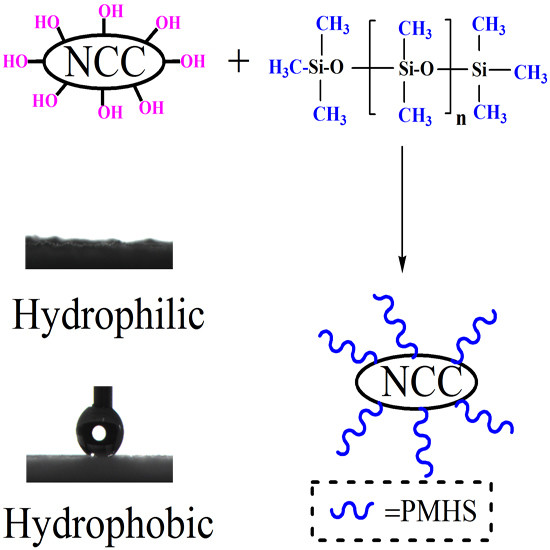
An Effective, Economical and Ultra-Fast Method for Hydrophobic Modification of NCC Using Poly(Methylhydrogen)Siloxane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NCC
2.3. Hydrophobic Modification of NCC
2.4. Characterization
3. Results and Discussion
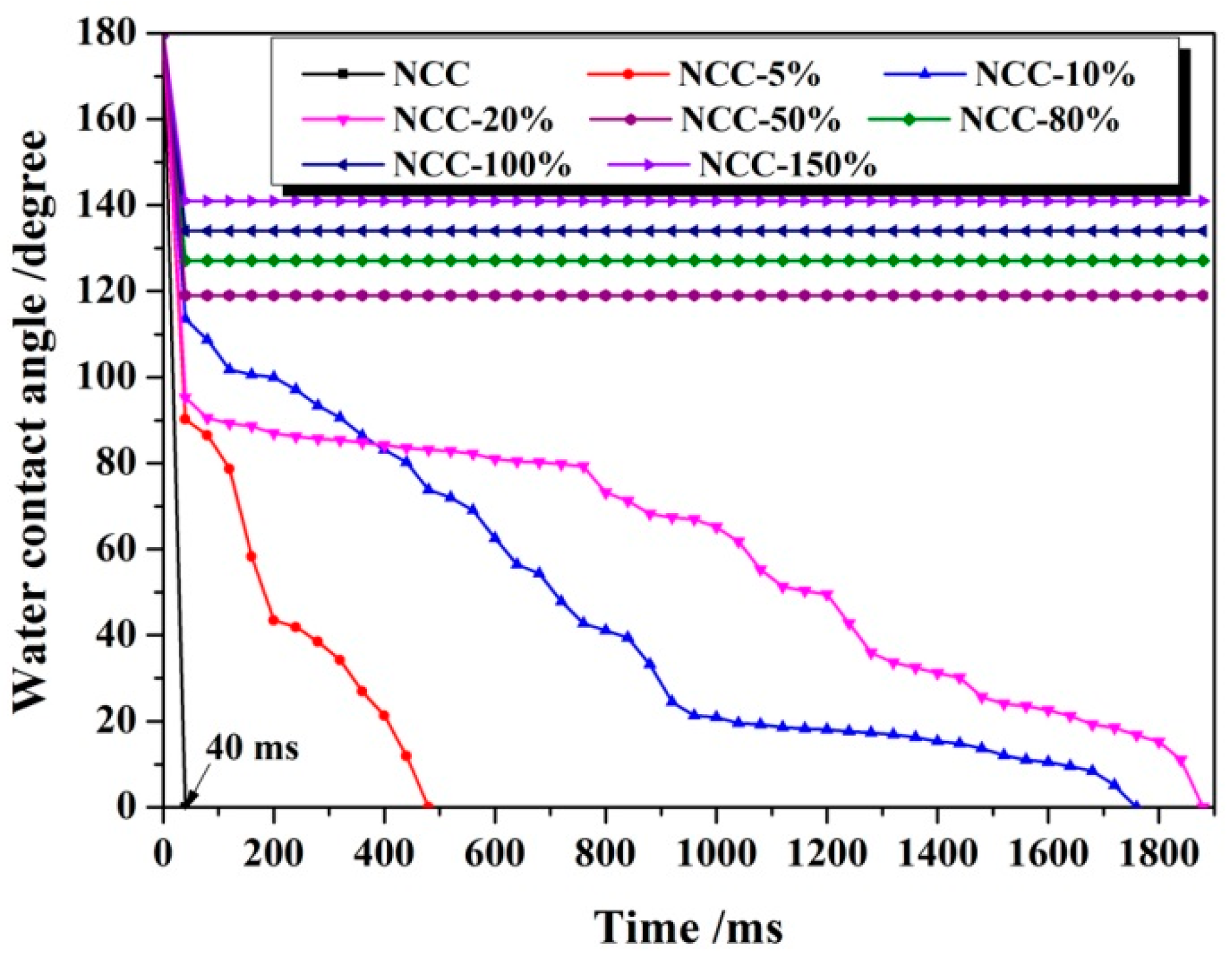
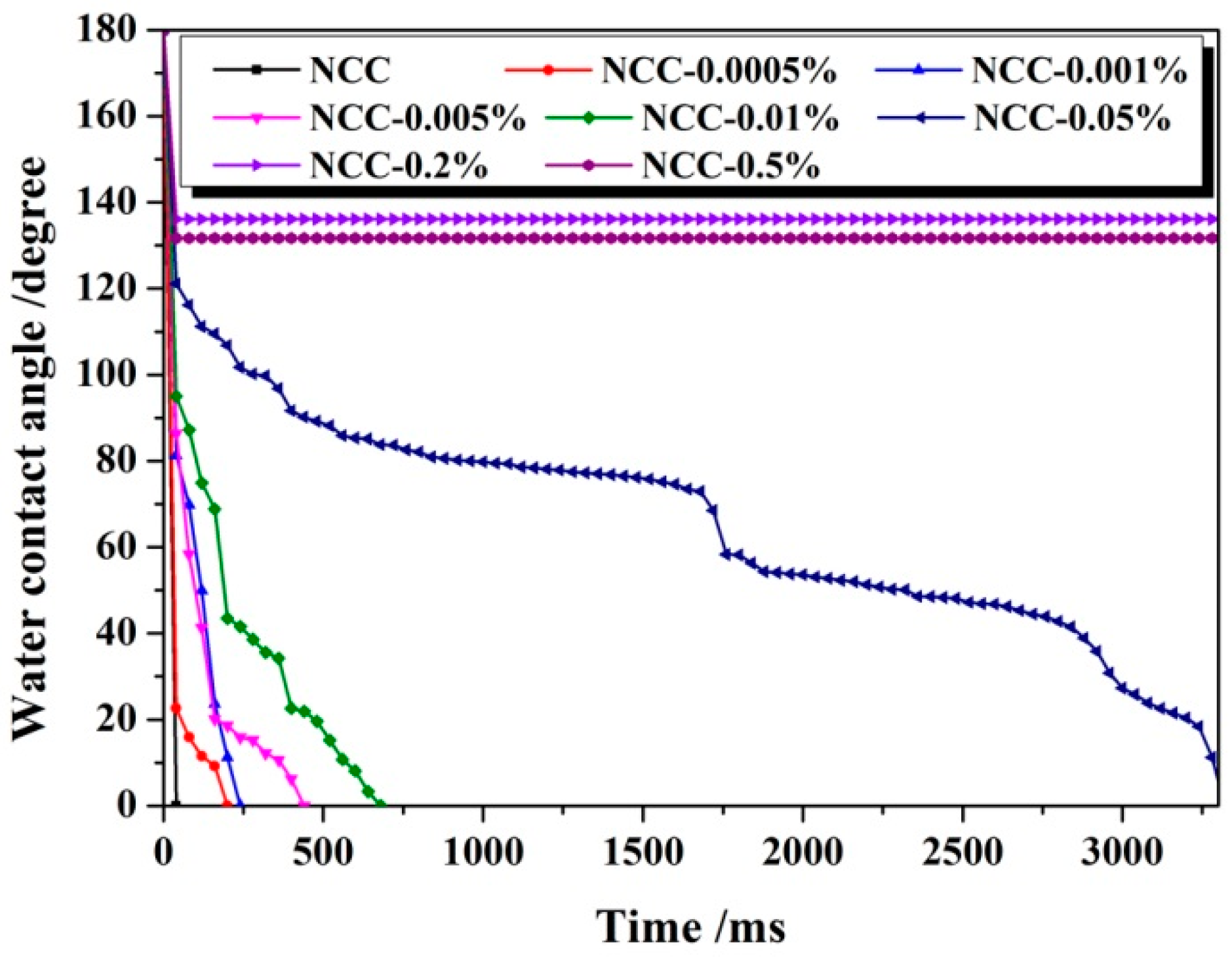
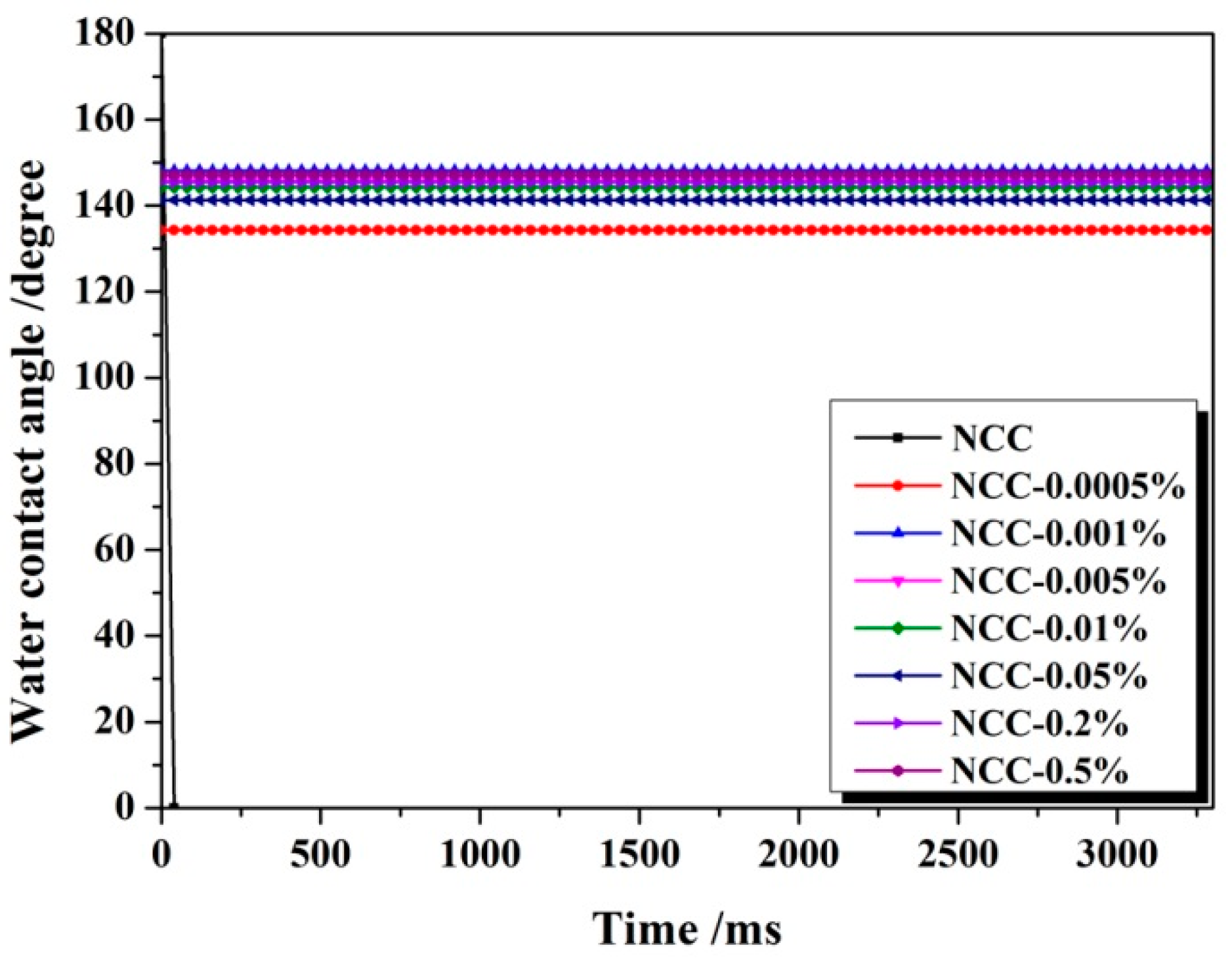
3.1. Hydrophobicity of NCC
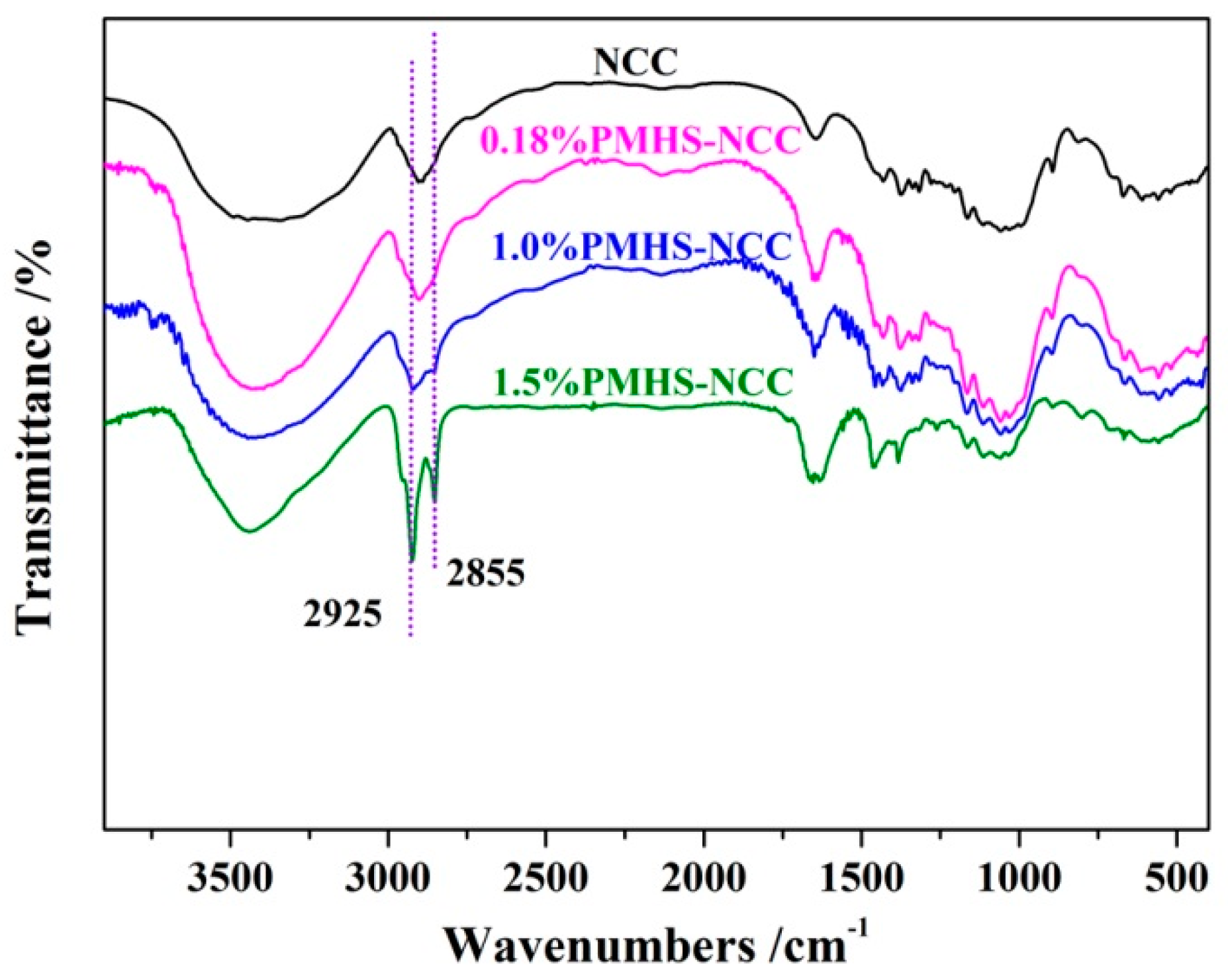
3.2. FTIR Characterization
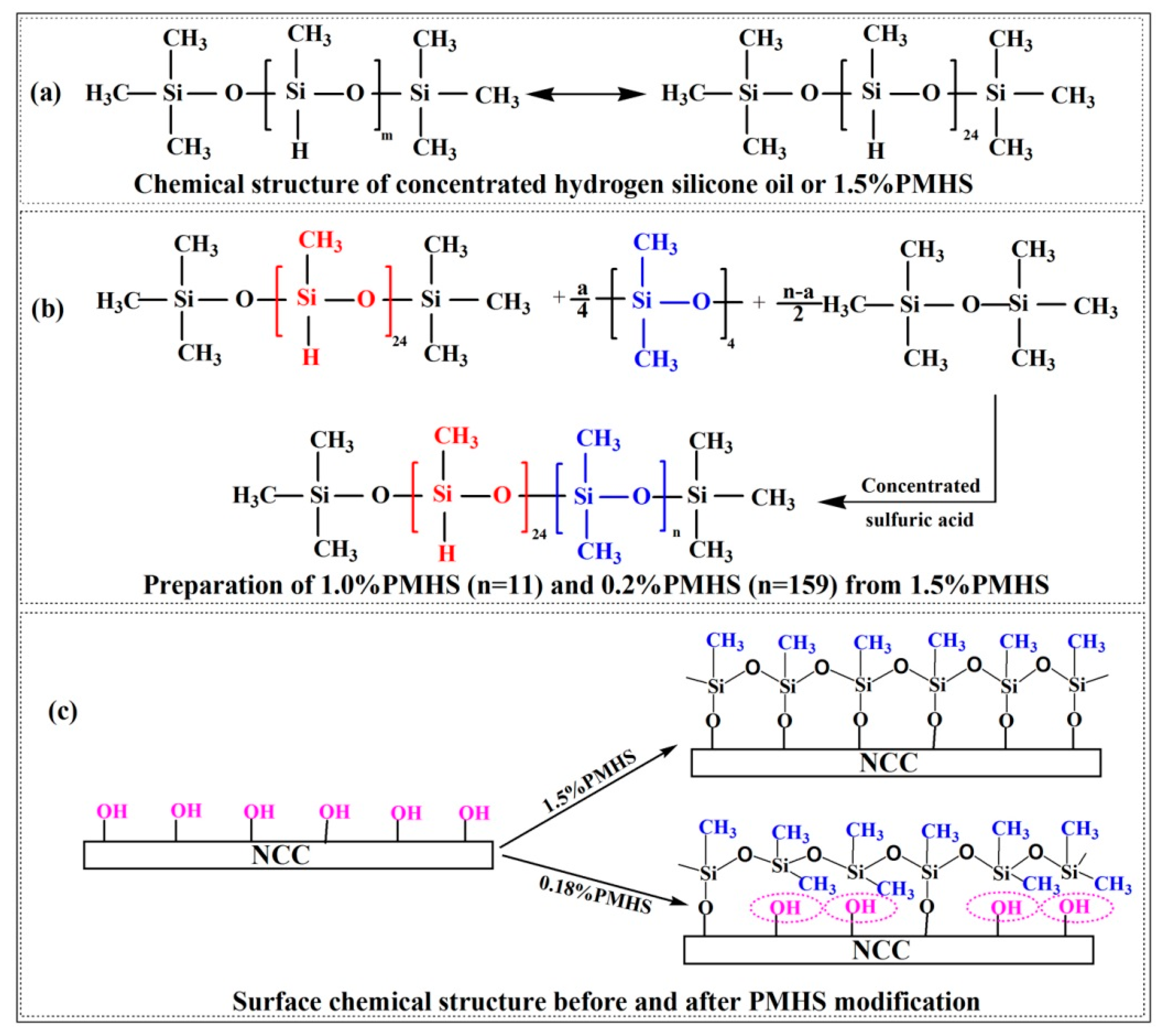
3.3. Difference in PMHS Structure
3.4. Crystallinity of NCC
3.5. Thermal Stability of NCC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dufresne, A. Nanocellulose: a new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Shang, W.L.; Huang, J.; Luo, H.; Chang, P.R.; Feng, J.W.; Xie, G.Y. Hydrophobic modification of cellulose nanocrystal via covalently grafting of castor oil. Cellulose 2013, 20, 179–190. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: a review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Han, S.Y.; Park, C.W.; Chae, H.M.; Lee, S.H. Preparation and properties of cellulose nanofiber films with various chemical compositions impregnated by ultraviolet-curable resin. Bioresources 2017, 12, 1767–1778. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. New Process of Chemical Grafting of Cellulose Nanoparticles with a Long Chain Isocyanate. Langmuir 2010, 26, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale 2014, 6, 5384–5393. [Google Scholar] [CrossRef] [PubMed]
- Goussé, C.; Chanzy, H.; Excoffier, G.; Soubeyrand, L.; Fleury, E. Stable suspensions of partially silylated cellulose whiskers dispersed in organic solvent. Polymer 2002, 43, 2645–2651. [Google Scholar] [CrossRef]
- Frone, A.N.; Berlioz, S.; Chailan, J.F.; Panaitescu, D.M.; Donescu, D. Cellulose fiber-reinforced polylactic acid. Polym. Compos. 2011, 32, 976–985. [Google Scholar] [CrossRef]
- De Oliveira Taipina, M.; Ferrarezi, M.M.F.; Yoshida, I.V.P.; do Carmo Gonçalves, M. Surface modification of cotton nanocrystals with a silane agent. Cellulose 2013, 20, 217–226. [Google Scholar] [CrossRef]
- Lin, W.; Hu, X.; You, X.; Sun, Y.; Wen, Y.; Yang, W.; Zhang, X.; Li, Y.; Chen, H. Hydrophobic modification of nanocellulose via a two-step silanation method. Polymers 2018, 10, 1035. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Inamochi, T.; Funahashi, R.; Nakamura, Y.; Saito, T.; Isogai, A. Effect of coexisting salt on TEMPO-mediated oxidation of wood cellulose for preparation of nanocellulose. Cellulose 2017, 24, 4097–4101. [Google Scholar] [CrossRef]
- Huang, F.; Wu, X.; Yu, Y.; Lu, Y.; Chen, Q. Acylation of cellulose nanocrystals with acids/trifluoroacetic anhydride and properties of films from esters of CNCs. Carbohydr. Polym. 2017, 155, 525–534. [Google Scholar] [CrossRef]
- Johnson, R.K.; Zink-Sharp, A.; Glasser, W.G. Preparation and characterization of hydrophobic derivatives of TEMPO-oxidized nanocelluloses. Cellulose 2011, 18, 1599–1609. [Google Scholar] [CrossRef]
- De Menezes, A.J.; Siqueira, G.; Curvelo, A.A.S.; Dufresne, A. Extrusion and characterization of functionalized cellulose whiskers reinforced polyethylene nanocomposites. Polymer 2009, 50, 4552–4563. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; GindlAltmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef]
- Saini, S.; Naceur, M.; Bras, J. Effect of variable aminoalkyl chains on chemical grafting of cellulose nanofiber and their antimicrobial activity. Mater. Sci. Eng. C 2017, 75, 760–768. [Google Scholar] [CrossRef]
- Tang, L.R.; Huang, B.; Ou, W.; Chen, X.R.; Chen, Y.D. Manufacture of cellulose nanocrystals by cation exchange resin-catalyzed hydrolysis of cellulose. Bioresour. Technol. 2011, 102, 10973–10977. [Google Scholar] [CrossRef]
- Segal, L.; Cheely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhang, L.; Zha, D.C.; Chen, L.Q.; Chen, X.X.; Qi, Z.M. Biosorption of malachite green onto Haematococcus pluvialis observed through synchrotron Fourier-transform infrared microspectroscopy. Lett. Appl. Microbiol. 2018, 67, 348–353. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, J.; Huang, R.; Zhang, X.; Chen, C.; Jiang, B.; Chen, H.X.; Yan, L.H.; Yang, W.B. A simple method to control the microstructure and properties of sol–gel silica antireflective coatings. RSC Adv. 2017, 7, 31950–31959. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crop. Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Lee, K.Y.; Quero, F.; Blaker, J.J.; Hill, C.A.S.; Eichhorn, S.J.; Bismarck, A. Surface only modification of bacterial cellulose nanofibres with organic acids. Cellulose 2011, 18, 595–605. [Google Scholar] [CrossRef]
- Goswami, D.; Medda, S.K.; De, G. Superhydrophobic films on glass surface derived from trimethylsilanized silica gel nanoparticles. ACS Appl. Mater. Interfaces 2011, 3, 3440–3447. [Google Scholar] [CrossRef] [PubMed]


| Sample | χc | Crystalline Dimensions (nm) | N0·10−3 (mmol·m−2) | Weight/% (238–416 °C) | ||
|---|---|---|---|---|---|---|
| {101} | {10ī} | {002} | ||||
| NCC | 78.51% | 6.7 | 8.3 | 8.0 | 6.656 | 46.35 |
| 0.18%PMHS-NCC | 76.59% | 7.0 | 8.4 | 8.3 | 6.472 | 46.24 |
| 1.0%PMHS-NCC | 74.90% | 7.5 | 8.0 | 8.2 | 6.430 | 53.97 |
| 1.5%PMHS-NCC | 73.71% | 7.4 | 11.5 | 8.2 | 5.273 | 54.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, X.; Hu, Q.; Hu, X.; Chen, H.; Yang, W.; Zhang, X. An Effective, Economical and Ultra-Fast Method for Hydrophobic Modification of NCC Using Poly(Methylhydrogen)Siloxane. Polymers 2019, 11, 963. https://doi.org/10.3390/polym11060963
You X, Hu Q, Hu X, Chen H, Yang W, Zhang X. An Effective, Economical and Ultra-Fast Method for Hydrophobic Modification of NCC Using Poly(Methylhydrogen)Siloxane. Polymers. 2019; 11(6):963. https://doi.org/10.3390/polym11060963
Chicago/Turabian StyleYou, Xueqing, Qingjian Hu, Xiaoyong Hu, Hanxian Chen, Wenbin Yang, and Xinxiang Zhang. 2019. "An Effective, Economical and Ultra-Fast Method for Hydrophobic Modification of NCC Using Poly(Methylhydrogen)Siloxane" Polymers 11, no. 6: 963. https://doi.org/10.3390/polym11060963
APA StyleYou, X., Hu, Q., Hu, X., Chen, H., Yang, W., & Zhang, X. (2019). An Effective, Economical and Ultra-Fast Method for Hydrophobic Modification of NCC Using Poly(Methylhydrogen)Siloxane. Polymers, 11(6), 963. https://doi.org/10.3390/polym11060963