A Protein-Based Material from a New Approach Using Whole Defatted Larvae, and Its Interaction with Moisture
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Density and Extraction in Liquids
2.3. Infrared (IR) Spectroscopy
2.4. Thermogravimetry and Calorimetry
2.5. Dynamic Vapor Sorption (DVS)
3. Results and Discussion
3.1. Larva Constituents
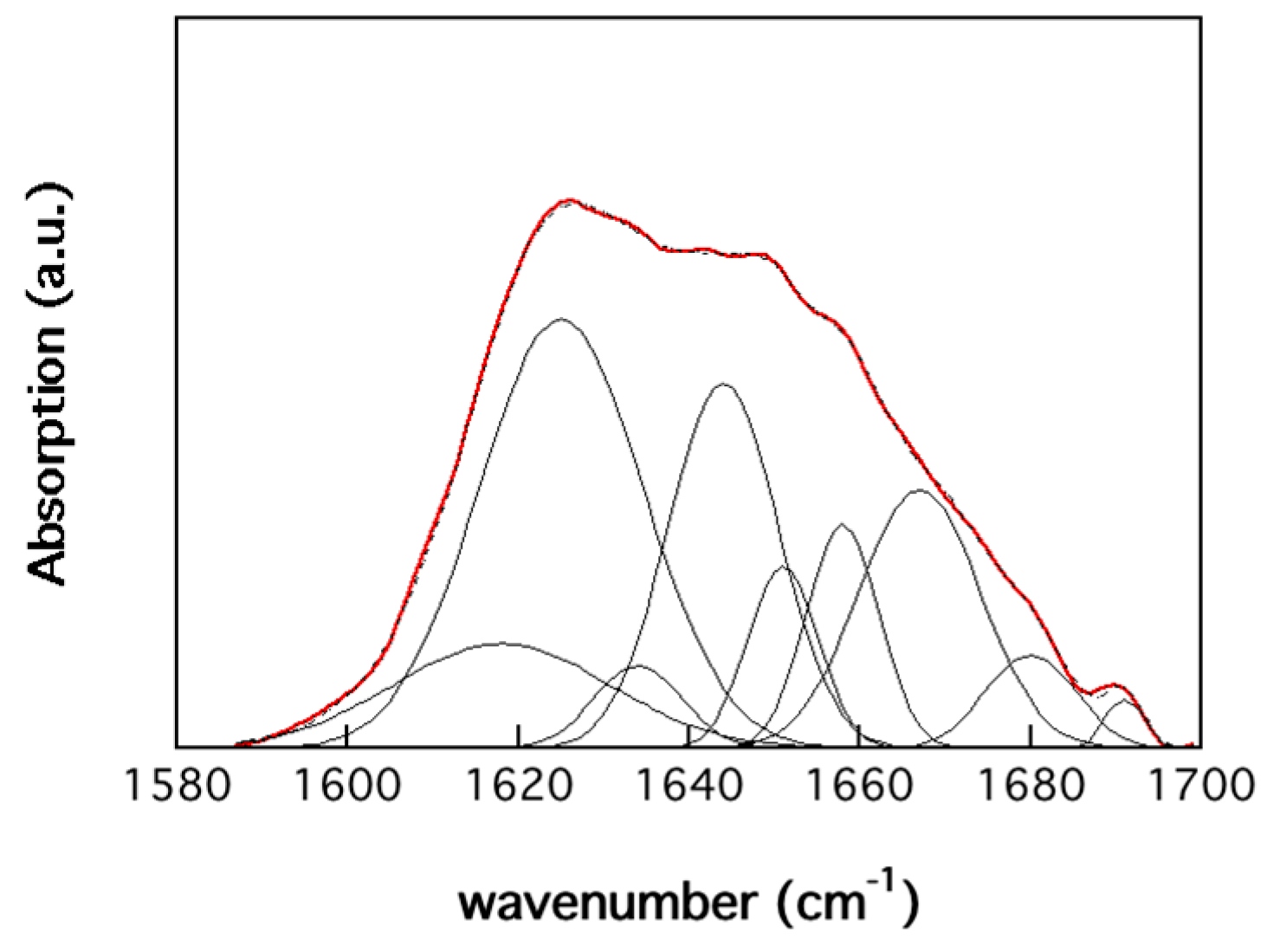
3.2. Protein Structure
3.3. Thermogravimetry and Calorimetry
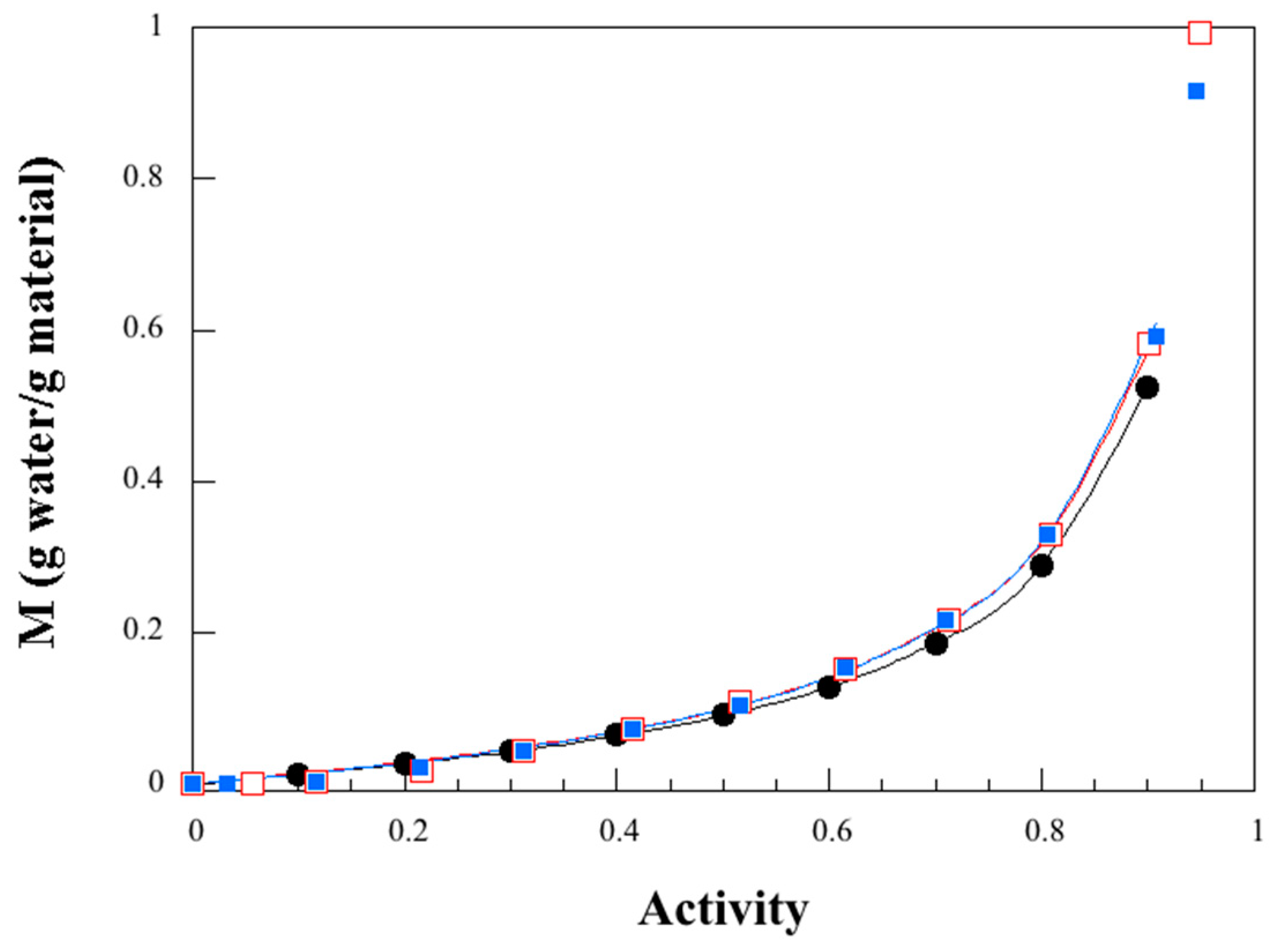
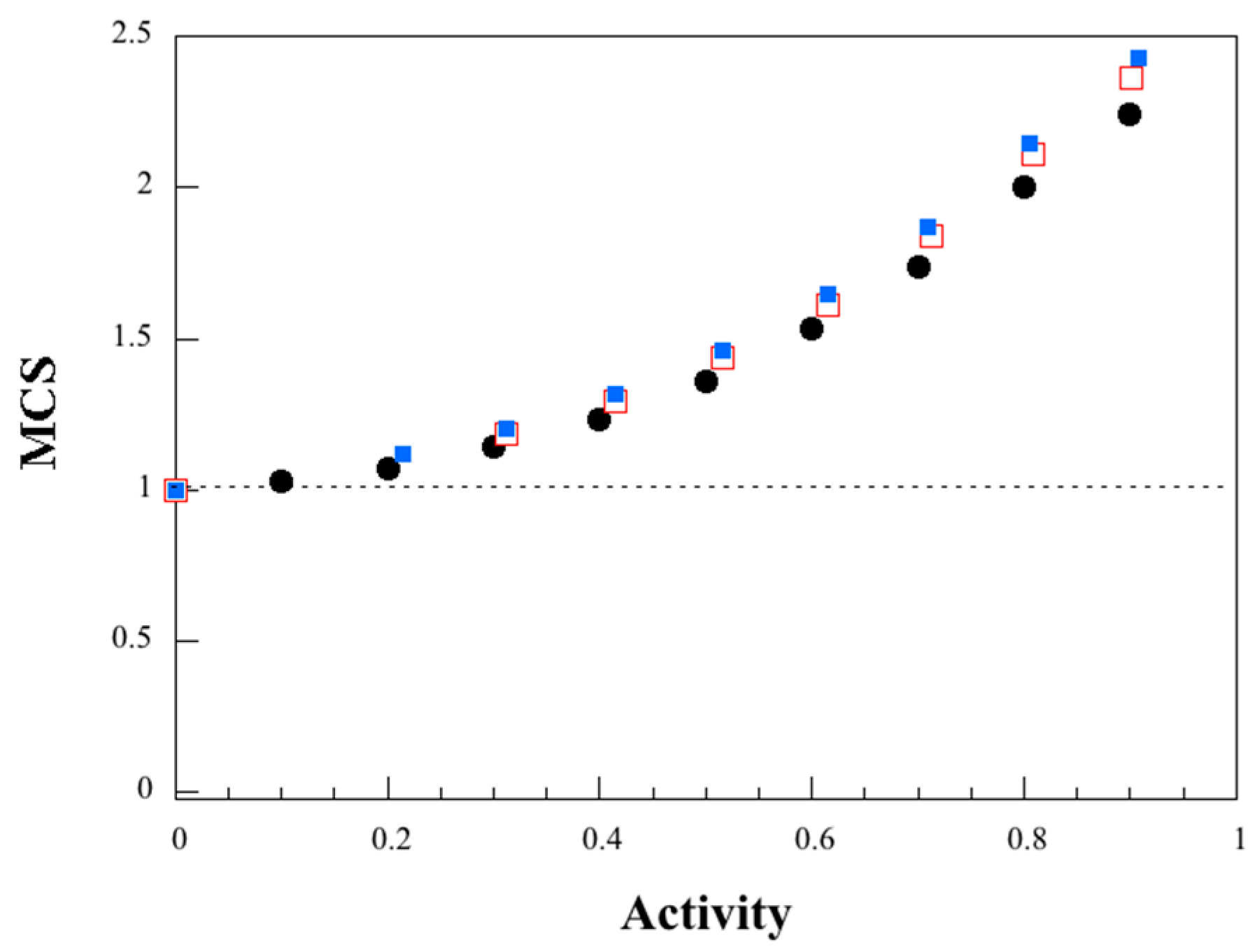
3.4. Dynamic Water Vapor Sorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- May 2017 Was Second Warmest May on Record. Available online: https://data.giss.nasa.gov/gistemp/news/20170615/ (accessed on 7 February 2019).
- Posen, I.D.; Jaramillo, P.; Landis, A.E.; Griffin, W.M. Greenhouse gas mitigation for U.S. Plastics production: Energy first, feedstocks later. Environ. Res. Lett. 2017, 12, 034024. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Fernandes, E.G.; Kenawy, E.-R.S.; Lazzeri, A. Gelatin-based blends and composites. Morphological and thermal mechanical characterization. Biomacromolecules 2001, 2, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Gennadios, A. Protein-Based Films and Coatings; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Baldwin, A.D.; Kiick, K.L. Polysaccharide-modified synthetic polymeric biomaterials. Peptide Sci. 2010, 94, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Čičková, H.; Newton, G.L.; Lacy, R.C.; Kozánek, M. The use of fly larvae for organic waste treatment. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Stamer, A. Insect proteins—A new source for animal feed. EMBO Rep. 2015, 16, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Waśko, A.; Bulak, P.; Polak-Berecka, M.; Nowak, K.; Polakowski, C.; Bieganowski, A. The first report of the physicochemical structure of chitin isolated from hermetia illucens. Int. J. Biol. Macromol. 2016, 92, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Diener, S.; Zurbrügg, C.; Roa-Gutiérrez, F.; Hong Dang, N.; Morel, A.; Koottatep, T.; Tockner, K. Black soldier fly larvae for organic waste treatment—Prospects and constraints. In Proceedings of the International Conference on Solid Waste Management in Developing Countries, Khulna, Bangladesh, 13–15 February 2011. [Google Scholar]
- Diener, S.; Studt Solano, N.M.; Roa Gutiérrez, F.; Zurbrügg, C.; Tockner, K. Biological treatment of municipal organic waste using black soldier fly larvae. Waste Biomass Valoriz. 2011, 2, 357–363. [Google Scholar] [CrossRef]
- Lalander, C.H.; Fidjeland, J.; Diener, S.; Eriksson, S.; Vinnerås, B. High waste-to-biomass conversion and efficient salmonella spp. Reduction using black soldier fly for waste recycling. Agron. Sustain. Develop. 2015, 35, 261–271. [Google Scholar] [CrossRef]
- Dortmans, B. Valorisation of Organic Waste—Effect of the Feeding Regime on Process Parameters in a Continuous Black Soldier Fly Larvae Composting System. Master’s Thesis, SLU, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2015. [Google Scholar]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.K.; Irving, S.; Newton, L.; McGuire, M.A.; Mosley, E.E.; Hardy, R.W.; Sealey, W. Fly prepupae as a feedstuff for rainbow trout, oncorhynchus mykiss. J. World Aquacult. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Surendra, K.C.; Olivier, R.; Tomberlin, J.K.; Jha, R.; Khanal, S.K. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renewable Energy 2016, 98, 197–202. [Google Scholar] [CrossRef]
- Kroeckel, S.; Harjes, A.G.E.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the black soldier fly (hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (psetta maxima). Aquaculture 2012, 364–365, 345–352. [Google Scholar] [CrossRef]
- Al-Qazzaz, M.F.A.; Ismail, D.; Akit, H.; Idris, L.H. Effect of using insect larvae meal as a complete protein source on quality and productivity characteristics of laying hens. Rev. Brasil. Zootec. 2016, 45, 518–523. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Bosch, G.; Vervoort, J.J.M.; Hendriks, W.H. In vitro digestibility and fermentability of selected insects for dog foods. Anim. Feed Sci. Technol. 2016, 221, 174–184. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Bioaccumulation of heavy metals in the black soldier fly, Hermetia illucens and effects on its life cycle. J. Insects Food Feed 2015, 1, 261–270. [Google Scholar] [CrossRef]
- Lalander, C.; Senecal, J.; Gros Calvo, M.; Ahrens, L.; Josefsson, S.; Wiberg, K.; Vinnerås, B. Fate of pharmaceuticals and pesticides in fly larvae composting. Sci. Total Environ. 2016, 565, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-I.; Chang, B.S.; Yoe, S.M. Detection of antimicrobial substances from larvae of the black soldier fly, hermetia illucens (diptera: Stratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Park, K.H.; Kwak, K.W.; Nam, S.H.; Choi, J.Y.; Lee, S.H.; Kim, H.G.; Kim, S.H. Antibacterial activity of larval extract from the black soldier fly hermetia illucens (diptera: Stratiomyidae) against plant pathogens. J. Entomol. Zool. Stud. 2015, 3, 176–179. [Google Scholar]
- Wang, H.; Li, H.; Gilbert, J.A.; Li, H.; Wu, L.; Liu, M.; Wang, L.; Zhou, Q.; Yuan, J.; Zhang, Z. Housefly larva vermicomposting efficiently attenuates antibiotic resistance genes in swine manure, with concomitant bacterial population changes. Appl. Environ. Microbiol. 2015, 81, 7668–7679. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Available online: https://www.plasticseurope.org (accessed on 7 February 2019).
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, hermetiaillucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Zheng, L.; Hou, Y.; Li, W.; Yang, S.; Li, Q.; Yu, Z. Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 2012, 47, 225–229. [Google Scholar] [CrossRef]
- Yu, S.; Chen, F.; Wu, Q.; Roth, S.V.; Brüning, K.; Schneider, K.; Kuktaite, R.; Hedenqvist, M.S. Structural changes of gluten/glycerol plastics under dry and moist conditions and during tensile tests. ACS Sustain. Chem. Eng. 2016, 4, 3388–3397. [Google Scholar] [CrossRef]
- Ullsten, H.; Gällstedt, M.; Hedenqvist, M. Plasticizers for protein-based materials. In Viscoelastic and Viscoplastic Materials; El-Amin, M., Ed.; IntechOpen: London, UK, 2016; pp. 81–101. [Google Scholar]
- Choi, S.G.; Kim, K.M.; Hanna, M.A.; Weller, C.L.; Kerr, W.L. Molecular dynamics of soy-protein isolate films plasticized by water and glycerol. J. Food Sci. 2006, 68, 2516–2522. [Google Scholar] [CrossRef]
- Bonnaillie, M.L.; Zhang, H.; Akkurt, S.; Yam, L.K.; Tomasula, M.P. Casein films: The effects of formulation, environmental conditions and the addition of citric pectin on the structure and mechanical properties. Polymers 2014, 6, 2018–2036. [Google Scholar] [CrossRef]
- Javanmard, M.; Golestan, L. Effect of olive oil and glycerol on physical properties of whey protein concentrate films. J. Food Process Eng. 2008, 31, 628–639. [Google Scholar] [CrossRef]
- Johansson, E.; Spencer, G.M.; Bettini, E.; Cho, S.-W.; Marttila, S.; Kuktaite, R.; Gällstedt, M.; Hedenqvist, M.S. Biobased materials production from biodiesel residuals of rapeseed. ISRN Mater. Sci. 2012, 2012, 6. [Google Scholar] [CrossRef]
- Lu, S.; Wang, X.; Lu, Q.; Zhang, X.; Kluge, J.A.; Uppal, N.; Omenetto, F.; Kaplan, D.L. Insoluble and flexible silk films containing glycerol. Biomacromolecules 2010, 11, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Thomazine, M.; Carvalho, R.A.; Sobral, P.J.A. Physical properties of gelatin films plasticized by blends of glycerol and sorbitol. J. Food Sci. 2006, 70, E172–E176. [Google Scholar] [CrossRef]
- Xu, H.; Chai, Y.; Zhang, G. Synergistic effect of oleic acid and glycerol on zein film plasticization. J. Agric. Food Chem. 2012, 60, 10075–10081. [Google Scholar] [CrossRef]
- Gällstedt, M.; Mattozzi, A.; Johansson, E.; Hedenqvist, M.S. Transport and tensile properties of compression-molded wheat gluten films. Biomacromolecules 2004, 5, 2020–2028. [Google Scholar] [CrossRef]
- Lagrain, B.; Goderis, B.; Brijs, K.; Delcour, J.A. Molecular basis of processing wheat gluten toward biobased materials. Biomacromolecules 2010, 11. [Google Scholar] [CrossRef] [PubMed]
- Newson, W.R.; Kuktaite, R.; Hedenqvist, M.S.; Gällstedt, M.; Johansson, E. Oilseed meal based plastics from plasticized, hot pressed crambe abyssinica and brassica carinata residuals. J. Am. Oil Chem. Soc. 2013, 90, 1229–1237. [Google Scholar] [CrossRef]
- Cho, S.W.; Gallstedt, M.; Johansson, E.; Hedenqvist, M.S. Injection-molded nanocomposites and materials based on wheat gluten. Int. J. Biol. Macromol. 2011, 48, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Türe, H.; Blomfeldt, T.O.J.; Gällstedt, M.; Hedenqvist, M.S.; Farris, S. Nanostructured silica/wheat gluten hybrid materials prepared by catalytic sol–gel chemistry. Macromol. Chem. Phys. 2013, 214, 1131–1139. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbo, R.; Torstensen, B.E.; Lock, E.J.A. Modulation of nutrient composition of black soldier fly (hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient content of four species of feeder insects. Zoo Biol. 2013, 32, 27–36. [Google Scholar] [CrossRef]
- Khalfaoui, M.; Knani, S.; Hachicha, M.A.; Lamine, A.B. New theoretical expressions for the five adsorption type isotherms classified by bet based on statistical physics treatment. J. Colloid Interface Sci. 2003, 263, 350–356. [Google Scholar] [CrossRef]
- Vopička, O.; Friess, K. Analysis of gas sorption in glassy polymers with the gab model: An alternative to the dual mode sorption model. J. Polym. Sci., Part B 2014, 52, 1490–1495. [Google Scholar] [CrossRef]
- Zeppa, C.; Gouanvé, F.; Espuche, E. Effect of a plasticizer on the structure of biodegradable starch/clay nanocomposites: Thermal, water-sorption, and oxygen-barrier properties. J. Appl. Polym. Sci. 2009, 112, 2044–2056. [Google Scholar] [CrossRef]
- Timmermann, E.O. Multilayer sorption parameters: Bet or gab values? Colloids Surf. Physicochem. Eng. Aspects 2003, 220, 235–260. [Google Scholar] [CrossRef]
- Angellier-Coussy, H.; Gastaldi, E.; Gontard, N.; Guillard, V. Influence of processing temperature on the water vapour transport properties of wheat gluten based agromaterials. Ind. Crops Prod. 2011, 33, 457–461. [Google Scholar] [CrossRef]
- Zimm, B.H.; Lundberg, J.L. Sorption of vapors by high polymers. J. Phys. Chem. 1956, 60, 425–428. [Google Scholar] [CrossRef]
- Mali, S.; Sakanaka, L.S.; Yamashita, F.; Grossmann, M.V.E. Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect. Carbohydr. Polym. 2005, 60, 283–289. [Google Scholar] [CrossRef]
- Roy, S.; Gennadios, A.; Weller, C.L.; Testin, R.F. Water vapor transport parameters of a cast wheat gluten film. Ind. Crops Prod. 2000, 11, 43–50. [Google Scholar] [CrossRef]
- Masclaux, C.; Gouanvé, F.; Espuche, E. Experimental and modelling studies of transport in starch nanocomposite films as affected by relative humidity. J. Membr. Sci. 2010, 363, 221–231. [Google Scholar] [CrossRef]
- Hedenqvist, M.S.; Gedde, U.W. Parameters affecting the determination of transport kinetics data in highly swelling polymers above tg. Polymer 1999, 40, 2381–2393. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; Taylor and Francis: Hoboken, NJ, USA, 2012. [Google Scholar]





| Essential Amino Acids (wt.%) | Non-Essential Amino Acids (wt.%) | ||
|---|---|---|---|
| Arginine | 5.6 ± 0.2 | Alanine | 6.3 ± 0.1 |
| Histidine | 3.7 ± 0.3 | Asparagine/Aspartic acid | 10.3 ± 0.3 |
| Isoleucine | 5.1 ± 0.2 | Cystine/cysteine | 0.6 ± 0 |
| Leucine | 7.6 ± 0.2 | Glutamine/Glutamic acid | 11.1 ± 0.1 |
| Lysine | 7.1 ± 0.1 | Glycine | 6.3 ± 0.1 |
| Methionine | 2.0 ± 0.1 | Proline | 5.6 ± 0.1 |
| Phenyl alanine | 4.2 ± 0.5 | Serine | 4.5 ± 0.2 |
| Threonine | 4.2 ± 0.2 | Tyrosine | 8.9 ± 1.1 |
| Valine | 6.9 ± 0.2 |
| Present a | Reference b | |
|---|---|---|
| Capric acid (C10:0) | 1 | - |
| Lauric (C12:0) | 48 | 45 |
| Myristic (C14:0) | 7 | 8 |
| Palmitic (C16:0) | 14 | 14 |
| Stearic (C18:0) | 2 | 2 |
| Palmitoeic (C16:1 n-7) | 2 | 2 |
| Oleic (C18:1 n-9) | 13 | 12 |
| Linoleic (C18:2 n-6) | 8 | 10 |
| α-linolenic (C18:2 n-3) | 2 | 0.1 |
| Saturated fatty acids | 72 | 70 |
| Monounsaturated fatty acids | 16 | 15 |
| Polyunsaturated fatty acids | 10 | 12.5 |
| Peak | Position (cm−1) | Size (%) | Origin | Size (%) a |
|---|---|---|---|---|
| 1 | 1618 | 10.8 | β-sheets (strongly bonded) | 37.7 |
| 2 | 1625 | 33.3 | β-sheets (strongly bonded) | 4.5 |
| 3 | 1634 | 3.4 | β-sheets (weakly bonded) | 7.9 |
| 4 | 1644 | 19.0 | unordered | 8.9 |
| 5 | 1651 | 6.0 | α-helices and random coils | 8.3 |
| 6 | 1658 | 7.7 | α-helices | 3.9 |
| 7 | 1667 | 14.8 | β-turns | 22.3 |
| 8 | 1680 | 4.0 | β-sheets (weakly bonded) | 4.2 |
| 9 | 1691 | 0.9 | β-turns | 2.3 |
| Peak | Larva | WG 80 °C a | WG 120 °C a |
|---|---|---|---|
| Mm | 0.083 | 0.095 | 0.096 |
| Cg | 1.47 | 1.359 | 1.31 |
| K | 0.953 | 0.947 | 0.948 |
| MRD (%) | 1.32 | 2.27 | 6.56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alipour, N.; Vinnerås, B.; Gouanvé, F.; Espuche, E.; Hedenqvist, M.S. A Protein-Based Material from a New Approach Using Whole Defatted Larvae, and Its Interaction with Moisture. Polymers 2019, 11, 287. https://doi.org/10.3390/polym11020287
Alipour N, Vinnerås B, Gouanvé F, Espuche E, Hedenqvist MS. A Protein-Based Material from a New Approach Using Whole Defatted Larvae, and Its Interaction with Moisture. Polymers. 2019; 11(2):287. https://doi.org/10.3390/polym11020287
Chicago/Turabian StyleAlipour, Nazanin, Björn Vinnerås, Fabrice Gouanvé, Eliane Espuche, and Mikael S. Hedenqvist. 2019. "A Protein-Based Material from a New Approach Using Whole Defatted Larvae, and Its Interaction with Moisture" Polymers 11, no. 2: 287. https://doi.org/10.3390/polym11020287
APA StyleAlipour, N., Vinnerås, B., Gouanvé, F., Espuche, E., & Hedenqvist, M. S. (2019). A Protein-Based Material from a New Approach Using Whole Defatted Larvae, and Its Interaction with Moisture. Polymers, 11(2), 287. https://doi.org/10.3390/polym11020287








