Computer Simulation of Anisotropic Polymeric Materials Using Polymerization-Induced Phase Separation under Combined Temperature and Concentration Gradients
Abstract
1. Introduction
2. Model Development
3. Results and Discussion
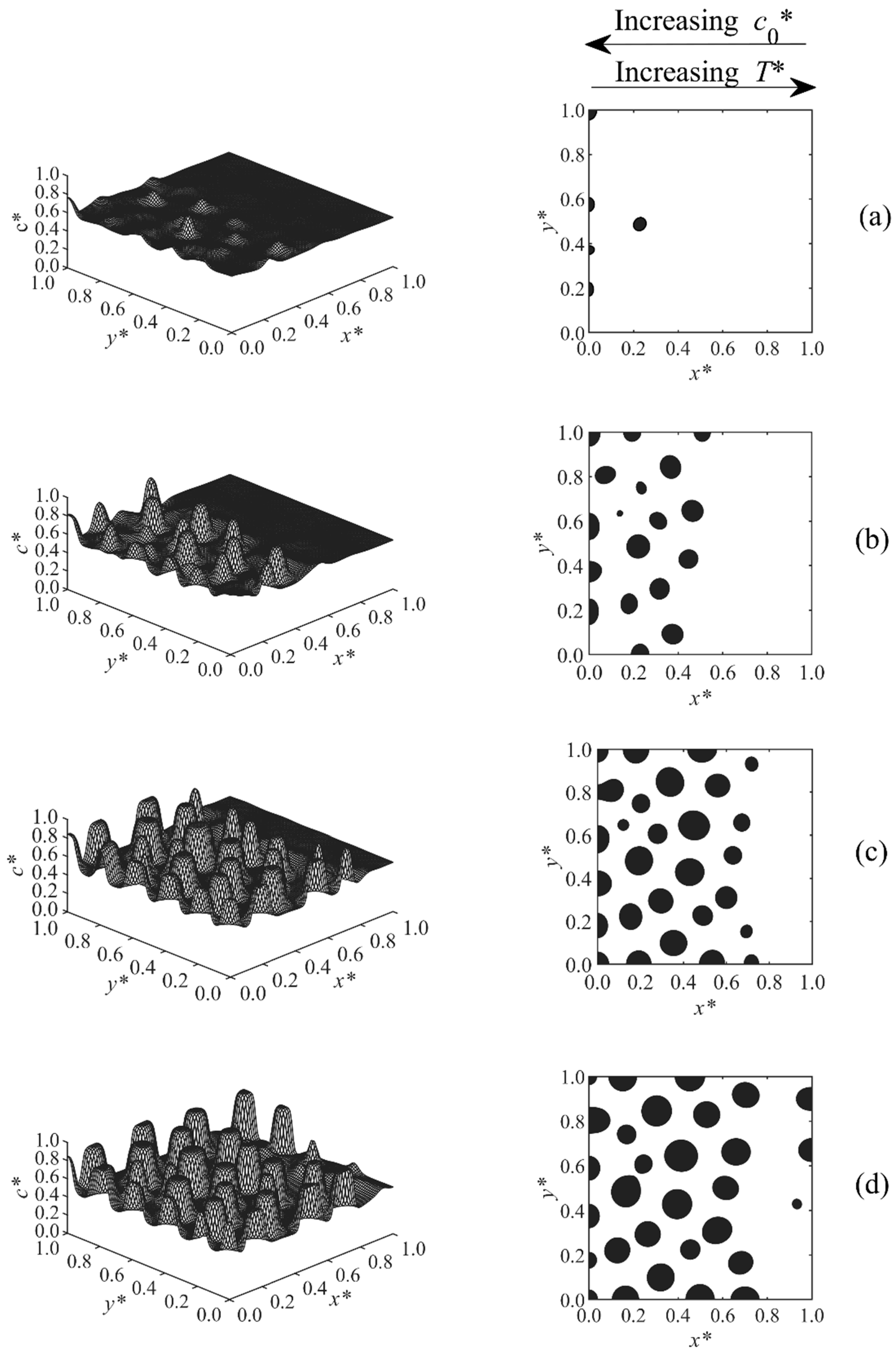
3.1. Phase-Separated Structure and Morphology Development
3.2. Size and Morphology Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chan, P.K.; Rey, A.D. Polymerization-Induced Phase Separation. 1. Droplet Size Selection Mechanism. Macromolecules 1996, 29, 8934–8941. [Google Scholar] [CrossRef]
- Chan, P.K.; Rey, A.D. Polymerization-Induced Phase Separation. 2. Morphological Analysis. Macromolecules 1997, 30, 2135–2143. [Google Scholar] [CrossRef]
- Sundararaj, U.; Macosko, C.W.; Shih, C.K. Evidence for Inversion of Phase Continuity during Morphology Development in Polymer Blending. Polym. Eng. Sci. 1996, 36, 1769–1781. [Google Scholar] [CrossRef]
- Lyu, S.P.; Cernohous, J.J.; Bates, F.S.; Macosko, C.W. Interfacial Reaction Induced Roughening in Polymer Blends. Macromolecules 1999, 32, 106–110. [Google Scholar] [CrossRef]
- Lee, J.; Macosko, C.W.; Urry, D.W. Phase Transition and Elasticity of Protein-Based Hydrogels. J. Biomater. Sci. Polym. Ed. 2001, 12, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Van-Pham, D.T.; Tran-Cong-Miyata, Q. Phase Separation Kinetics and Morphology Induced by Photopolymerization of 2-Hydroxyehyl Methacrylate (HEMA) in Poly(Ethyl Acrylate)/HEMA Mixtures. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4. [Google Scholar] [CrossRef]
- Loera, A.G.; Cara, F.; Dumon, M.; Pascault, J.P. Porous Epoxy Thermosets Obtained by a Polymerization-Induced Phase Separation Process of a Degradable Thermoplastic Polymer. Macromolecules 2002, 35, 6291–6297. [Google Scholar] [CrossRef]
- Romeo, H.E.; Vílchez, A.; Esquena, J.; Hoppe, C.E.; Williams, R.J.J. Polymerization-Induced Phase Separation as a One-Step Strategy to Self-Assemble Alkanethiol-Stabilized Gold Nanoparticles inside Polystyrene Domains Dispersed in an Epoxy Matrix. Eur. Polym. J. 2012, 48, 1101–1109. [Google Scholar] [CrossRef]
- Seo, M.; Hillmyer, M.A. Reticulated Nanoporous Polymers by Controlled Polymerization-Induced Microphase Separation. Science 2012, 336. [Google Scholar] [CrossRef]
- Williams, R.J.J.; Hoppe, C.E.; Zucchi, I.A.; Romeo, H.E.; Dell’Erba, I.E.; Gómez, M.L.; Puig, J.; Leonardi, A.B. Reprint of: Self-Assembly of Nanoparticles Employing Polymerization-Induced Phase Separation. J. Colloid Interface Sci. 2014, 447, 129–138. [Google Scholar] [CrossRef]
- Duran, H.; Meng, S.; Kim, N.; Hu, J.; Kyu, T.; Natarajan, L.V.; Tondiglia, V.P.; Bunning, T.J. Kinetics of Photopolymerization-Induced Phase Separation and Morphology Development in Mixtures of a Nematic Liquid Crystal and Multifunctional Acrylate. Polymer 2008, 49, 534–545. [Google Scholar] [CrossRef]
- Caneba, G.T.; Soong, D.S. Polymer Membrane Formation through the Thermal-Inversion Process. 1. Experimental Study of Membrane Structure Formation. Macromolecules 1985, 18, 2538–2545. [Google Scholar] [CrossRef]
- Caneba, G.T.; Soong, D.S. Polymer Membrane Formation through the Thermal-Inversion Process. 2. Mathematical Modeling of Membrane Structure Formation. Macromolecules 1985, 18, 2545–2555. [Google Scholar] [CrossRef]
- Matsuyama, H.; Berghmans, S.; Lloyd, D.R. Formation of Anisotropic Membranes via Thermally Induced Phase Separation. Polymer 1999, 40, 2289–2301. [Google Scholar] [CrossRef]
- Matsuyama, H.; Yuasa, M.; Kitamura, Y.; Teramoto, M.; Lloyd, D.R. Structure Control of Anisotropic and Asymmetric Polypropylene Membrane Prepared by Thermally Induced Phase Separation. J. Membr. Sci. 2000, 179, 91–100. [Google Scholar] [CrossRef]
- Natarajan, L.V.; Shepherd, C.K.; Brandelik, D.M.; Sutherland, R.L.; Chandra, S.; Tondiglia, V.P.; Tomlin, D.; Bunning, T.J. Switchable Holographic Polymer-Dispersed Liquid Crystal Reflection Gratings Based on Thiol-Ene Photopolymerization. Chem. Mater. 2003, 15, 2477–2484. [Google Scholar] [CrossRef]
- Wagner, A.J.; Yeomans, J.M. Phase Separation under Shear in Two-Dimensional Binary Fluids. Phys. Rev. E 1999, 59, 4366. [Google Scholar] [CrossRef]
- Wirtz, D.; Fuller, G.G. Phase Transitions Induced by Electric Fields in Near-Critical Polymer Solutions Denis. Phys. Rev. Lett. 1993, 71, 2236–2239. [Google Scholar] [CrossRef]
- Venugopal, G.; Krause, S. Development of Phase Morphologies of Poly(Methyl Methacrylate)-Polystyrene-Toluene Mixtures in Electric Fields. Macromolecules 1992, 25, 4626–4634. [Google Scholar] [CrossRef]
- Vaynzof, Y.; Kabra, D.; Zhao, L.; Chua, L.L.; Steiner, U.; Friend, R.H. Surface-Directed Spinodal Decomposition in Poly[3-Hexylthiophene] and C61-Butyric Acid Methyl Ester Blends. ACS Nano 2011, 5, 329–336. [Google Scholar] [CrossRef]
- Jamie, E.A.G.; Dullens, R.P.A.; Aarts, D.G.A.L. Spinodal Decomposition of a Confined Colloid-Polymer System. J. Chem. Phys. 2012, 137. [Google Scholar] [CrossRef]
- Tabatabaieyazdi, M.; Chan, P.K.; Wu, J. A Computational Study of Short-Range Surface-Directed Phase Separation in Polymer Blends under a Linear Temperature Gradient. Chem. Eng. Sci. 2015, 137, 884–895. [Google Scholar] [CrossRef]
- Tabatabaieyazdi, M.; Chan, P.K.; Wu, J. A Computational Study of Long Range Surface-Directed Phase Separation in Polymer Blends under a Temperature Gradient. Comput. Mater. Sci. 2016, 111, 387–394. [Google Scholar] [CrossRef]
- Tabatabaieyazdi, M.; Chan, P.K.; Wu, J. A Computational Study of Multiple Surface-Directed Phase Separation in Polymer Blends under a Temperature Gradient. Model. Simul. Mater. Sci. Eng. 2015, 23, 387–394. [Google Scholar] [CrossRef]
- Tran-Cong, Q.; Kawai, J.; Nishikawa, Y.; Jinnai, H. Phase Separation with Multiple Length Scales in Polymer Mixtures Induced by Autocatalytic Reactions. Phys. Rev. E 1999, 60, 1150–1153. [Google Scholar] [CrossRef]
- Jaiswal, P.K.; Binder, K.; Puri, S. Phase Separation of Binary Mixtures in Thin Films: Effects of an Initial Concentration Gradient across the Film. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2012, 85, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mino, Y.; Ishigami, T.; Kagawa, Y.; Matsuyama, H. Three-Dimensional Phase-Field Simulations of Membrane Porous Structure Formation by Thermally Induced Phase Separation in Polymer Solutions. J. Membr. Sci. 2015, 483, 104–111. [Google Scholar] [CrossRef]
- Tran-cong, Q.; Okinaka, J. Polymer Blends With Spatially Graded Structures Prepared by Phase Separation Under a Temperature Gradient. Polym. Eng. Sci. 1999, 39, 365–374. [Google Scholar] [CrossRef]
- Lee, K.W.D.; Chan, P.K.; Feng, X. A Computational Study into Thermally Induced Phase Separation in Polymer Solutions under a Temperature Gradient. Macromol. Theory Simul. 2002, 11, 996–1005. [Google Scholar] [CrossRef]
- Lee, K.W.D.; Chan, P.K.; Feng, X. Morphology Development and Characterization of the Phase-Separated Structure Resulting from the Thermal-Induced Phase Separation Phenomenon in Polymer Solutions under a Temperature Gradient. Chem. Eng. Sci. 2004, 59, 1491–1504. [Google Scholar] [CrossRef]
- Chan, P.K. Effect of Concentration Gradient on the Thermal-Induced Phase Separation Phenomenon in Polymer Solutions. Model. Simul. Mater. Sci. Eng. 2006, 14, 41–51. [Google Scholar] [CrossRef]
- Jiang, B.T.; Chan, P.K. Effect of Concentration Gradient on the Morphology Development in Polymer Solutions Undergoing Thermally Induced Phase Separation. Macromol. Theory Simul. 2007, 16, 690–702. [Google Scholar] [CrossRef]
- Hong, S.; Chan, P.K. Simultaneous Use of Temperature and Concentration Gradients to Control Polymer Solution Morphology Development during Thermal-Induced Phase Separation. Model. Simul. Mater. Sci. Eng. 2010, 18, 1–12. [Google Scholar] [CrossRef]
- Oh, J.; Rey, A.D. Computational Simulation of Polymerization-Induced Phase Separation under a Temperature Gradient. Comput. Polym. Sci. 2001, 11, 205–217. [Google Scholar] [CrossRef]
- Lee, K.W.D.; Chan, P.K.; Feng, X. A Computational Study of the Polymerization-Induced Phase Separation Phenomenon in Polymer Solutions under a Temperature Gradient. Macromol. Theory Simul. 2003, 12, 413–424. [Google Scholar] [CrossRef]
- Norisuye, T.; Fujiki, D.; Tran-Cong-Miyata, Q.; Van-Pham, D.-T.; Jing, C.; Nakanishi, H. Polymer Materials with Spatially Graded Morphologies: Preparation, Characterization and Utilization. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 1, 043003. [Google Scholar] [CrossRef]
- Cahn, J.W. Phase Separation by Spinodal Decomposition in Isotropic Systems. J. Chem. Phys. 1965, 42, 93–99. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University: Ithaca, NY, USA, 1953. [Google Scholar]
- Kurata, M. Thermodynamics of Polymer Solutions; Harwood Academic Publisher: Chur, Switzerland; London, UK; New York, NY, USA, 1982. [Google Scholar]
- Chan, P.K.; Rey, A.D. Computational Analysis of Spinodal Decomposition Dynamics in Polymer Solutions. Macromol. Theory Simul. 1995, 4, 873–899. [Google Scholar] [CrossRef]
- Chan, P.K.; Rey, A.D. A Numerical Method for the Nonlinear Cahn-Hilliard Equation with Nonperiodic Boundary Conditions. Comput. Mater. Sci. 1995, 3, 377–392. [Google Scholar] [CrossRef]
- Chan, P.K. Comparison of Mobility Modes in Polymer Solutions Undergoing Thermal-Induced Phase Separation. In MRS Online Proceedings Library Archive; 2001; Volume 710. [Google Scholar] [CrossRef]
- Chen, J.P.; Lee, Y.D. A Real-Time Study of the Phase-Separation Process during Polymerization of Rubber-Modified Epoxy. Polymer 1995, 36, 55–65. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ, U. S. National Institutes of Health, Bethesda, MD, USA, 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 20 June 2019).
- Kurzydlowski, K.J.; Ralph, B. The Quantitative Description of the Microstructure of Materials; CRC Press: New York, NY, USA, 1995. [Google Scholar]
- Puri, S.; Binder, K.; Dattagupta, S. Dynamical Scaling in Anisotropic Phase-Separating Systems in a Gravitational Field. Phys. Rev. B 1992, 46, 98–105. [Google Scholar] [CrossRef] [PubMed]
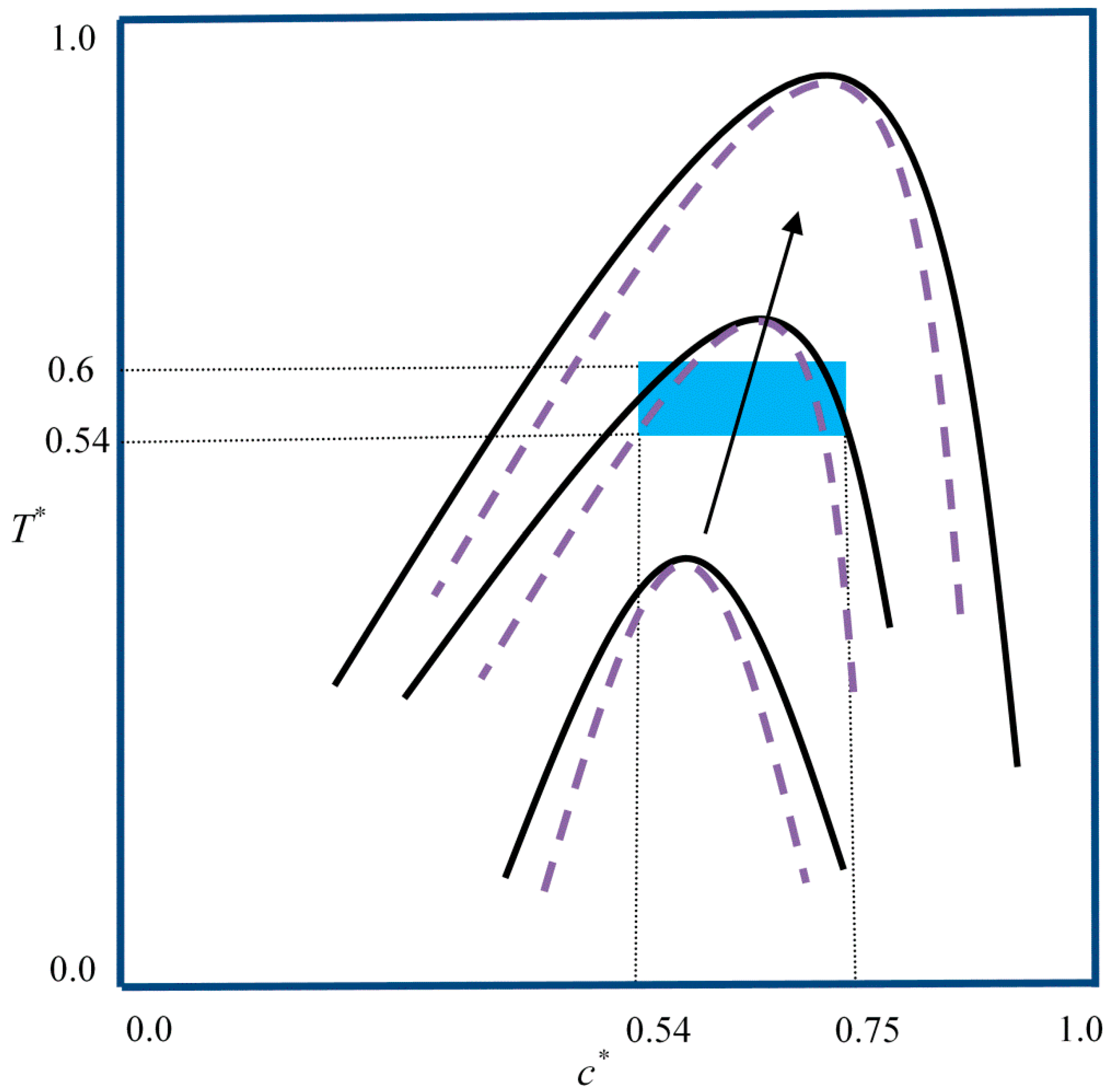
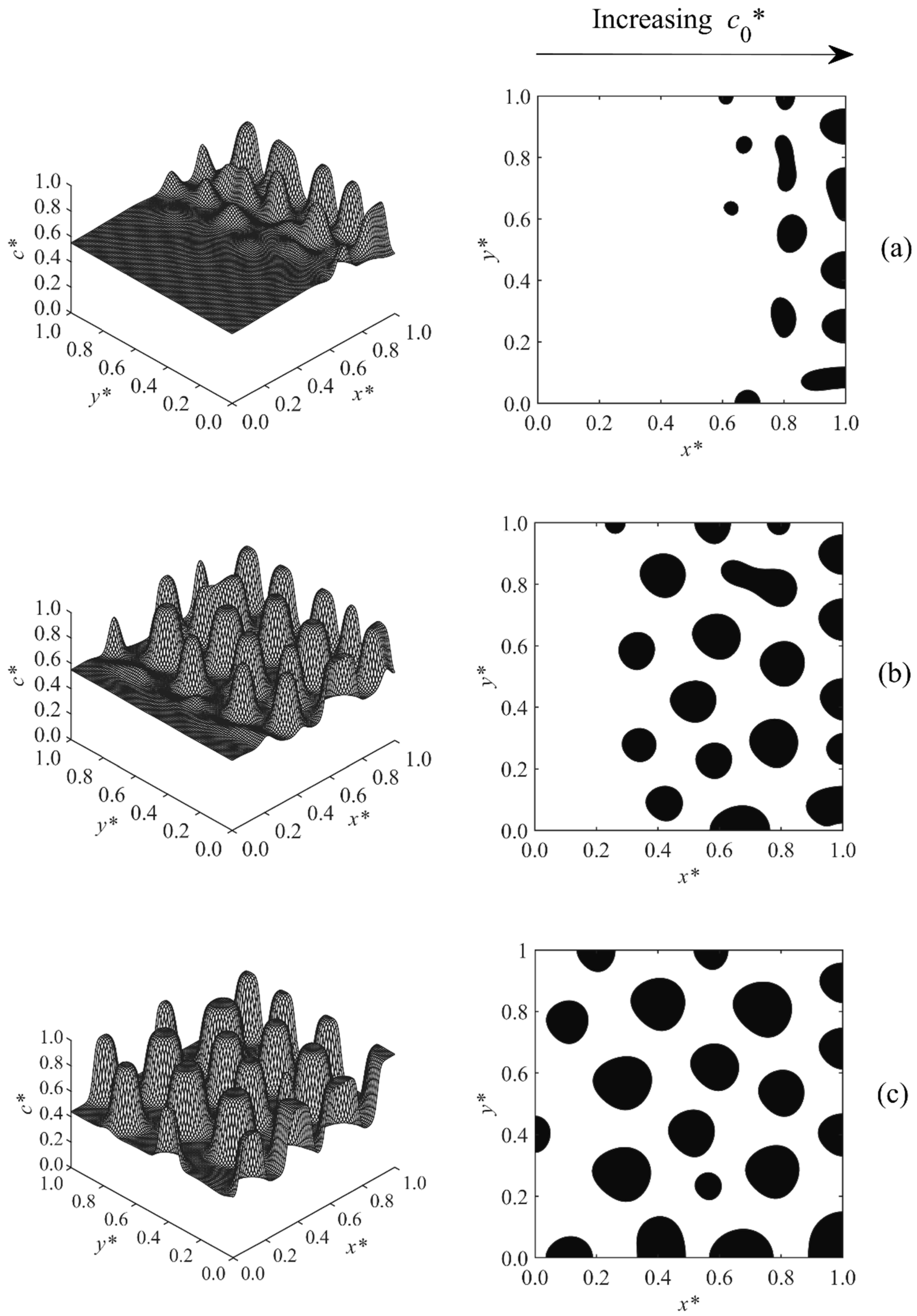



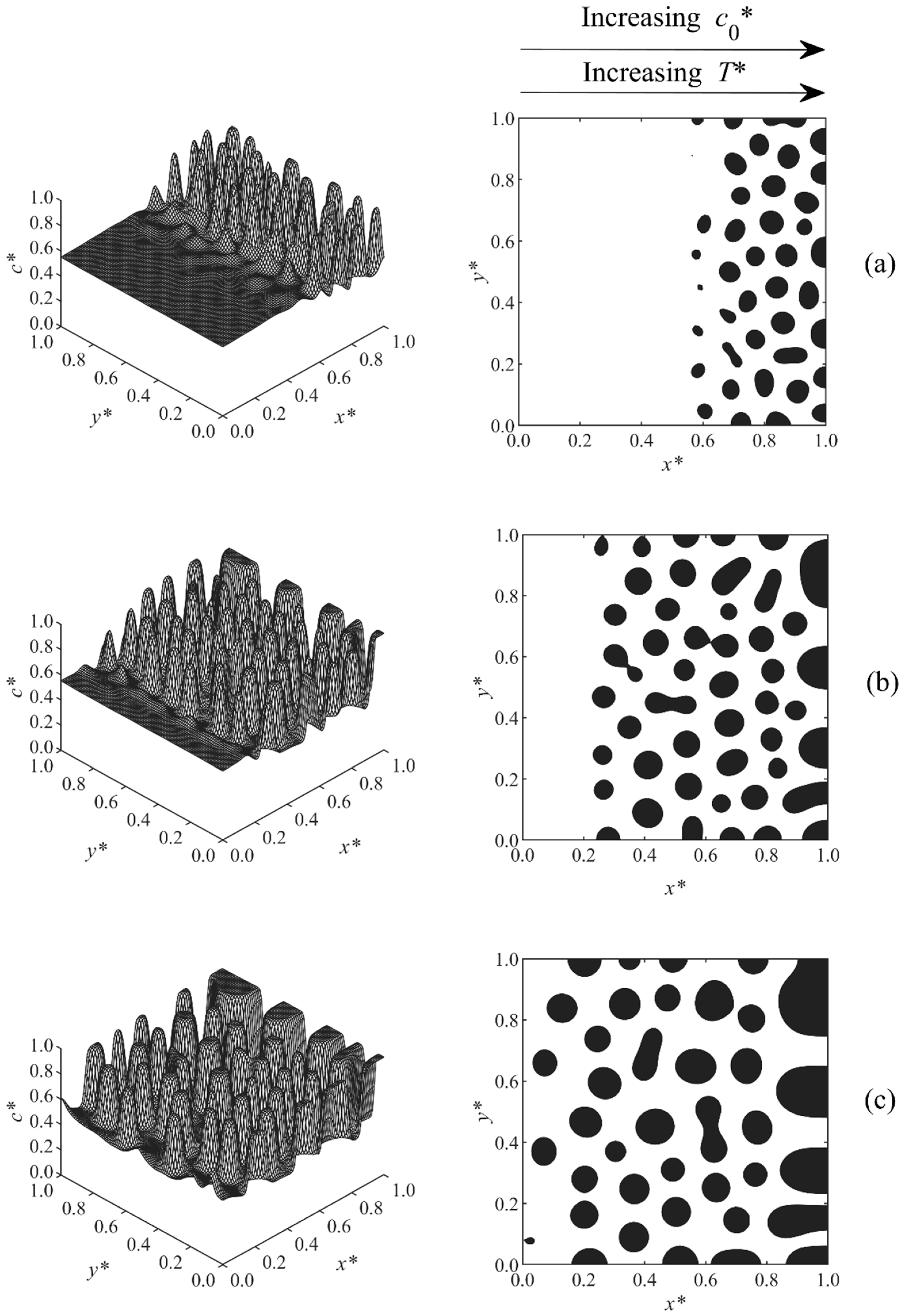
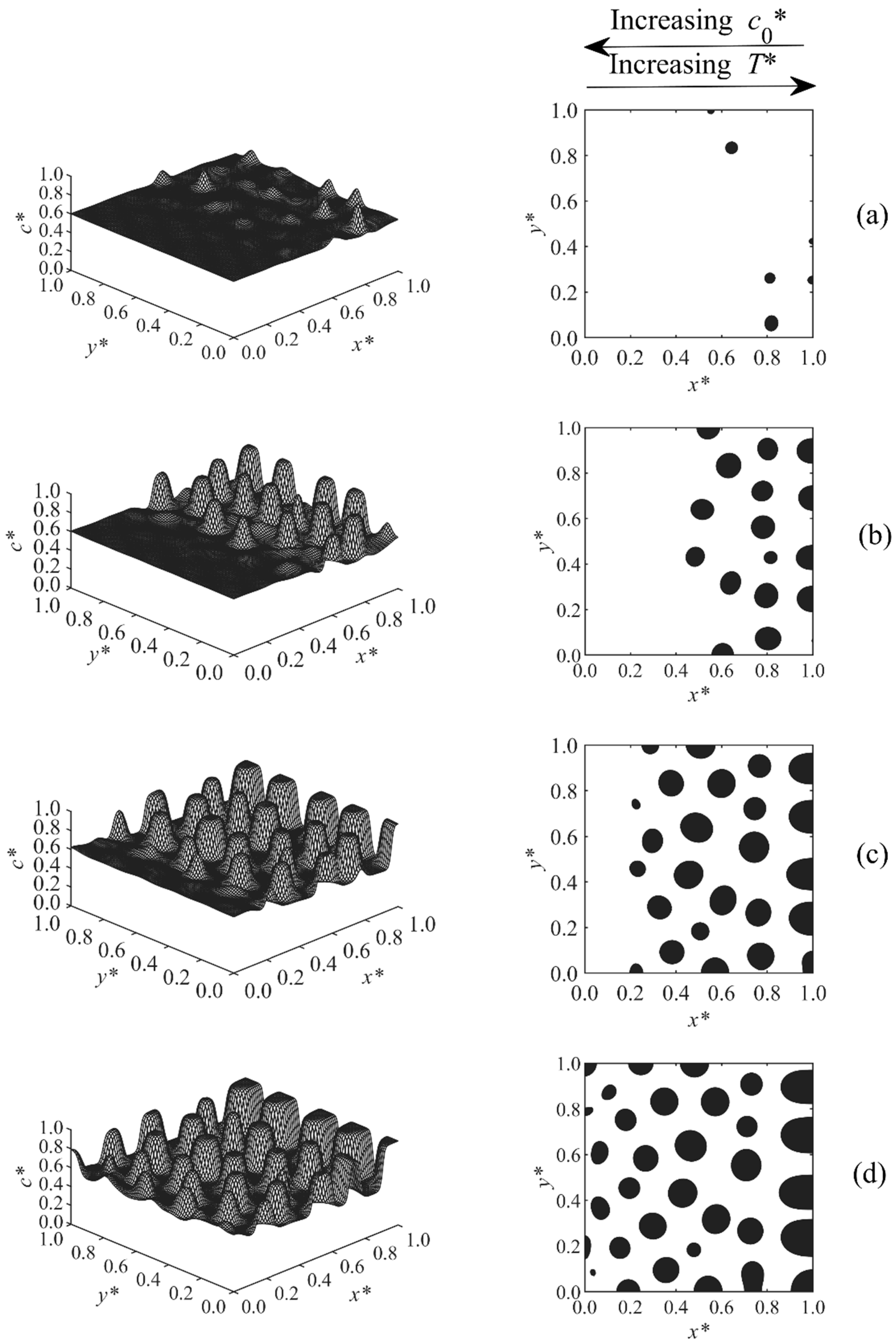


| Case | Condition | T* | co* | D | A* |
|---|---|---|---|---|---|
| Case 1 | • Δco* • Left to the critical region | 0.6 | 0.55–0.6 | 2 × 105 | 5 × 1010 |
| Case 2 | • Δco* • Across the critical region | 0.6 | 0.6–0.7 | 4 × 105 | 1011 |
| Case 3 | • Δco* • Right to the critical region | 0.6 | 0.7–0.75 | 4 × 105 | 1011 |
| Case 4 | • ΔT* • Left to the critical region | 0.54–0.55 | 0.55 | 4 × 105 | 1011 |
| Case 5 | • ΔT*+ Δco* • Same Direction • Left to the critical region | 0.595–0.6 | 0.55–0.6 | 4 × 105 | 2 × 1011 |
| Case 6 | • ΔT*+ Δco* • Opposite directions • Left to the critical region | 0.595–0.6 | 0.56–0.6 | 4 × 105 | 1011 |
| Case 7 | • ΔT*+ Δco* • Opposite directions • Left to the critical region | 0.595–0.6 | 0.54–0.6 | 4 × 105 | 2 × 1011 |
| Case 8 | • ΔT*+ Δco* • Opposite directions • Left to the critical region | 0.595–0.6 | 0.552–0.6 | 4 × 105 | 2 × 1011 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaffari, S.; Chan, P.K.; Mehrvar, M. Computer Simulation of Anisotropic Polymeric Materials Using Polymerization-Induced Phase Separation under Combined Temperature and Concentration Gradients. Polymers 2019, 11, 1076. https://doi.org/10.3390/polym11061076
Ghaffari S, Chan PK, Mehrvar M. Computer Simulation of Anisotropic Polymeric Materials Using Polymerization-Induced Phase Separation under Combined Temperature and Concentration Gradients. Polymers. 2019; 11(6):1076. https://doi.org/10.3390/polym11061076
Chicago/Turabian StyleGhaffari, Shima, Philip K. Chan, and Mehrab Mehrvar. 2019. "Computer Simulation of Anisotropic Polymeric Materials Using Polymerization-Induced Phase Separation under Combined Temperature and Concentration Gradients" Polymers 11, no. 6: 1076. https://doi.org/10.3390/polym11061076
APA StyleGhaffari, S., Chan, P. K., & Mehrvar, M. (2019). Computer Simulation of Anisotropic Polymeric Materials Using Polymerization-Induced Phase Separation under Combined Temperature and Concentration Gradients. Polymers, 11(6), 1076. https://doi.org/10.3390/polym11061076






