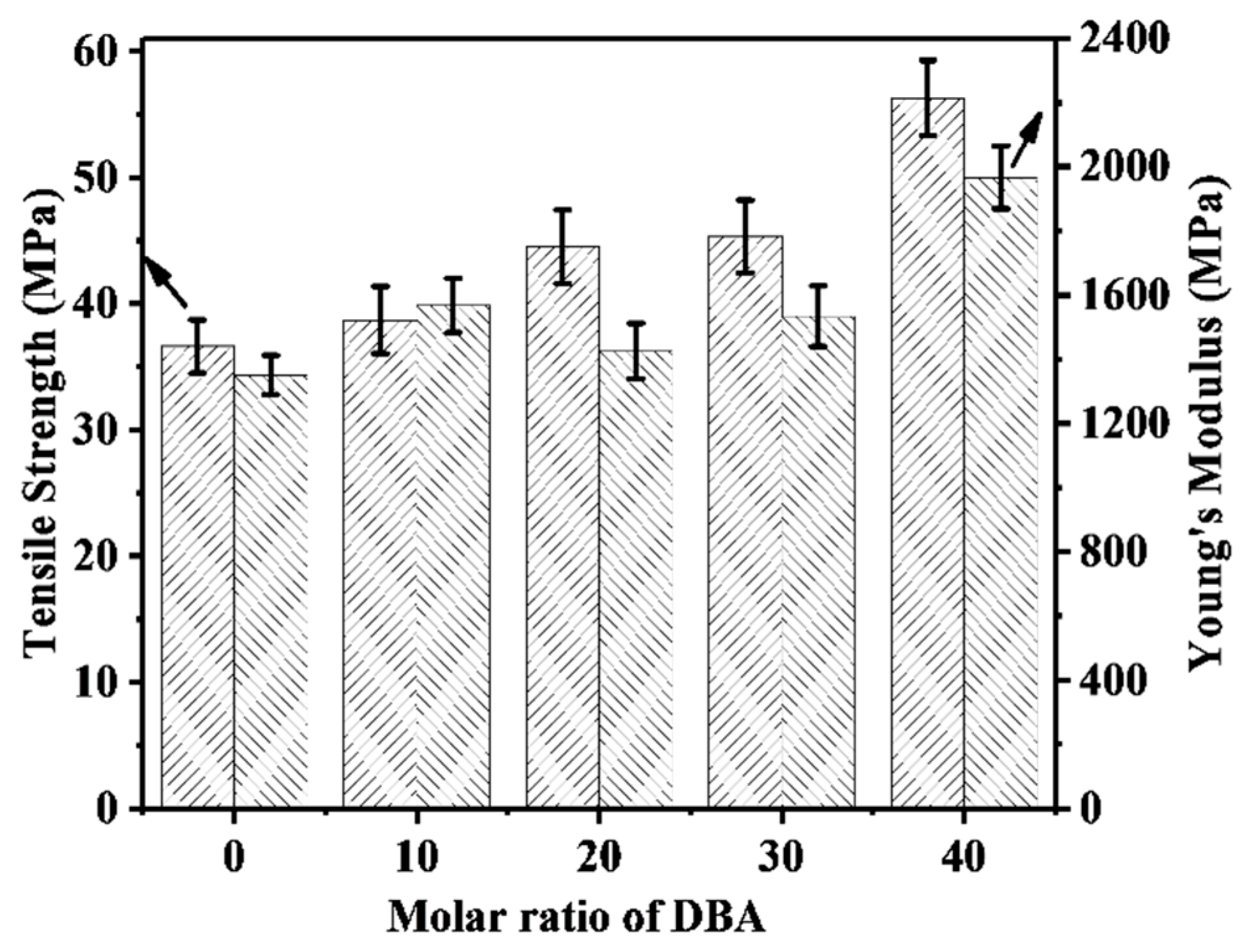
3.4. Mechanical Properties
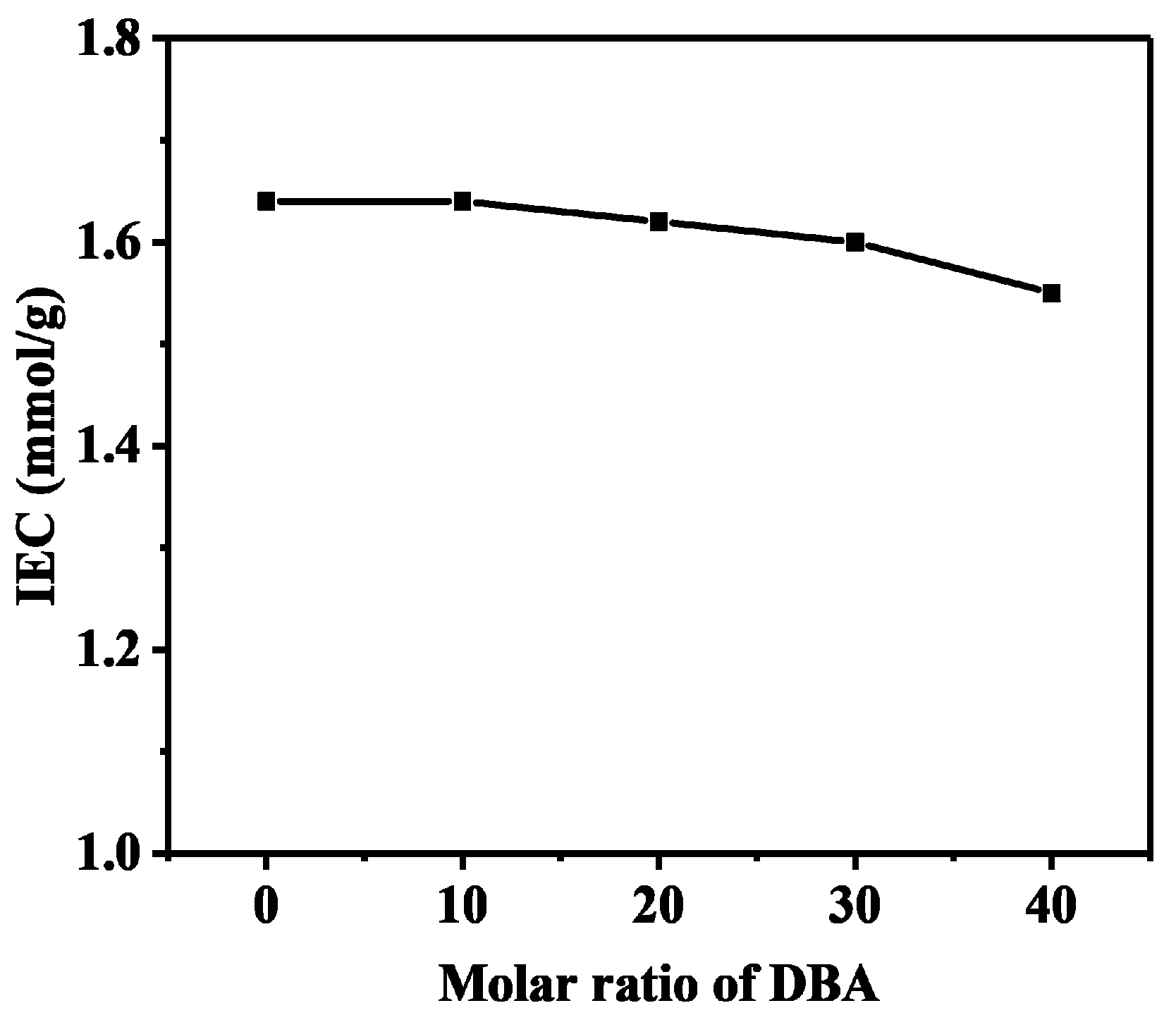
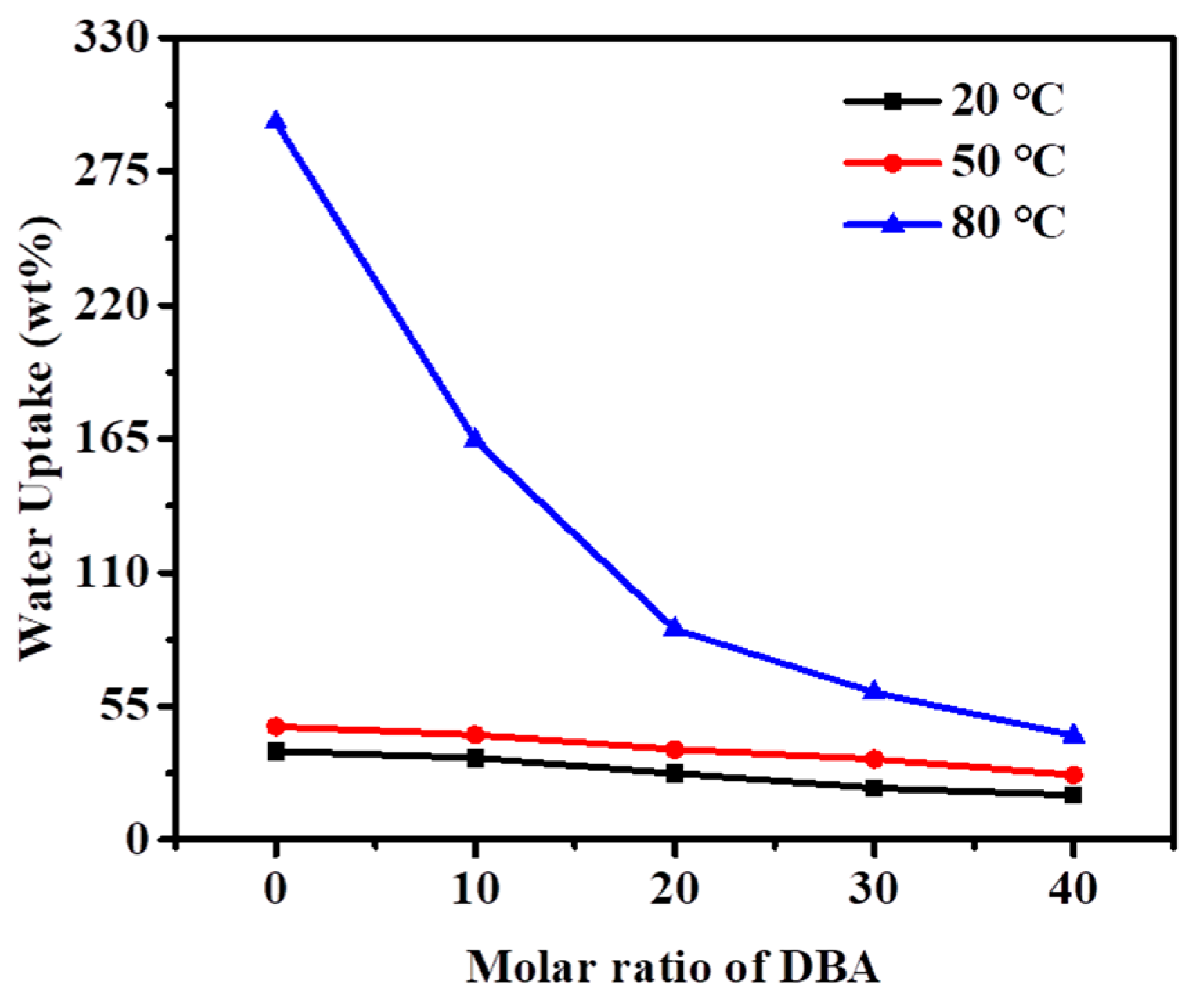
3.5. Ion Exchange Capacity, Water Uptake and Swelling Ratio
3.6. Proton Conductivity
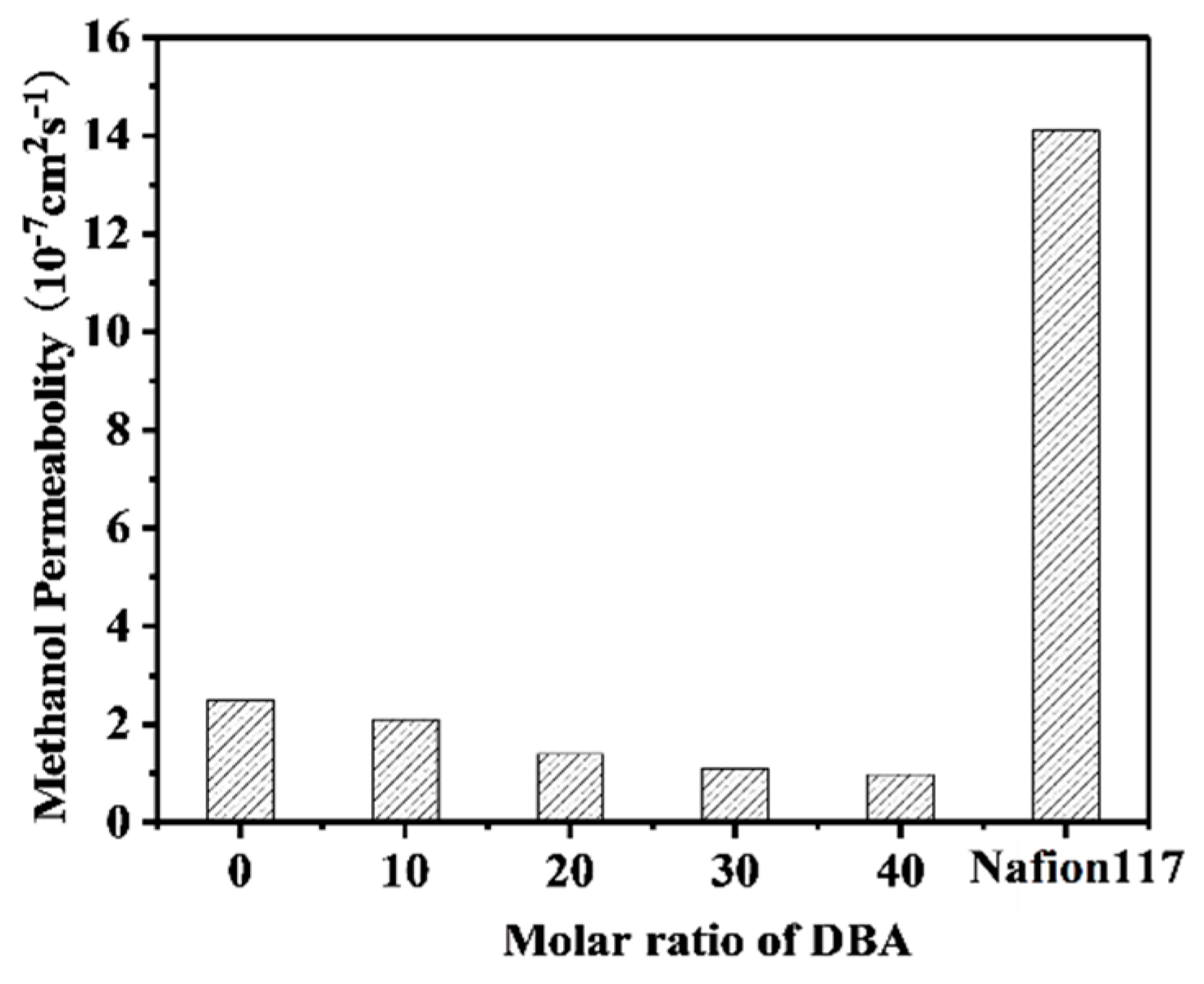
3.7. Methanol Permeability and Selectivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mallick, R.K.; Thombre, S.B.; Shrivastava, N.K. Vapor feed direct methanol fuel cells (DMFCs): A review. Renew. Sust. Energ. Rev. 2016, 56, 51–74. [Google Scholar] [CrossRef]
- Li, Q.; Wang, T.; Havas, D.; Zhang, H.; Xu, P.; Han, J.; Cho, J.; Wu, G. High Performance Direct Methanol Fuel Cells with Precious Metal Free Cathode. Adv. Sci. 2016, 3, 1600140. [Google Scholar] [CrossRef]
- Yan, X.H.; Wu, R.; Xu, J.B.; Luo, Z.; Zhao, T.S. A monolayer graphene Nafion sandwich membrane for direct methanol fuel cells. J. Power Sources 2016, 311, 188–194. [Google Scholar] [CrossRef]
- Radenahmad, N.; Afif, A.; Petra, P.I.; Rahamn, S.M.H.; Eriksson, S.G.; Azad, A.K. Proton-conducting electrolytes for direct methanol and direct urea fuel cells: A state of the art review. Renew. Sust. Energ. Rev. 2016, 57, 1347–1358. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Liu, C.; Li, Y.; Xing, W.; Sun, J. Self-healing Proton Exchange Membranes Composed of Nafion Poly (vinyl alcohol) Complexes for Durable Direct Methanol Fuel Cells. Adv. Mater. 2018, 30, 1707146. [Google Scholar] [CrossRef]
- Vecchio, C.L.; Sebastián, D.; Alegre, C.; Aricò, A.S.; Baglio, V. Carbon-supported Pd and Pd-Co cathode catalysts for direct methanol fuel cells (DMFCs) operating with high methanol concentration. J. Electroanal. Chem. 2018, 808, 464–473. [Google Scholar] [CrossRef]
- Ru, C.; Gu, Y.; Duan, Y.; Zhao, C.; Na, H. Enhancement in proton conductivity and methanol resistance of Nafion membrane induced by blending sulfonated poly (arylene ether ketones) for direct methanol fuel cells. J. Membr. Sci. 2019, 573, 439–447. [Google Scholar] [CrossRef]
- Rambabu, G.; Bhat, S.D. Amino acid functionalized graphene oxide based nanocomposite membrane electrolytes for direct methanol fuel cells. J. Membr. Sci. 2018, 551, 1–11. [Google Scholar] [CrossRef]
- Fu, C.; Yang, W.; Chen, X.; Evans, D.G. Direct electrochemistry of glucose oxidase on a graphite nanosheet–Nafion composite film modified electrode. Electrochem. Commun. 2009, 11, 997–1000. [Google Scholar] [CrossRef]
- Shu, C.; Song, B.; Wei, X.; Liu, Y.; Tan, Q.; Chong, S.; Chen, Y.Z.; Yang, X.D.; Yang, W.H.; Liu, Y. Mesoporous 3D nitrogen-doped yolk-shelled carbon spheres for direct methanol fuel cells with polymer fiber membranes. Carbon 2018, 129, 613–620. [Google Scholar] [CrossRef]
- Maiti, J.; Kakati, N.; Woo, S.P.; Yoon, Y.S. Nafion® based hybrid composite membrane containing GO and dihydrogen phosphate functionalized ionic liquid for high temperature polymer electrolyte membrane fuel cell. Compos. Sci. Technol. 2018, 155, 189–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, R.; Zhong, Y.; Jiang, F.; Hu, M.; Yu, Q. Nafion/IL Intermediate Temperature Proton Exchange Membranes Improved by Mesoporous Hollow Silica Spheres. Fuel Cells 2018, 18, 389–396. [Google Scholar] [CrossRef]
- Liu, J.; Yu, L.; Cai, X.; Khan, U.; Cai, Z.; Xi, J.; Liu, B.; Kang, F. Sandwiching h-BN Monolayer Films between Sulfonated Poly (ether ether ketone) and Nafion for Proton Exchange Membranes with Improved Ion Selectivity. ACS Nano 2019, 13, 2094–2102. [Google Scholar] [CrossRef]
- Sharma, R.; Grahl-Madsen, L.; Andersen, S.M. Influence of dispersion media on Nafion® ionomer distribution in proton exchange membrane fuel cell catalyst carbon support. Mater. Chem. Phys. 2019, 226, 66–72. [Google Scholar] [CrossRef]
- Zhang, S.; Li, D.; Kang, J.; Ma, G.; Liu, Y. Electrospinning preparation of a graphene oxide nanohybrid proton-exchange membrane for fuel cells. J. Appl. Polym. Sci. 2018, 135, 46443. [Google Scholar] [CrossRef]
- Xu, G.; Wei, Z.; Li, S.; Li, J.; Yang, Z.; Grigoriev, S.A. In-situ sulfonation of targeted silica-filled Nafion for high temperature PEM fuel cell application. Int. J. Hydrogen Energ. 2019. in Press. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Wei, S.; Geng, C.; Kang, M.; Lu, A. Chemically stable thioether cross-linked membranes derived from sulfonated poly (arylene ether ketone) s for direct methanol fuel cells. High Perform. Polym. 2018, 30, 129–138. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Chen, D.; Ye, Z. Sulfonated binaphthyl containing poly (arylene ether ketone) s with rigid backbone and excellent film forming capability for proton exchange membranes. Polymers 2018, 10, 1287. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Xia, L.; Huang, D.; Fu, X.; Zhang, R.; Hu, S.; Zhao, F.; Li, X.; Bao, X. Poly (2, 5-benzimidazole)/sulfonated sepiolite composite membranes with low phosphoric acid doping levels for PEMFC applications in a wide temperature range. J. Membr. Sci. 2019, 574, 282–298. [Google Scholar] [CrossRef]
- Lysova, A.A.; Stenina, I.A.; Volkov, A.O.; Ponomarev, I.I.; Yaroslavtsev, A.B. Proton conductivity of hybrid membranes based on polybenzimidazoles and surface-sulfonated silica. Solid State Ionics 2019, 329, 25–30. [Google Scholar] [CrossRef]
- Muthuraja, P.; Prakash, S.; Susaimanickam, A.; Manisankar, P. Potential membranes derived from poly (aryl hexafluoro sulfone benzimidazole) and poly (aryl hexafluoro ethoxy benzimidazole) for high-temperature PEM fuel cells. Int. J. Hydrogen Energ. 2018, 43, 21732–21741. [Google Scholar] [CrossRef]
- Ahn, M.K.; Lee, B.; Jang, J.; Min, C.M.; Lee, S.B.; Pak, C.; Lee, J.S. Facile preparation of blend proton exchange membranes with highly sulfonated poly (arylene ether) and poly (arylene ether sulfone) bearing dense triazoles. J. Membr. Sci. 2018, 560, 58–66. [Google Scholar] [CrossRef]
- Chen, R.M.; Xu, F.Z.; Fu, K.; Zhou, J.; Shi, Q.; Xue, C.; Lyu, Y.C.; Guo, B.K.; Li, G. Enhanced proton conductivity and dimensional stability of proton exchange membrane based on sulfonated poly (arylene ether sulfone) and graphene oxide. Mater. Res. Bull. 2018, 103, 142–149. [Google Scholar] [CrossRef]
- Han, J.; Kim, K.; Kim, J.; Kim, S.; Choi, S.W.; Lee, H.; Kim, J.; Kim, T.H.; Sung, Y.E.; Lee, J.C. Cross-linked highly sulfonated poly (arylene ether sulfone) membranes prepared by in-situ casting and thiol-ene click reaction for fuel cell application. J. Membr. Sci. 2019, 579, 70–78. [Google Scholar] [CrossRef]
- Hu, H.; Sui, Y.; Ueda, M.; Qian, J.; Wang, L.; Zhang, X. Multi-block sulfonated poly (arylene ether nitrile) polymers bearing oligomeric benzotriazole pendants with exceptionally high H2/O2 fuel cell performance. J. Membr. Sci. 2018, 564, 342–351. [Google Scholar] [CrossRef]
- Zheng, P.; Xu, M.; Liu, X.; Jia, K. Sulfonated poly (arylene ether nitrile) s containing cross-linkable nitrile groups for proton exchange membranes. Solid State Ionics 2018, 316, 110–117. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, X.; Ma, Y.; Huang, Y.; Liu, X. Constructing continuous proton-conducting highways within sulfonated poly (arylene ether nitrile) composite membrane by incorporating amino-sulfo-bifunctionalized GO. Polymers 2018, 10, 1005. [Google Scholar] [CrossRef]
- Wang, L.; Yu, L.; Mu, D.; Yu, L.; Wang, L.; Xi, J. Acid-base membranes of imidazole-based sulfonated polyimides for vanadium flow batteries. J. Membr. Sci. 2018, 552, 167–176. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, Z.; Hu, M.; Hou, J.; Wu, L.; Xu, T. Perylene-based sulfonated aliphatic polyimides for fuel cell applications: Performance enhancement by stacking of polymer chains. J. Membr. Sci. 2018, 547, 43–50. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, L.; Wang, L.; Yang, H.; Yu, X. High Proton Selectivity Sulfonated Polyimides Ion Exchange Membranes for Vanadium Flow Batteries. Polymers 2018, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Li, X.; Kaliaguine, S. Synthesis of copoly (aryl ether ether nitrile) s containing sulfonic acid groups for PEM application. Macromolecules 2005, 38, 3237–3245. [Google Scholar] [CrossRef]
- Gao, Y.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Li, X.; Kaliaguine, S. Low-swelling proton-conducting copoly (aryl ether nitrile) s containing naphthalene structure with sulfonic acid groups meta to the ether linkage. Polymer 2006, 47, 808–816. [Google Scholar] [CrossRef]
- Gao, Y.; Robertson, G.P.; Kim, D.S.; Guiver, M.D.; Mikhailenko, S.D.; Li, X.; Kaliaguine, S. Comparison of PEM properties of copoly (aryl ether ether nitrile)s containing sulfonic acid bonded to naphthalene in structurally different ways. Macromolecules 2007, 40, 1512–1520. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, T.; Zhang, X.; Zhang, W.; Liu, X. Novel composite proton exchange membrane with long-range proton transfer channels constructed by synergistic effect between acid and base functionalized graphene oxide. Polymer 2018, 149, 305–315. [Google Scholar] [CrossRef]
- Sumner, M.J.; Harrison, W.L.; Weyers, R.M.; Kim, Y.S.; McGrath, J.E.; Riffle, J.S.; Brink, A.; Brink, M.H. Novel proton conducting sulfonated poly(arylene ether) copolymers containing aromatic nitriles. J. Membr. Sci. 2004, 239, 199–211. [Google Scholar] [CrossRef]
- Gao, Y.; Robertson, G.P.; Guiver, M.D.; Wang, G.; Jian, X.; Mikhailenko, S.D.; Li, X.; Kaliaguine, S. Sulfonated copoly(phthalazinone ether ketone nitrile)s as proton exchange membrane materials. J. Membr. Sci. 2006, 278, 26–34. [Google Scholar] [CrossRef]
- Dong, W.S.; Lee, S.Y.; Na, R.K.; Lee, K.H.; Guiver, M.D.; Lee, Y.M. Durable sulfonated poly(arylene sulfide sulfone nitrile)s containing naphthalene units for direct methanol fuel cells (DMFCs). Macromolecules 2013, 46, 3452–3460. [Google Scholar]
- Kim, Y.S.; Kim, D.S.; Liu, B.; Guiver, M.D.; Pivovar, B.S. Copoly (arylene ether nitrile) s high performance polymer electrolytes for direct methanol fuel cells. J. Electrochem. Soc. 2008, 155, B21–B26. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, Y.S.; Guiver, M.D.; Pivovar, B.S. High performance nitrile copolymers for polymer electrolyte membrane fuel cells. J. Membr. Sci. 2008, 321, 199–208. [Google Scholar] [CrossRef]
- Wang, C.; Shen, B.; Zhou, Y.; Xu, C.; Chen, W.; Zhao, X.; Li, J. Sulfonated aromatic polyamides containing nitrile groups as proton exchange fuel cell membranes. Int. Hydrogen Energ. 2015, 40, 6422–6429. [Google Scholar] [CrossRef]
- Yang, T.; Li, Z.; Lyu, H.; Zheng, J.; Liu, J.; Liu, F.; Zhang, Z.; Rao, H. A graphene oxide polymer brush based cross-linked nanocomposite proton exchange membrane for direct methanol fuel cells. RSC Adv. 2018, 8, 15740–15753. [Google Scholar] [CrossRef]
- Cho, K.Y.; Jung, H.Y.; Shin, S.S.; Choi, N.S.; Sung, S.J.; Park, J.K.; Choi, J.H.; Park, K.W.; Sung, Y.E. Proton conducting semi-IPN based on Nafion and crosslinked poly (AMPS) for direct methanol fuel cell. Electrochim. Acta 2004, 50, 588–593. [Google Scholar] [CrossRef]
- Fu, T.; Zhao, C.; Zhong, S.; Zhang, G.; Shao, K.; Zhang, H.; Wang, J.; Na, H. SPEEK/epoxy resin composite membranes in situ polymerization for direct methanol fell cell usages. J. Power Sources 2007, 165, 708–716. [Google Scholar] [CrossRef]
- Qiao, J.; Hamaya, T.; Okada, T. New highly proton conductive polymer membranes poly (vinyl alcohol) 2-acrylamido-2-methyl-1-propanesulfonic acid (PVA-PAMPS). J. Mater. Chem. 2005, 15, 4414–4423. [Google Scholar] [CrossRef]
- Gasa, J.V.; Boob, S.; Weiss, R.A.; Shaw, M.T. Proton-exchange membranes composed of slightly sulfonated poly (ether ketone ketone) and highly sulfonated crosslinked polystyrene particles. J. Membr. Sci. 2006, 269, 177–186. [Google Scholar] [CrossRef]
- Yamaki, T.; Kobayashi, K.; Asano, M.; Kubota, H.; Yoshida, M. Preparation of proton exchange membranes based on crosslinked polytetrafluoroethylene for fuel cell applications. Polymer 2004, 45, 6569–6573. [Google Scholar] [CrossRef]
- Park, H.B.; Lee, C.H.; Sohn, J.Y.; Lee, Y.M.; Freeman, B.D.; Kim, H.J. Effect of crosslinked chain length in sulfonated polyimide membranes on water sorption, proton conduction, and methanol permeation properties. J. Membr. Sci. 2006, 285, 432–443. [Google Scholar] [CrossRef]
- Ye, Y.S.; Yen, Y.C.; Cheng, C.C.; Chen, W.Y.; Tsai, L.T.; Chang, F.C. Sulfonated poly (ether ether ketone) membranes crosslinked with sulfonic acid containing benzoxazine monomer as proton exchange membranes. Polymer 2009, 50, 3196–3203. [Google Scholar] [CrossRef]
- Zheng, P.; Xu, M.; Liu, X.; Jia, K. Novel cross-linked membrane for direct methanol fuel cell application: sulfonated poly (ether ether nitrile)s. Ionics 2017, 23, 87–94. [Google Scholar] [CrossRef]
- Heo, K.B.; Lee, H.J.; Kim, H.J.; Kim, B.S.; Lee, S.Y.; Cho, E.; Oh, I.H.; Hong, S.A.; Lim, T.H. Synthesis and characterization of cross-linked poly(ether sulfone) for a fuel cell membrane. J. Power Sources 2007, 172, 215–219. [Google Scholar] [CrossRef]
- Zheng, J.; He, Q.; Gao, N.; Yuan, T.; Zhang, S.; Yang, H. Novel proton exchange membranes based on cardo poly (arylene ether sulfone/nitrile)s with perfluoroalkyl sulfonic acid moieties for passive direct methanol fuel cells. J. Power Sources 2014, 261, 38–45. [Google Scholar] [CrossRef]
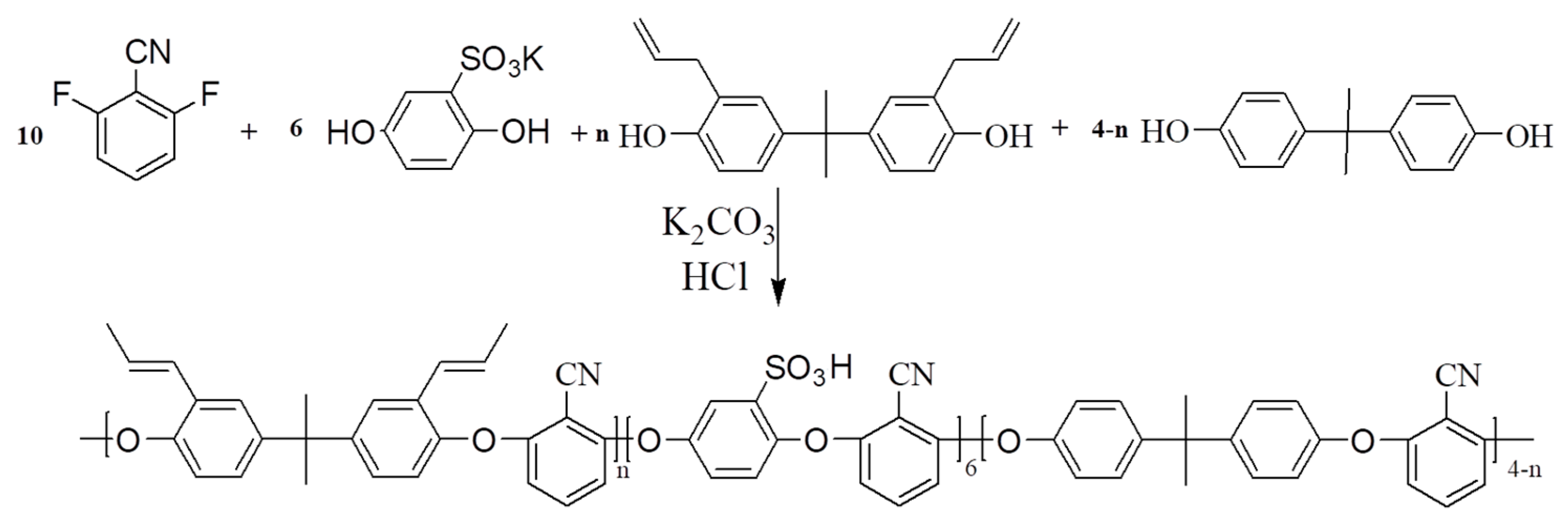
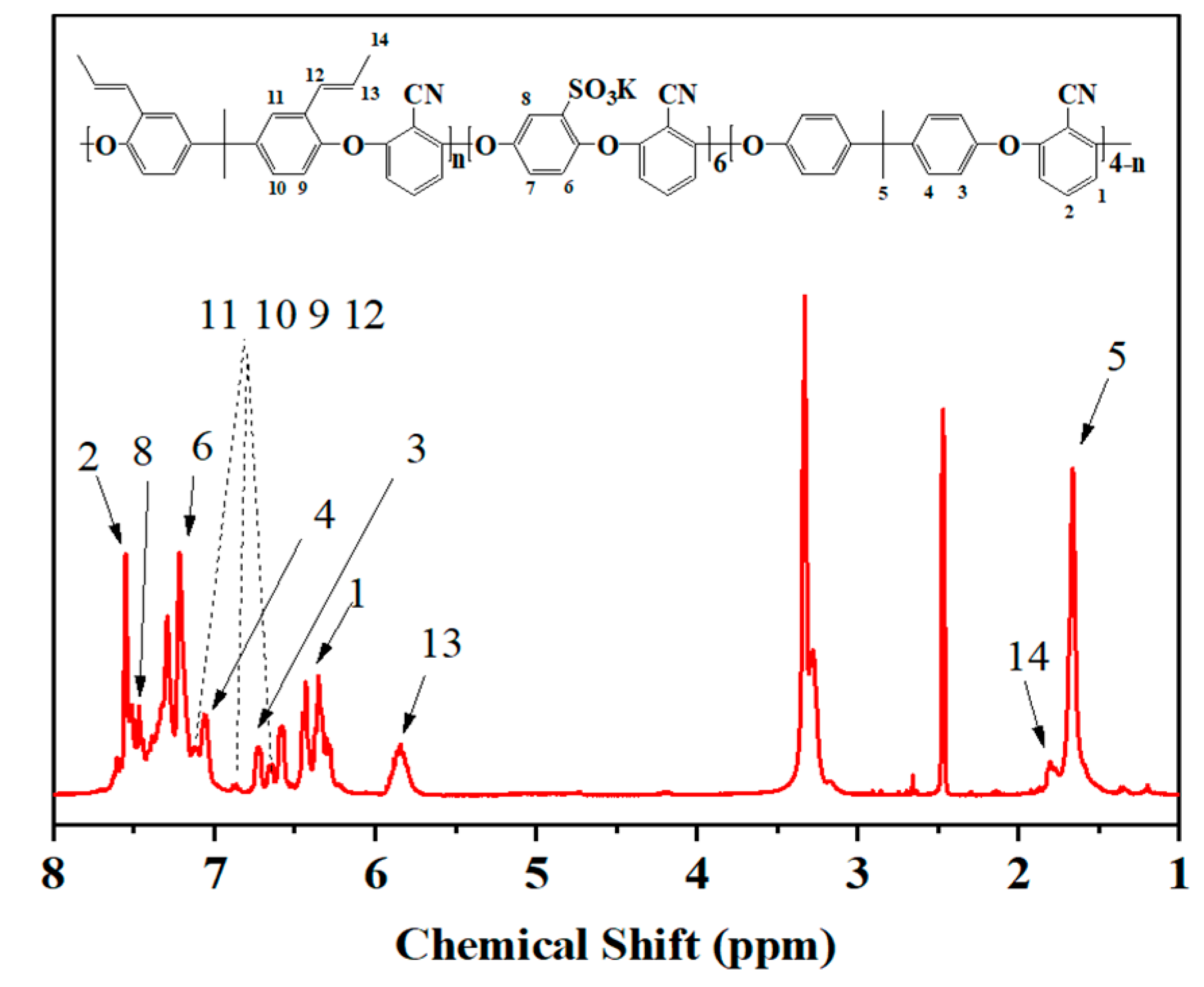

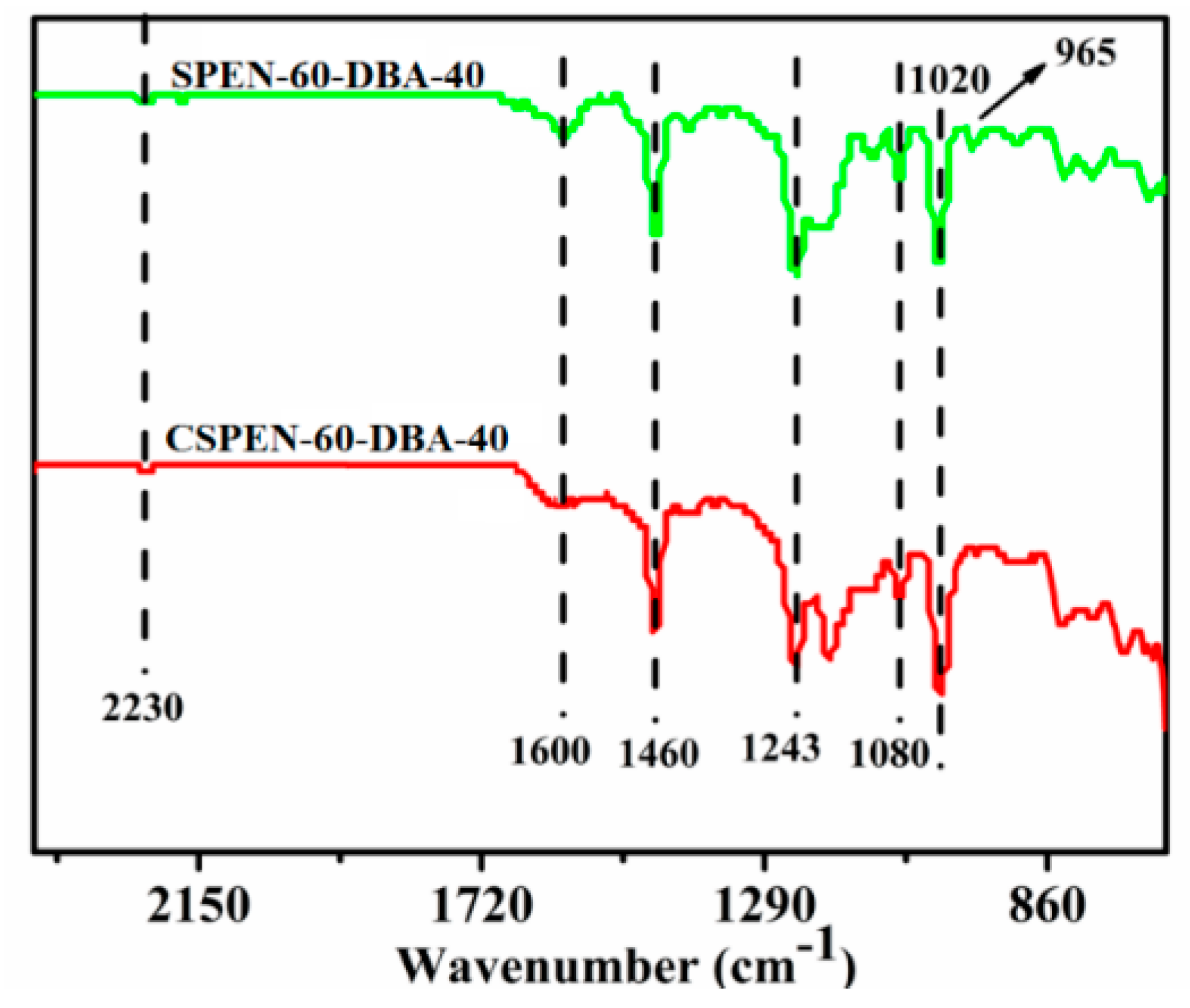
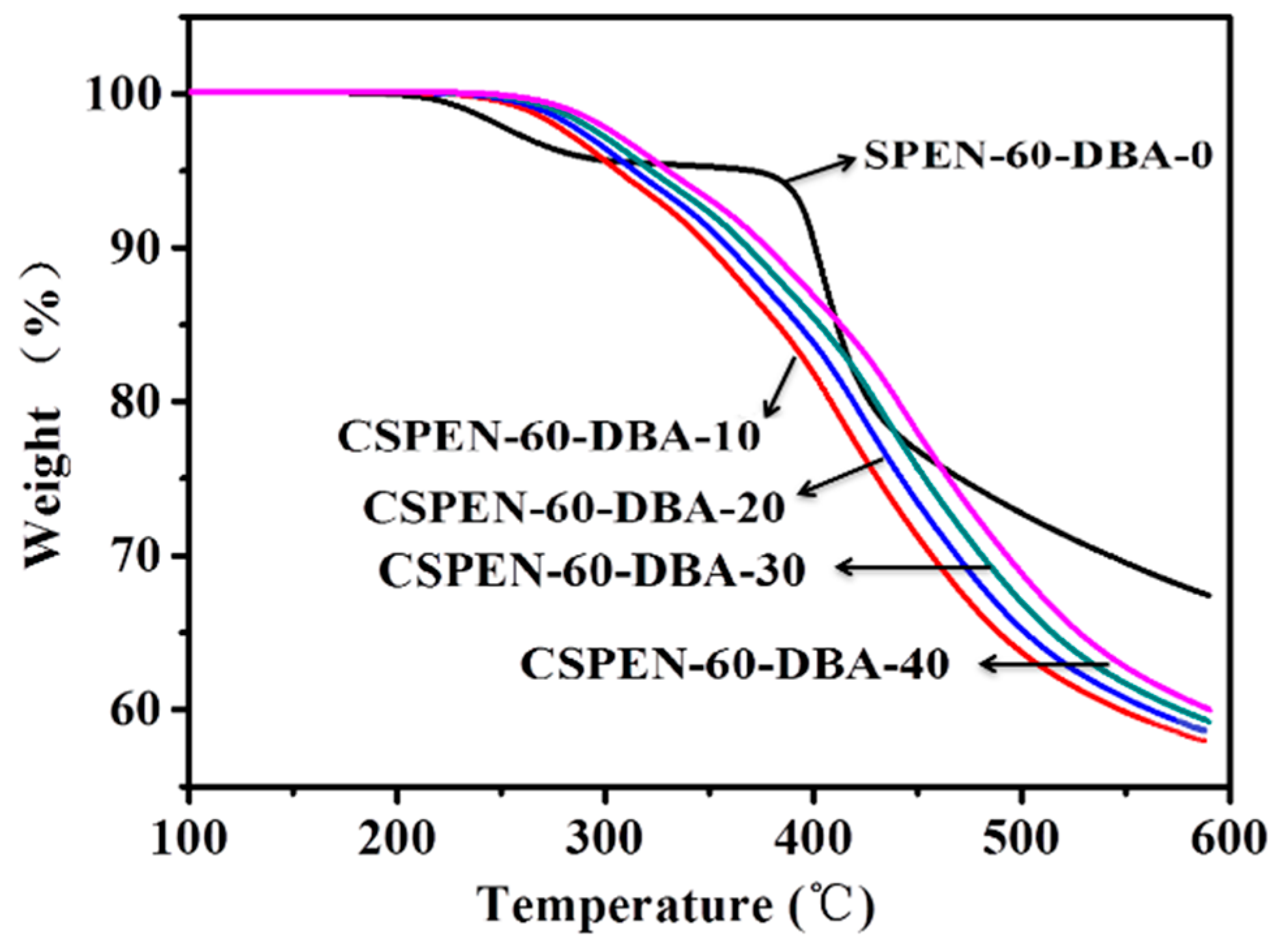



| Copolymers | Allyl bisphenol A (mol) | Bisphenol A (mol) | SHQ (mol) | K2CO3 (mol) | DFBN (mol) | NMP (mL) | Toluene (mL) |
|---|---|---|---|---|---|---|---|
| SPEN-60-DBA-0 | 0.00 | 0.04 | 0.06 | 0.2 | 0.1 | 65 | 25 |
| CSPEN-60-DBA-10 | 0.01 | 0.03 | 0.06 | 0.2 | 0.1 | 65 | 25 |
| CSPEN-60-DBA-20 | 0.02 | 0.02 | 0.06 | 0.2 | 0.1 | 65 | 25 |
| CSPEN-60-DBA-30 | 0.03 | 0.01 | 0.06 | 0.2 | 0.1 | 65 | 25 |
| CSPEN-60-DBA-40 | 0.04 | 0.00 | 0.06 | 0.2 | 0.1 | 65 | 25 |
| Membranes | SPEN-60-DBA-0 | CSPEN-60-DBA-10 | CSPEN-60-DBA-20 | CSPEN-60-DBA-30 | CSPEN-60-DBA-40 |
|---|---|---|---|---|---|
| The gel content (%) | 0 | 14 | 26 | 43 | 58 |
| Membranes | Proton conductivity (10−2 S·cm−1) | Methanol permeability (10−7 cm2·s−1) | Selectivity (105 S·s·cm−3) | ||
|---|---|---|---|---|---|
| 20 °C | 50 °C | 80 °C | |||
| SPEN-60-DBA-0 | 3.4 | 6.9 | 6.6 | 2.5 | 1.4 |
| CSPEN-60-DBA-10 | 3.2 | 6.4 | 8.6 | 2.1 | 1.5 |
| CSPEN-60-DBA-20 | 2.9 | 5.3 | 8.1 | 1.4 | 2.1 |
| CSPEN-60-DBA-30 | 2.9 | 5.1 | 7.2 | 1.1 | 2.6 |
| CSPEN-60-DBA-40 | 1.7 | 3.8 | 6.8 | 0.98 | 1.7 |
| Nafion117 | 6.4 | -- | -- | 14.1 | 0.45 |
| Disulfonated-6F-PAE-CN (35) [35] | 6.5 | -- | -- | 8.7 | 0.75 |
| FSPES-2 [51] | 7.4 | -- | -- | 8.0 | 0.93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, P.; Liu, Q.; Li, Z.; Wang, D.; Liu, X. Effect of Crosslinking Degree on Sulfonated Poly(aryl ether nitrile)s As Candidates for Proton Exchange Membranes. Polymers 2019, 11, 964. https://doi.org/10.3390/polym11060964
Zheng P, Liu Q, Li Z, Wang D, Liu X. Effect of Crosslinking Degree on Sulfonated Poly(aryl ether nitrile)s As Candidates for Proton Exchange Membranes. Polymers. 2019; 11(6):964. https://doi.org/10.3390/polym11060964
Chicago/Turabian StyleZheng, Penglun, Quanyi Liu, Zekun Li, Donghui Wang, and Xiaobo Liu. 2019. "Effect of Crosslinking Degree on Sulfonated Poly(aryl ether nitrile)s As Candidates for Proton Exchange Membranes" Polymers 11, no. 6: 964. https://doi.org/10.3390/polym11060964
APA StyleZheng, P., Liu, Q., Li, Z., Wang, D., & Liu, X. (2019). Effect of Crosslinking Degree on Sulfonated Poly(aryl ether nitrile)s As Candidates for Proton Exchange Membranes. Polymers, 11(6), 964. https://doi.org/10.3390/polym11060964




