The Stability of Intercalated Sericite by Cetyl Trimethylammonium Ion under Different Conditions and the Preparation of Sericite/Polymer Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Intercalation Process and the Stability Tests
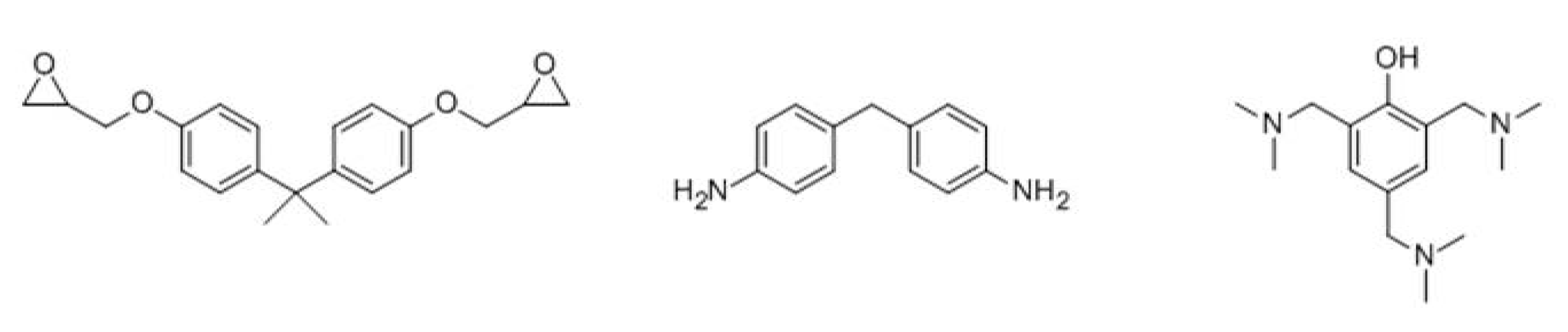
2.3. The Preparation Process of Sericite/Polymer Nanocomposites
2.4. Characterization
3. Results and Discussion
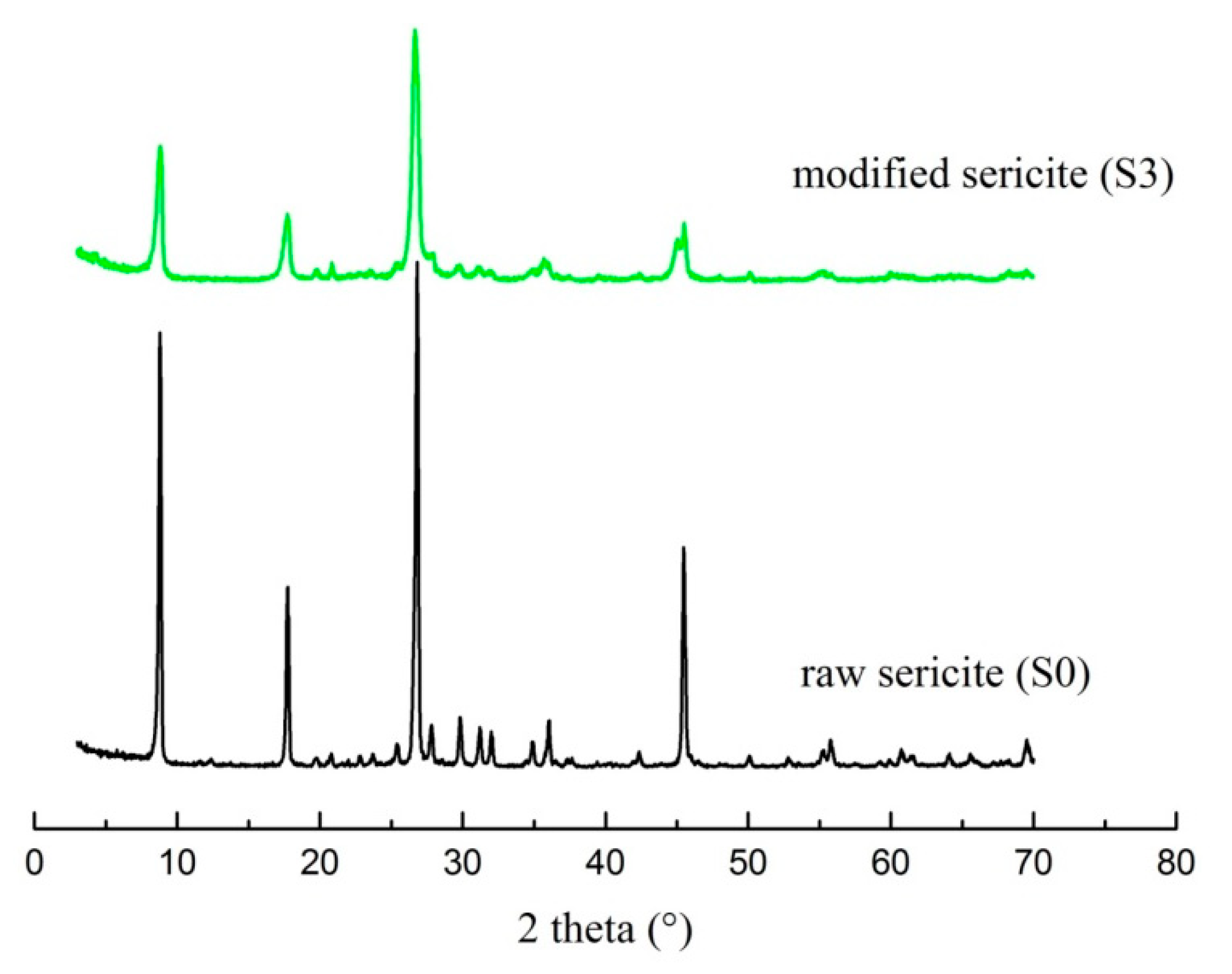
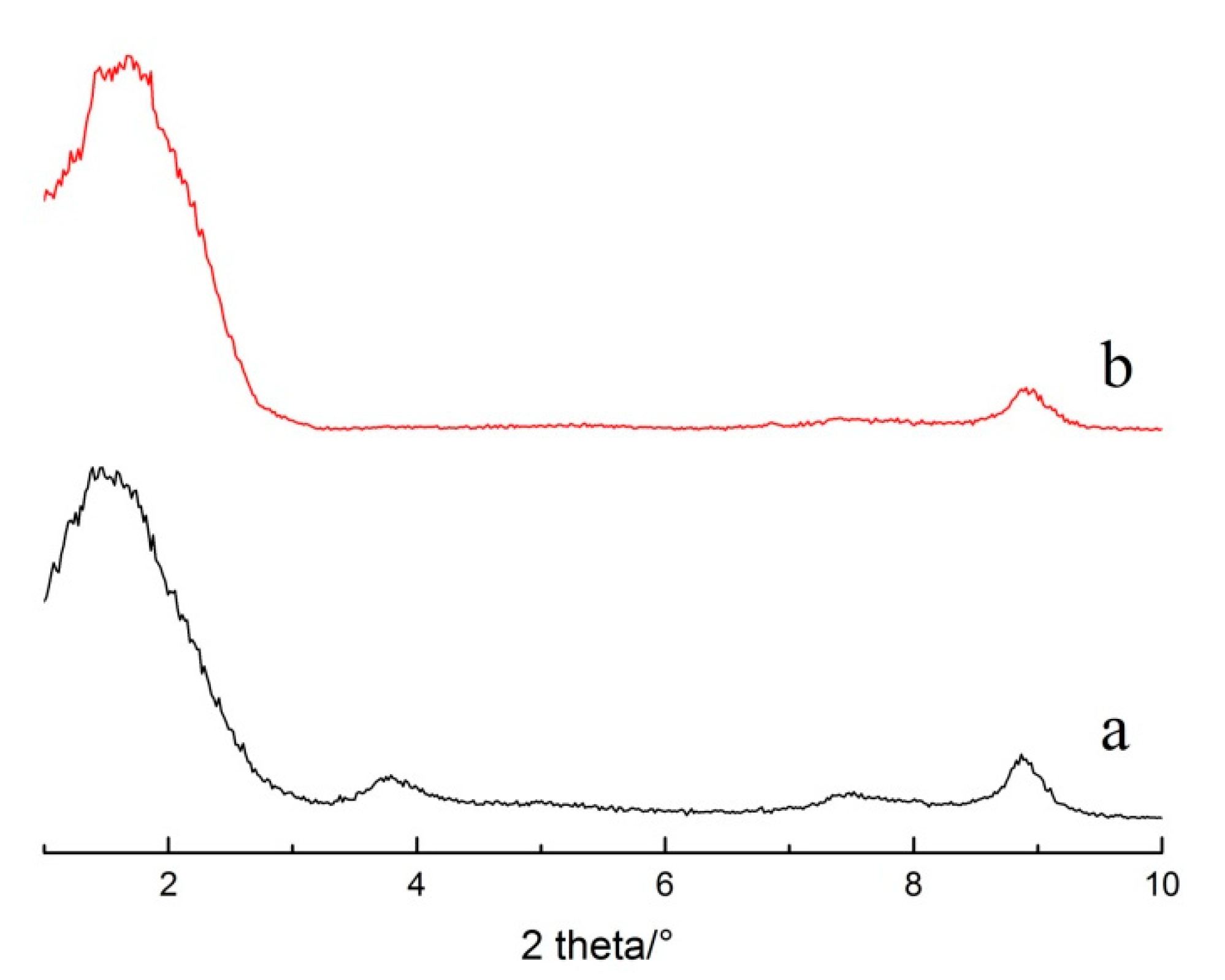
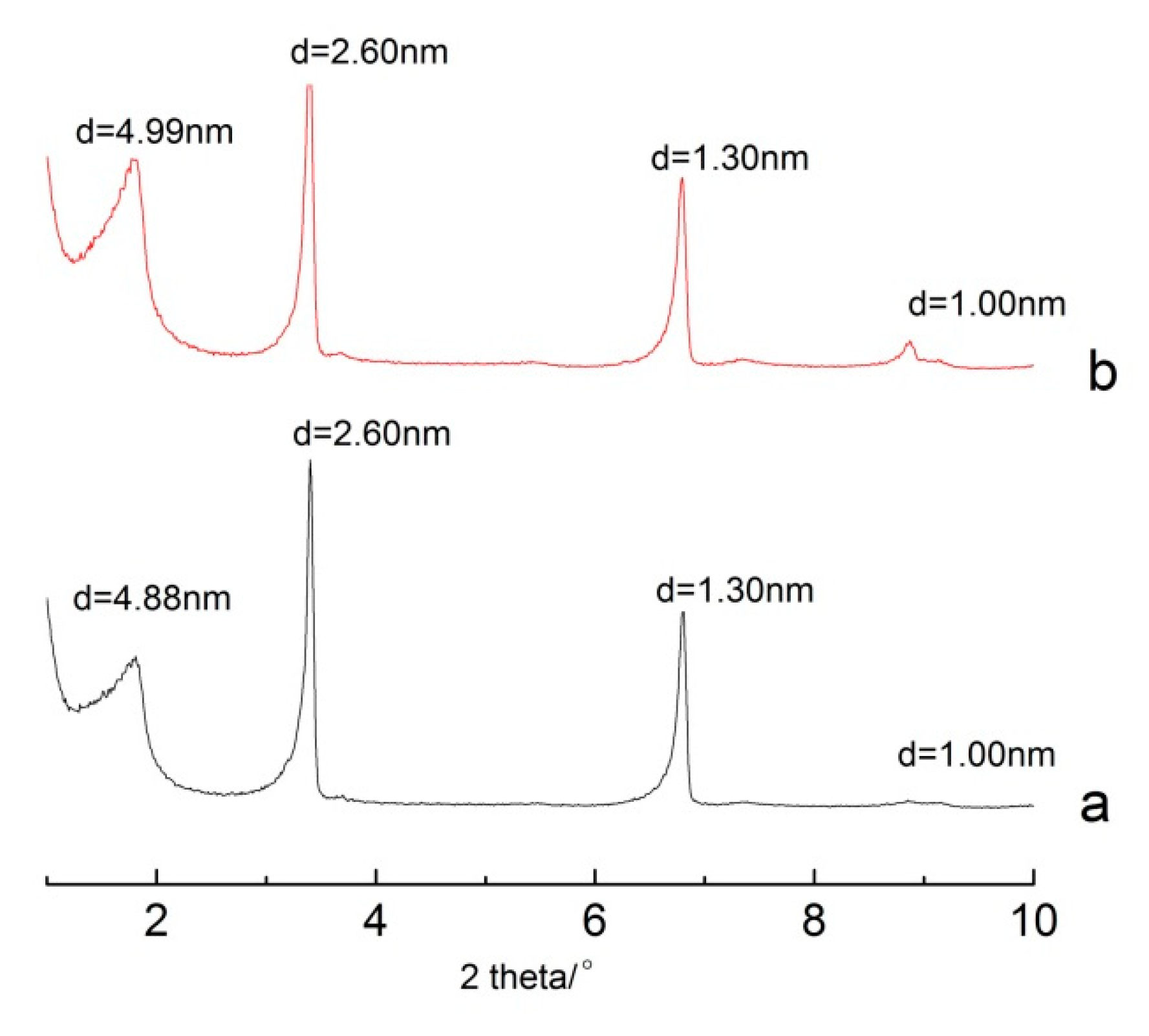
3.1. The Effect of Different Washing Solvents and Temperatures on the Stability of the Intercalated Product
3.2. The Effect of Ultrasonic Cleaning on the Stability of the Intercalated Product
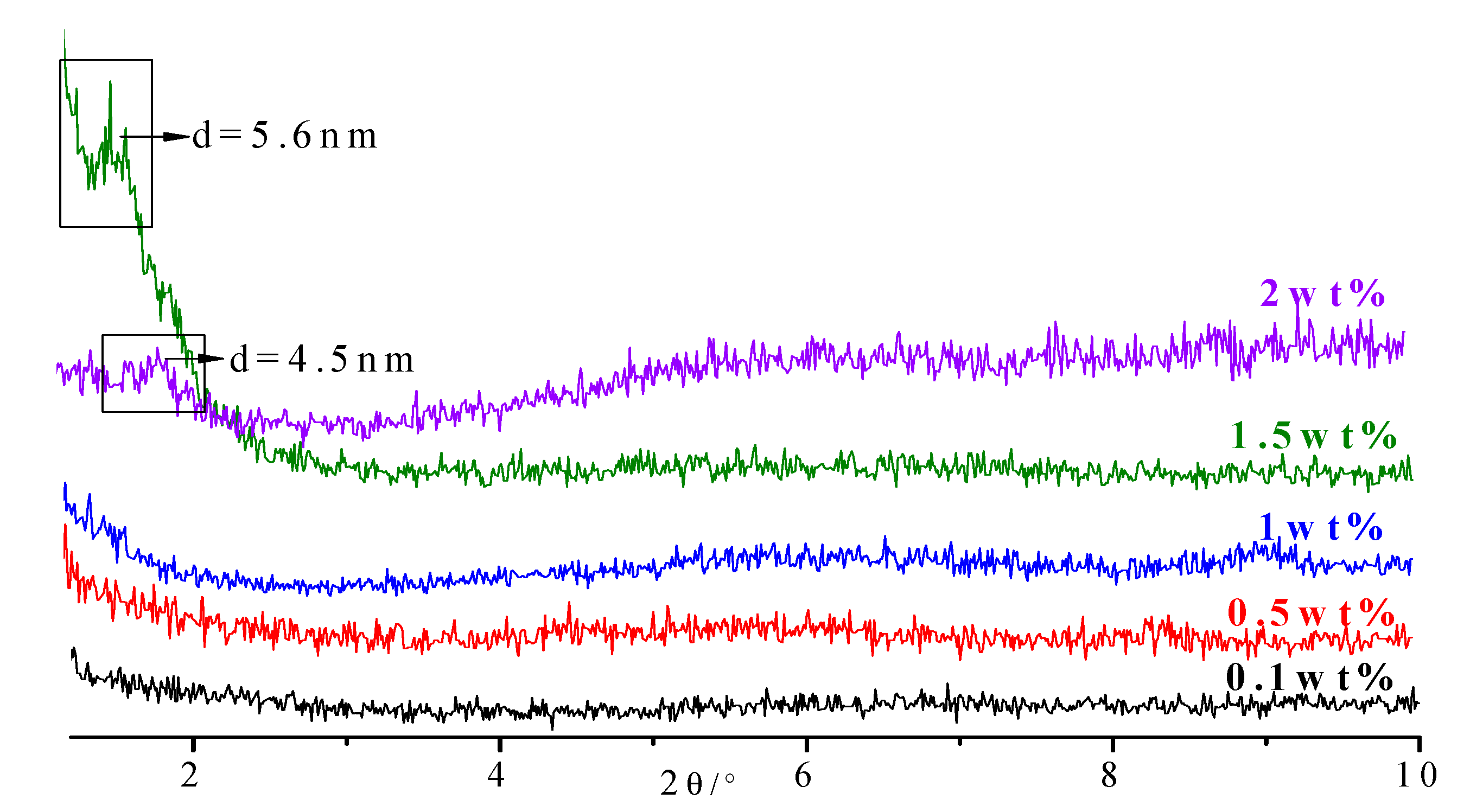
3.3. The Stability of the Intercalated Product in the Solution
3.4. The Choice of Raw Material for the Intercalation Based on Stability, Intercalation Rate, and Other Experimental Conditions
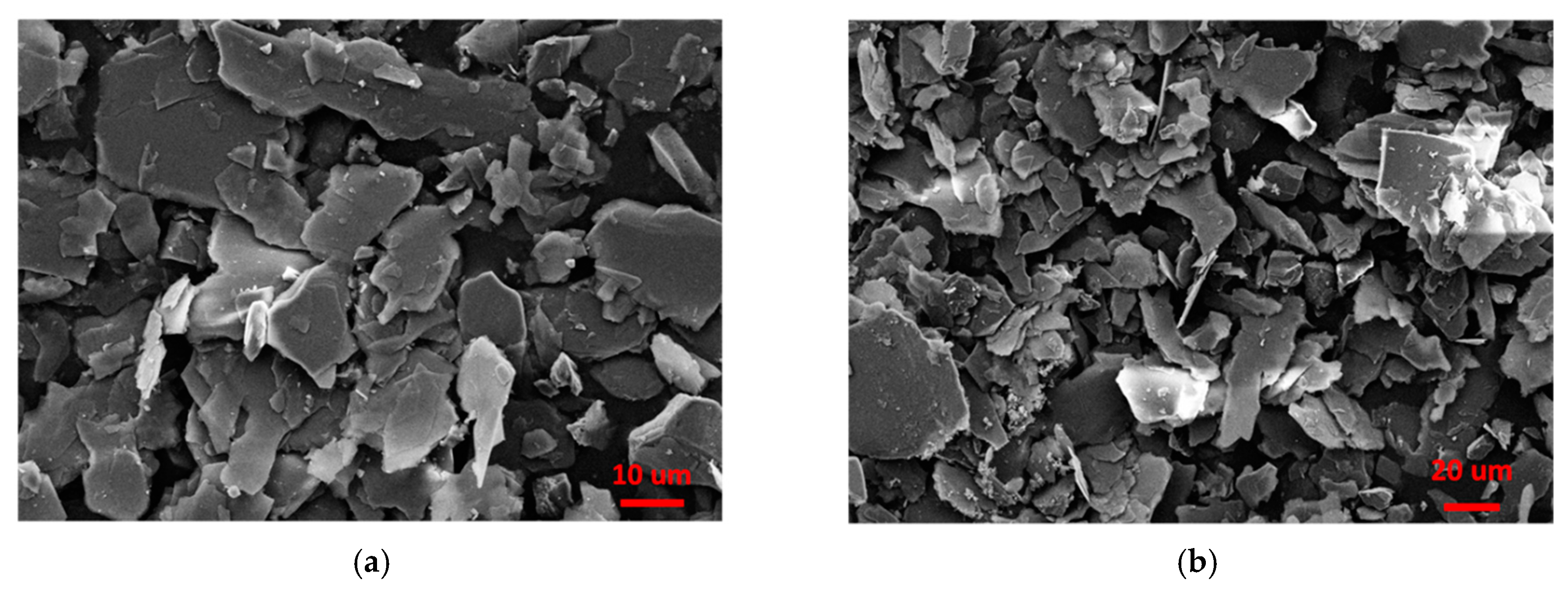
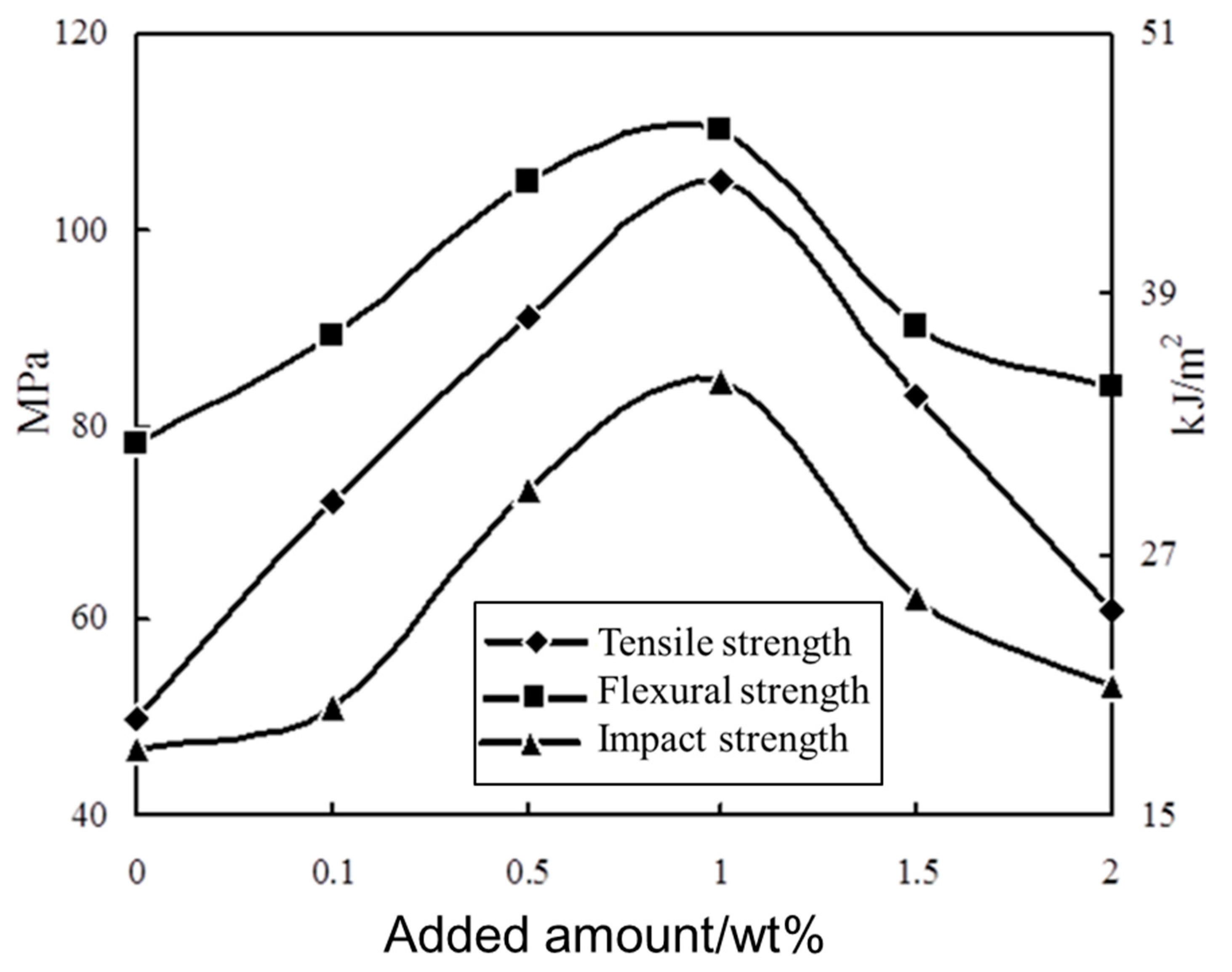
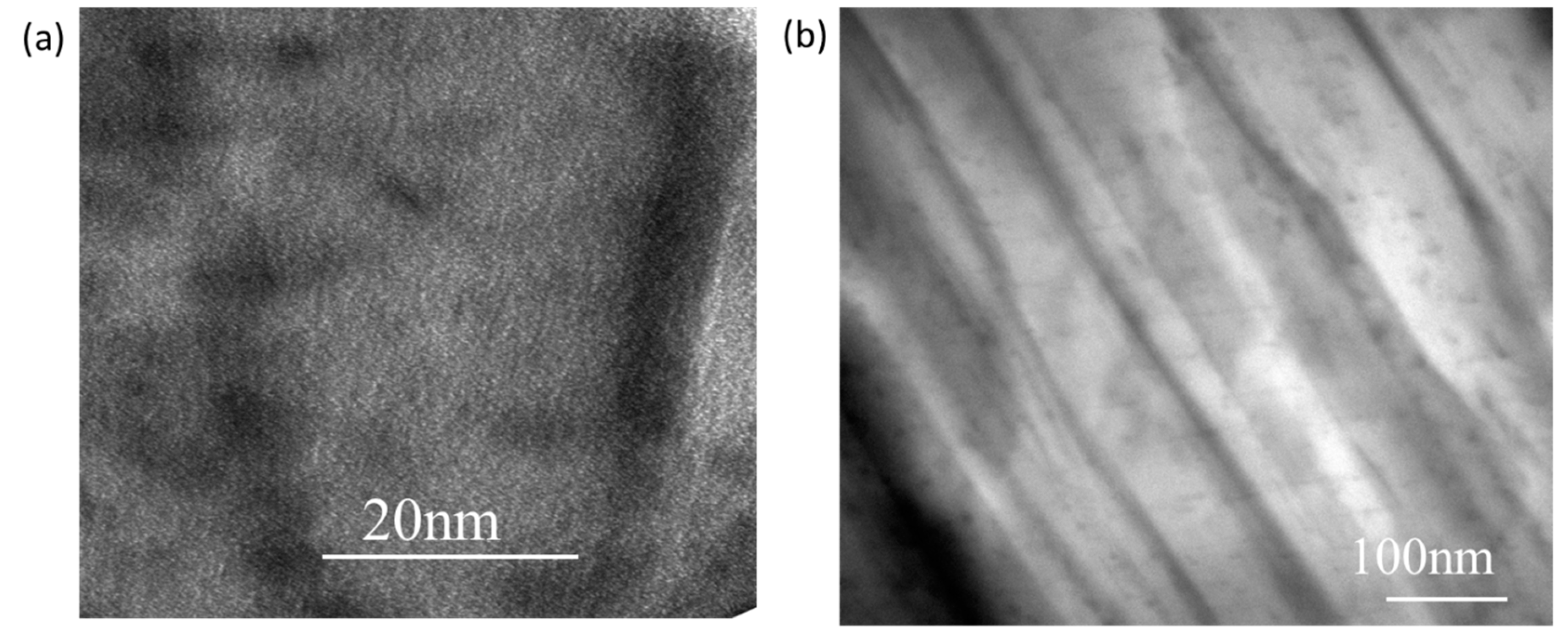
3.5. Properties of the Sericite/Polymer Nanocomposites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ha, C.S. Polymer Based Hybrid Nanocomposites; A Progress Toward Enhancing Interfacial Interaction and Tailoring Advanced Applications. Chem. Rec. 2017, 18, 759–775. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Chen, C.; Xi, J.; Han, Y.; Peng, L.; Gao, W.; Xu, Z.; Gao, C. Ultralight graphene micro-popcorns for multifunctional composite applications. Carbon 2018, 139, 545–555. [Google Scholar] [CrossRef]
- Ahn, C.; Kin, S.-M.; Jung, J.-W.; Park, J.; Kim, T.; Lee, S.; Jang, D.; Hong, J.-W.; Han, S.M.; Jeon, S. Multifunctional polymer nanocomposites reinforced by 3D continuous ceramic nanofillers. ACS Nano 2018, 12, 9126–9133. [Google Scholar] [CrossRef] [PubMed]
- Hrachova, J.; Komadel, P.; Chodak, I. Natural rubber nanocomposites with organo-modified bentonite. Clays Clay Miner. 2009, 57, 444–451. [Google Scholar] [CrossRef]
- Tan, X.; Xu, Y.; Cai, N.; Jia, G. Polypropylene/silica nanocomposites prepared by in-situ melt ultrasonication. Polym. Compos. 2009, 30, 835–840. [Google Scholar] [CrossRef]
- Carvalho, D.; Carvalho, J.; Oliveira, S.; Rosa, D. A new approach for flexible PBAT/PLA/CaCO3 film into agriculture. J. Appl. Polym. Sci. 2018, 135, 46660. [Google Scholar]
- Mao, Y.; Shao, C.; Shang, P.; Li, Q.; He, X.; Wu, C. Preparation of high strength PET/PE composites reinforced with continued long glass fibers. Mater. Res. Express 2019, 6, 045303. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Huang, T.-K.; Wang, Y.-C.; Alamani, B.G.; Lin, J.-J. Intercalation strategies in clay/polymer hybrids. Prog. Polym. Sci. 2014, 39, 443–485. [Google Scholar] [CrossRef]
- Ogawa, M.; Kuroda, K. Preparation of Inorganic–Organic Nanocomposites through Intercalation of Organoammonium Ions into Layered Silicates. Bull. Chem. Soc. Jpn. 1997, 70, 2593–2618. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Alateyah, A.I.; Dhakal, H.N.; Zhang, Z.Y. Processing, Properties, and Applications of Polymer Nanocomposites Based on Layer Silicates: A Review. Adv. Polym. Technol. 2013, 32, 21368. [Google Scholar] [CrossRef]
- Jian, X.; Xuebing, W.; Bingyao, D.; Qingsheng, L. Modification of montmorillonite by different surfactants and its use for the preparation of polyphenylene sulfide nanocomposites. High Perform. Polym. 2016, 28, 618–629. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, Y.; Yang, X.; Wang, X. Thermal and mechanical properties of polyurethane/montmorillonite nanocomposites based on a novel reactive modifier. Polym. Degrad. Stab. 2004, 86, 549–555. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, X.; Yu, J.; Xue, L. Effect of expanded vermiculite on microstructures and aging properties of styrene–butadiene–styrene copolymer modified bitumen. Constr. Build. Mater. 2013, 40, 224–230. [Google Scholar] [CrossRef]
- Liang, S.; Li, C.; Dai, L.; Tang, Q.; Cai, X.; Zhen, B.; Xie, X.; Wang, L. Selective modification of kaolinite with vinyltrimethoxysilane for stabilization of Pickering emulsions. Appl. Clay Sci. 2018, 161, 282–289. [Google Scholar] [CrossRef]
- Frost, R.L.; Kristof, J.; Horvath, E.; Kloprogge, J.T. Deintercalation of dimethylsulphoxide intercalated kaolinites: A DTA/TGA and Raman spectroscopic study. Thermochim. Acta 1999, 327, 155–166. [Google Scholar] [CrossRef]
- Tchoumene, R.; Dedzo, G.K.; Ngameni, E. Preparation of Methyl Viologen-Kaolinite Intercalation Compound: Controlled Release and Electrochemical Applications. ACS Appl. Mater. Interfaces 2018, 10, 34534–34542. [Google Scholar] [CrossRef]
- Isci, S.; Isci, Y. Characterization and comparison of thermal & mechanical properties of vermiculite polyvinylbutyral nanocomposites synthesized by solution casting method. Appl. Clay Sci. 2018, 151, 189–193. [Google Scholar]
- Isci, S. Intercalation of vermiculite in presence of surfactants. Appl. Clay Sci. 2017, 146, 7–13. [Google Scholar] [CrossRef]
- Su, X.; Ma, L.; Wei, J.; Zhu, R. Structure and thermal stability of organo-vermiculite. Appl. Clay Sci. 2016, 132, 261–266. [Google Scholar] [CrossRef]
- Wu, N.; Wu, L.; Liao, L.; Lv, G. Organic intercalation of structure modified vermiculite. J. Colloid Interface Sci. 2015, 457, 264–271. [Google Scholar] [CrossRef]
- Hattab, Y.; Benharrats, N. Thermal stability and structural characteristics of PTHF–Mmt organophile nanocomposite. Arab. J. Chem. 2015, 8, 285–292. [Google Scholar] [CrossRef]
- Belhouchat, N.; Zaghouaneboudiaf, H.; Viseras, C. Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads. Appl. Clay Sci. 2017, 135, 9–15. [Google Scholar] [CrossRef]
- Jin, J.; Tan, Y.; Liu, R.; Zheng, J.; Zhang, J. Synergy Effect of Attapulgite, Rubber, and Diatomite on Organic Montmorillonite-Modified Asphalt. J. Mater. Civ. Eng. 2019, 31, 04018388. [Google Scholar] [CrossRef]
- Solomon, M.J.; Almusallam, A.S.; Seefeldt, K.F.; Somwangthanaroj, A.; Varadan, P. Rheology of polypropylene/clay hybrid materials. Macromolecules 2001, 34, 1864–1872. [Google Scholar] [CrossRef]
- Mcnally, T.; Murphy, W.R.; Lew, C.Y.; Turner, R.J.; Brennan, G. Polyamide-12 layered silicate nanocomposites by melt blending. Polymer 2003, 44, 2761–2772. [Google Scholar] [CrossRef]
- Uno, H.; Tamura, K.; Yamada, H.; Umeyama, K.; Hatta, T.; Moriyoshi, Y. Preparation and mechanical properties of exfoliated mica-polyamide 6 nanocomposites using sericite mica. Appl. Clay Sci. 2009, 46, 81–87. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, H.; Wang, Y.; Liang, N.; Wang, G. Intercalation of cetyl trimethylammonium ion into sericite in the solvent of dimethyl sulfoxide. Appl. Clay Sci. 2013, 74, 109–114. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, H.; Sun, S.; Chen, Y. Microstructural Modification and Characterization of Sericite. Materials 2017, 10, 1182. [Google Scholar] [CrossRef]
- Gu, C.; Peng, T.; Sun, H.; Lv, X.; Luo, L. Assembled Structure and Characterization of TiO2/Montmorillonite nanocomposites. J. Synth. Cryst. 2012, 41, 771–778. [Google Scholar]


| S3 | Intercalated S3 | |
|---|---|---|
| Intercalation rate (%) | 98.20 | 94.83 |
| S3 | Intercalated S3 | |
|---|---|---|
| The amount of CTAB | 15 times the CEC of S3 | 30 times the CEC of S3 |
| Experiment time | 24 h | 24 h + drying time + 3 h |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Yang, D.; Yang, T.; Liang, N.; Ding, H. The Stability of Intercalated Sericite by Cetyl Trimethylammonium Ion under Different Conditions and the Preparation of Sericite/Polymer Nanocomposites. Polymers 2019, 11, 900. https://doi.org/10.3390/polym11050900
Liang Y, Yang D, Yang T, Liang N, Ding H. The Stability of Intercalated Sericite by Cetyl Trimethylammonium Ion under Different Conditions and the Preparation of Sericite/Polymer Nanocomposites. Polymers. 2019; 11(5):900. https://doi.org/10.3390/polym11050900
Chicago/Turabian StyleLiang, Yu, Dexin Yang, Tao Yang, Ning Liang, and Hao Ding. 2019. "The Stability of Intercalated Sericite by Cetyl Trimethylammonium Ion under Different Conditions and the Preparation of Sericite/Polymer Nanocomposites" Polymers 11, no. 5: 900. https://doi.org/10.3390/polym11050900
APA StyleLiang, Y., Yang, D., Yang, T., Liang, N., & Ding, H. (2019). The Stability of Intercalated Sericite by Cetyl Trimethylammonium Ion under Different Conditions and the Preparation of Sericite/Polymer Nanocomposites. Polymers, 11(5), 900. https://doi.org/10.3390/polym11050900






