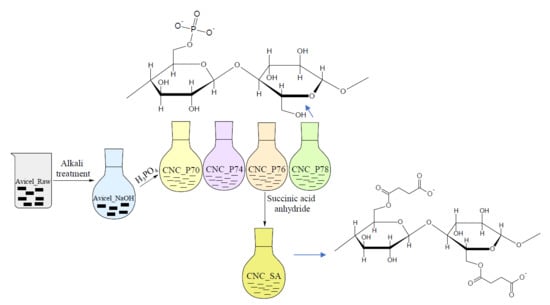
Surface Modification of Cellulose Nanocrystals with Succinic Anhydride
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cellulose Nanocrystals
2.3. Dynamic Laser Scattering (DLS)
2.4. Fourier Transform Infrared (FTIR)
2.5. X-ray Photoelectron Spectroscopy (XPS)
2.6. Wide-Angle X-ray Diffraction (WAXD)
2.7. Scanning Electron Microscopy (SEM)
2.8. Atomic Force Microscopy (AFM)
2.9. Thermogravimetric Analyses (TGA)
3. Results and Discussion
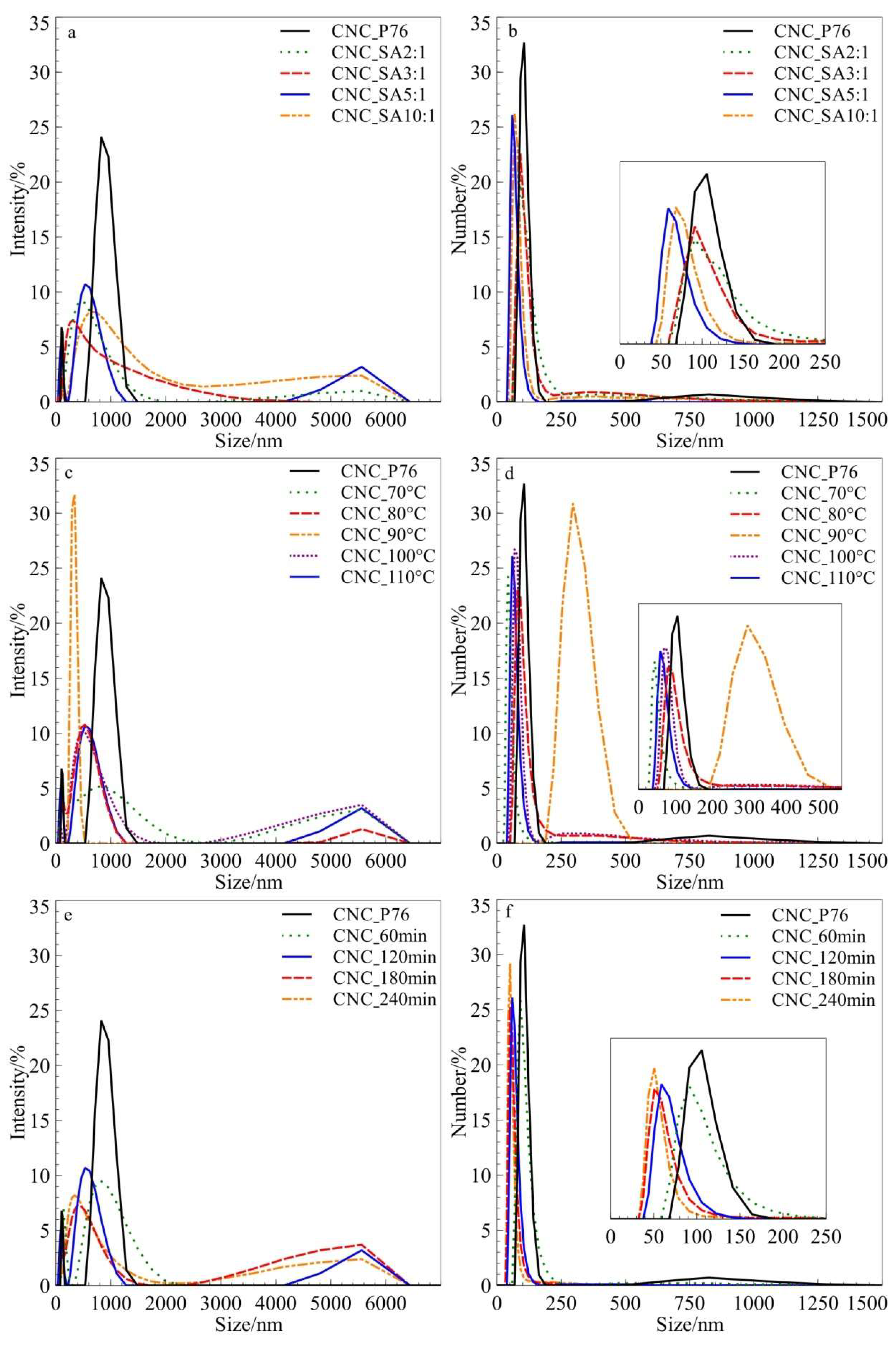
3.1. Particle Size Distribution by DLS Measurements
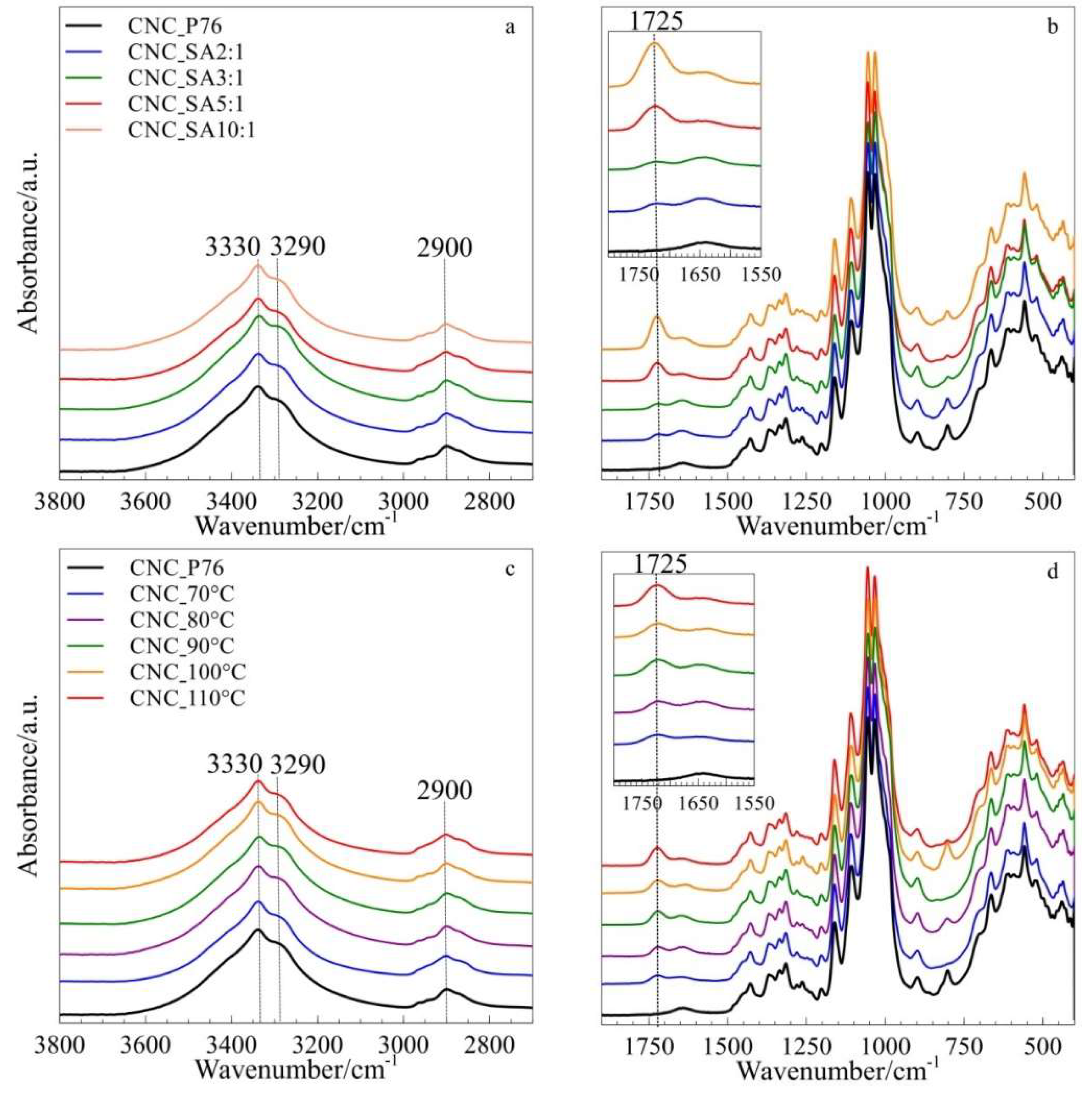
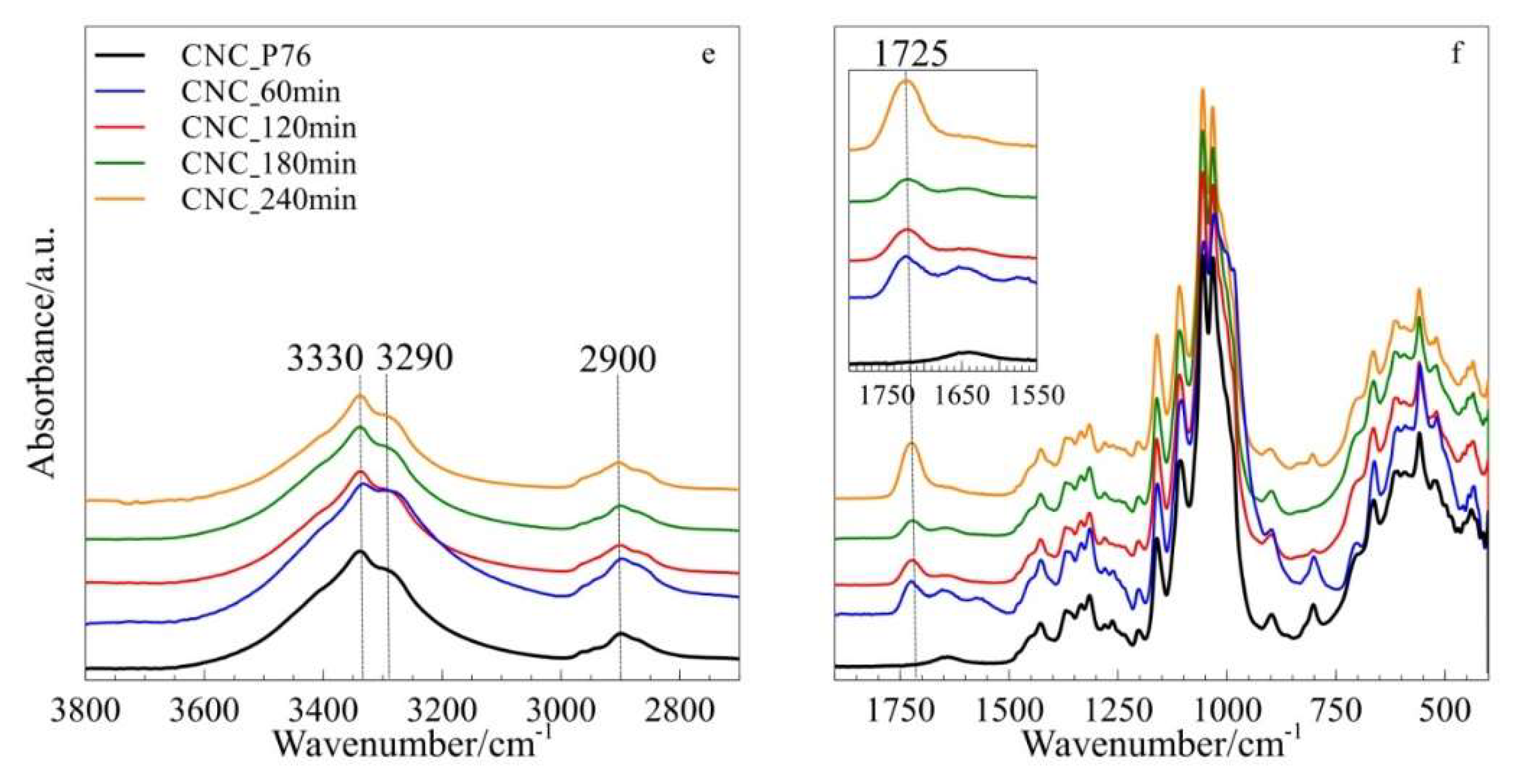
3.2. Structure Analysis by FTIR
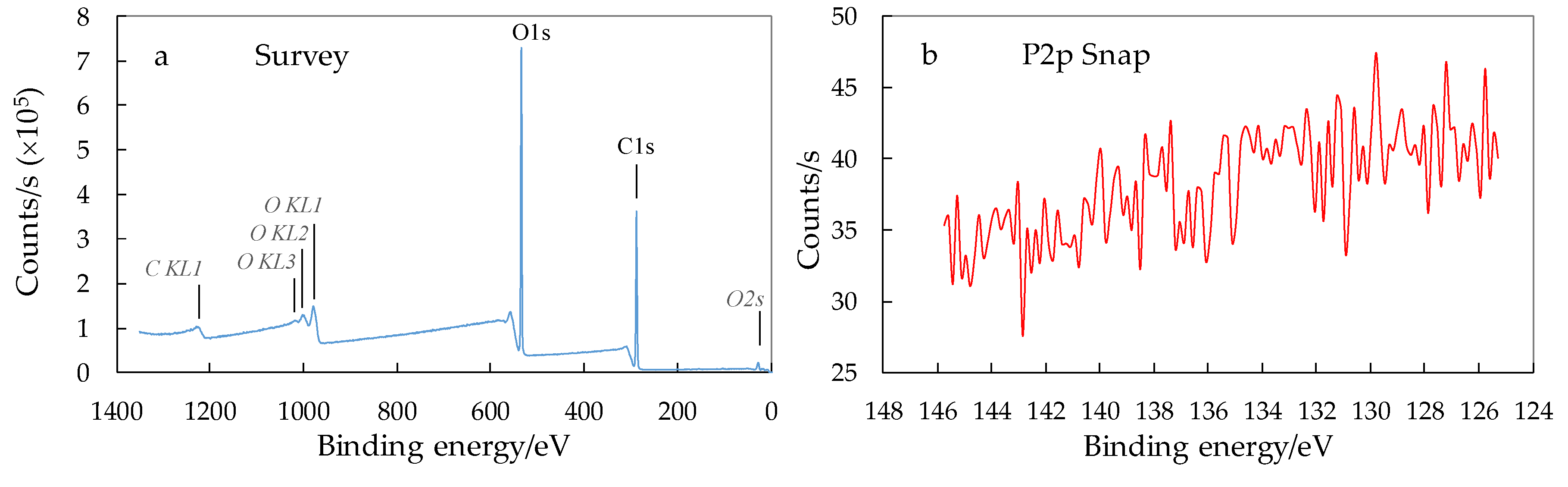
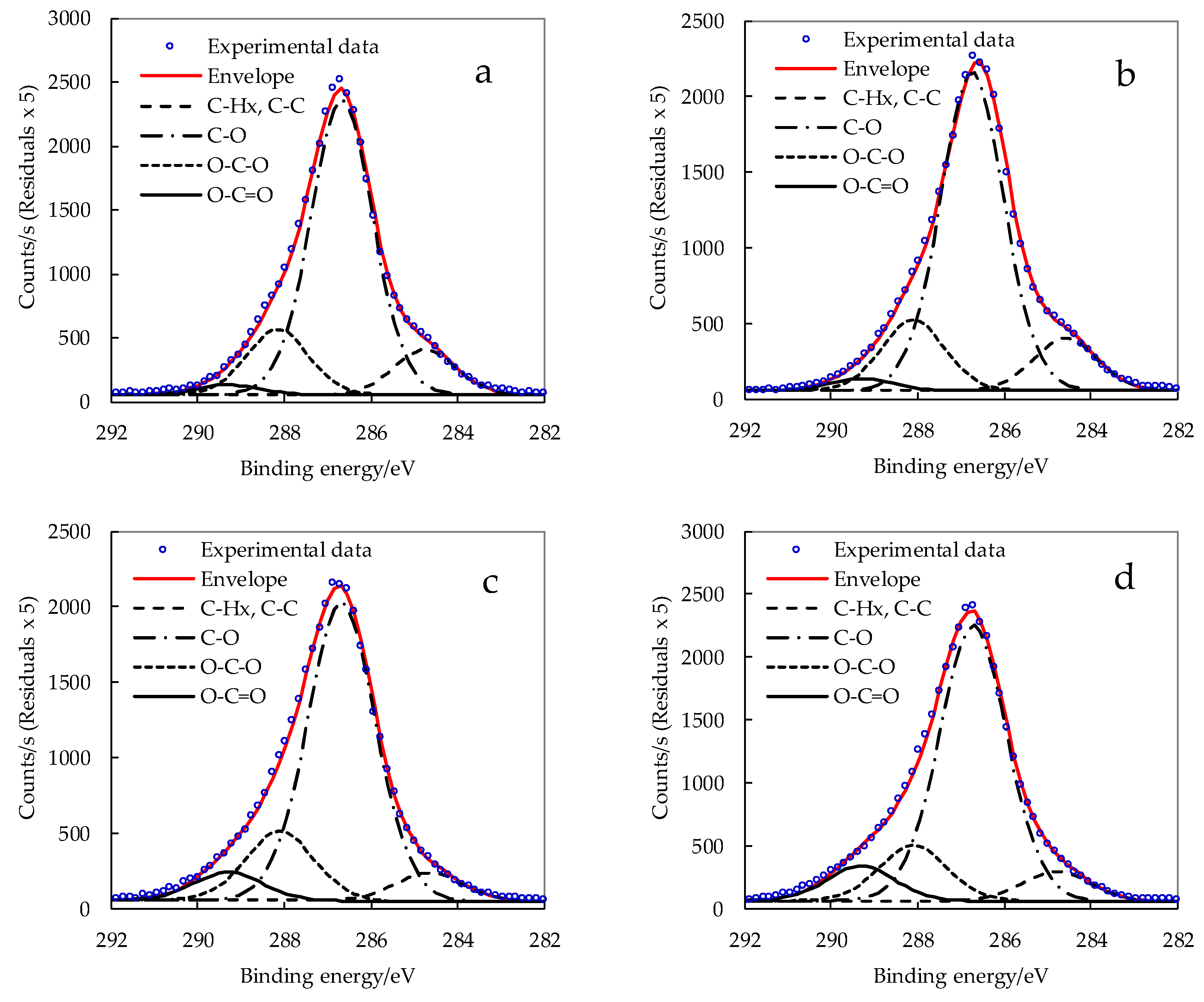
3.3. XPS Analysis of Surface Chemical Composition of CNCs
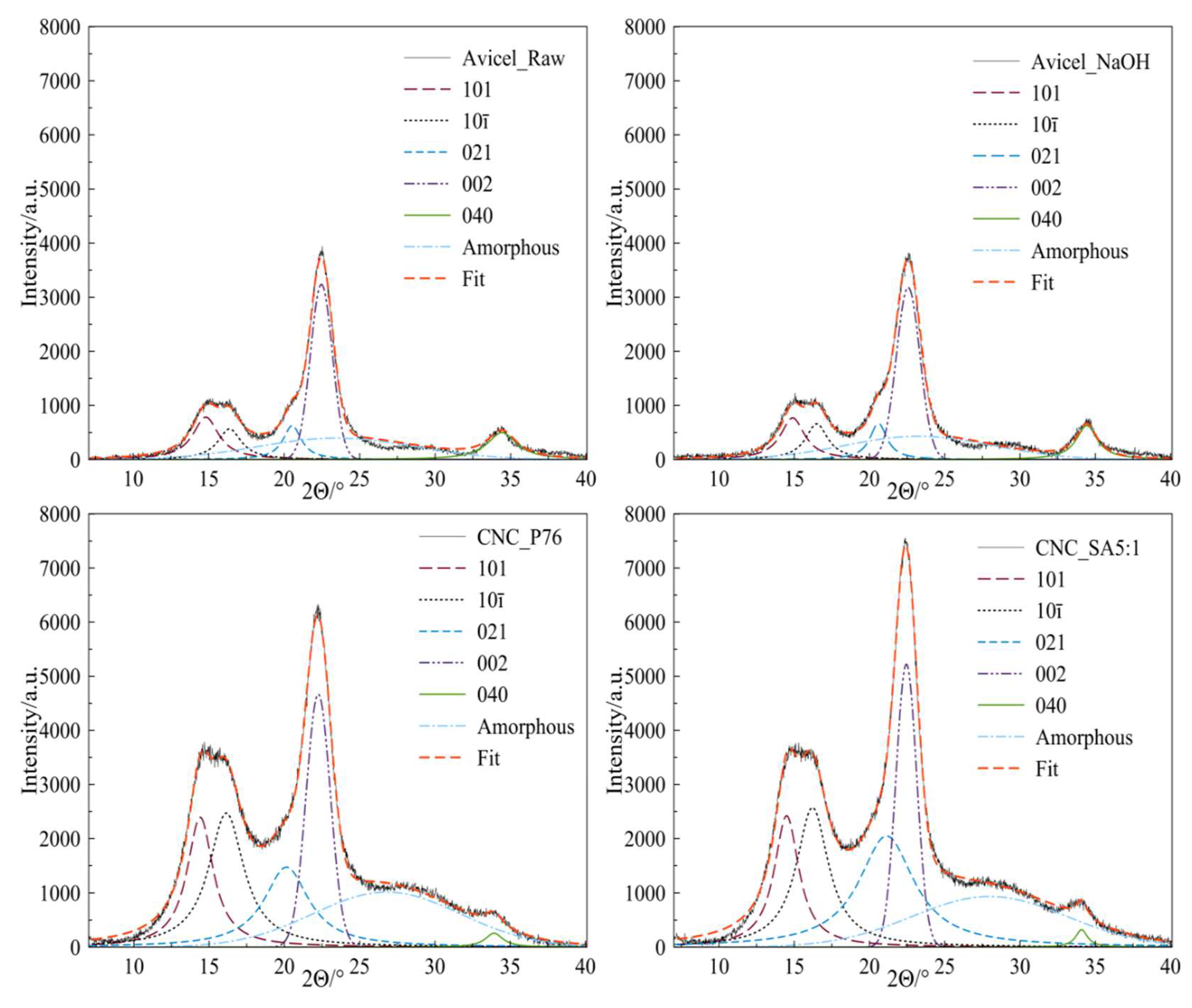
3.4. Crystalline Structure of CNC
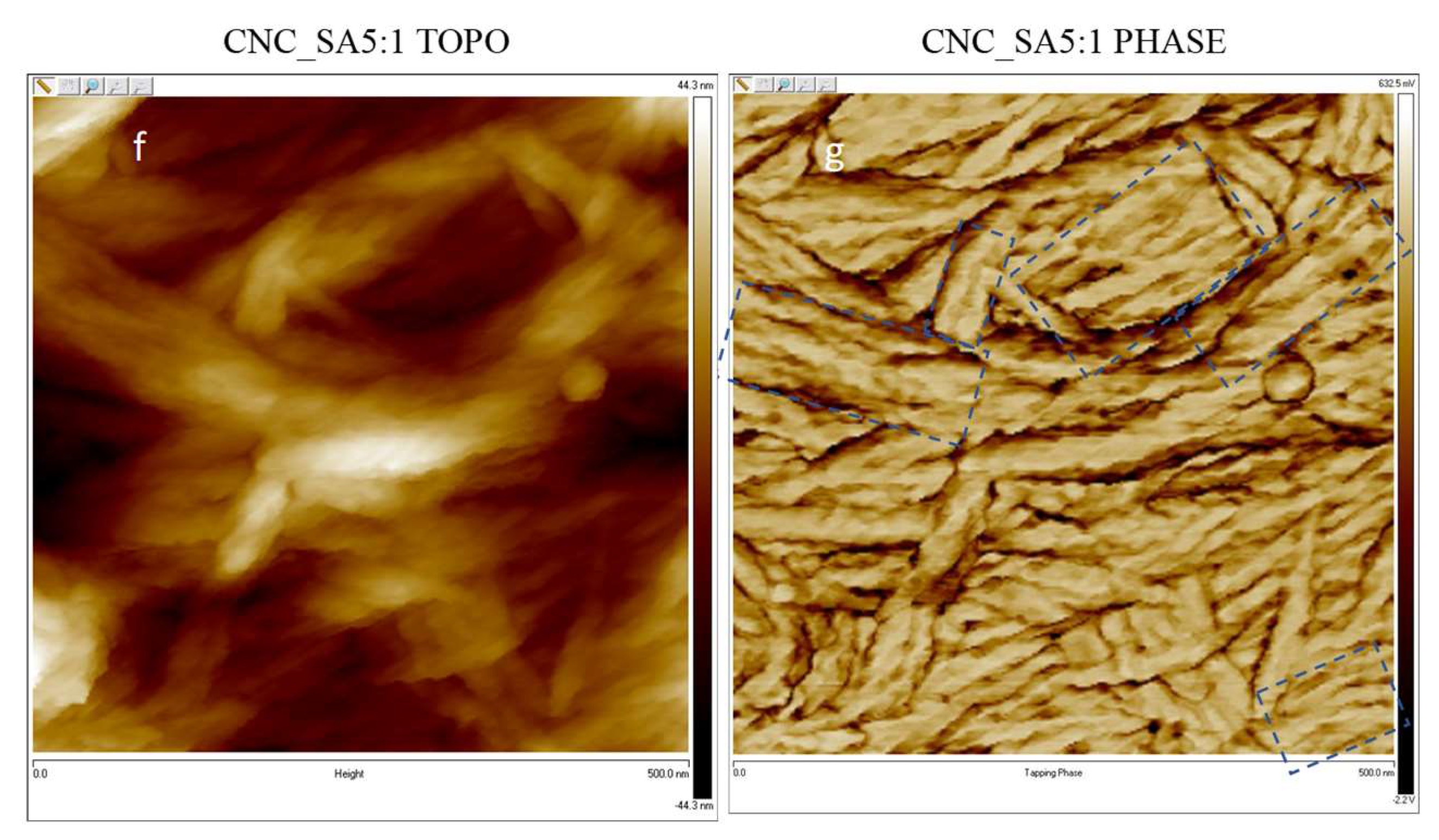
3.5. Microscopic Analysis of Cellulose Nanocrystals Morphology
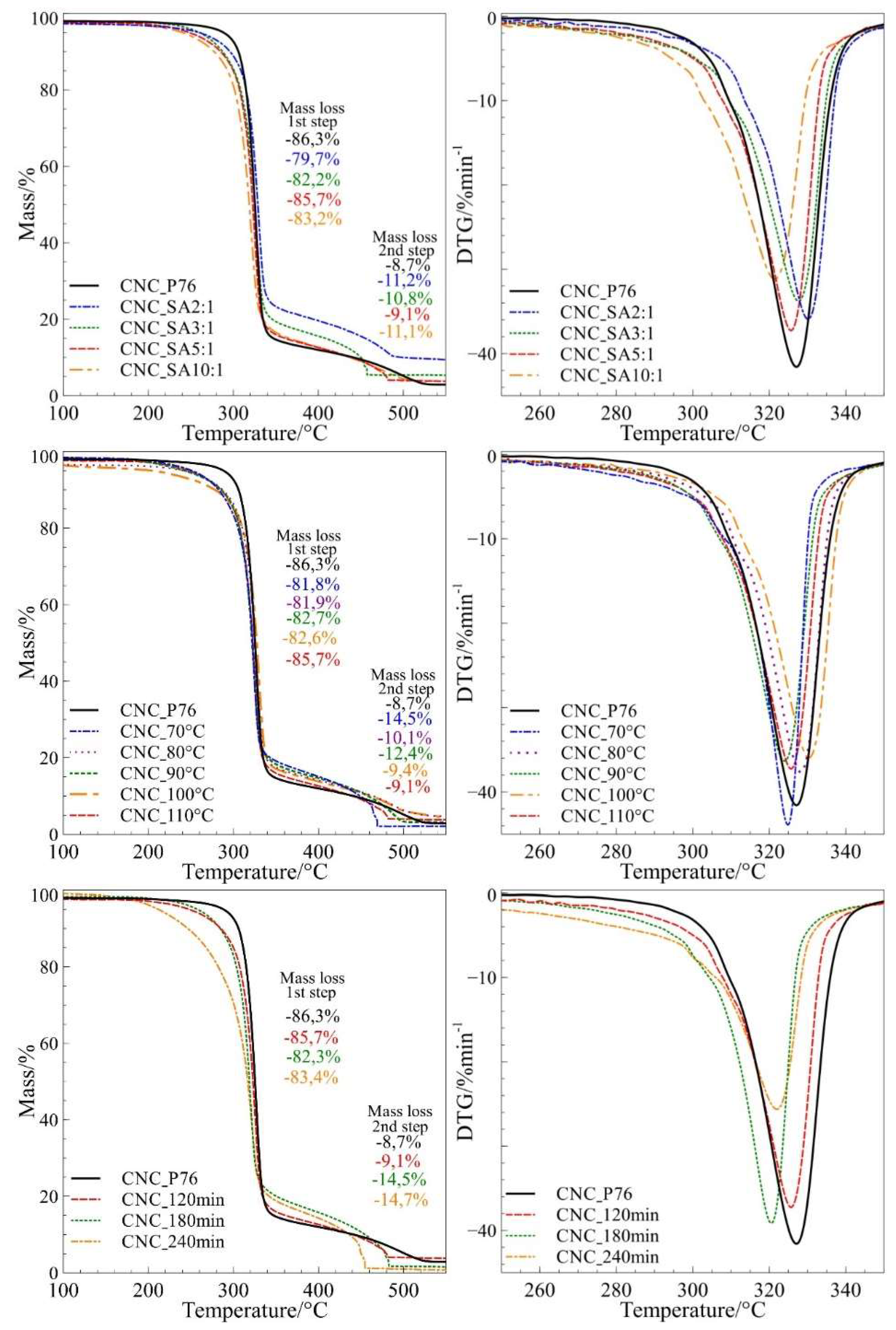
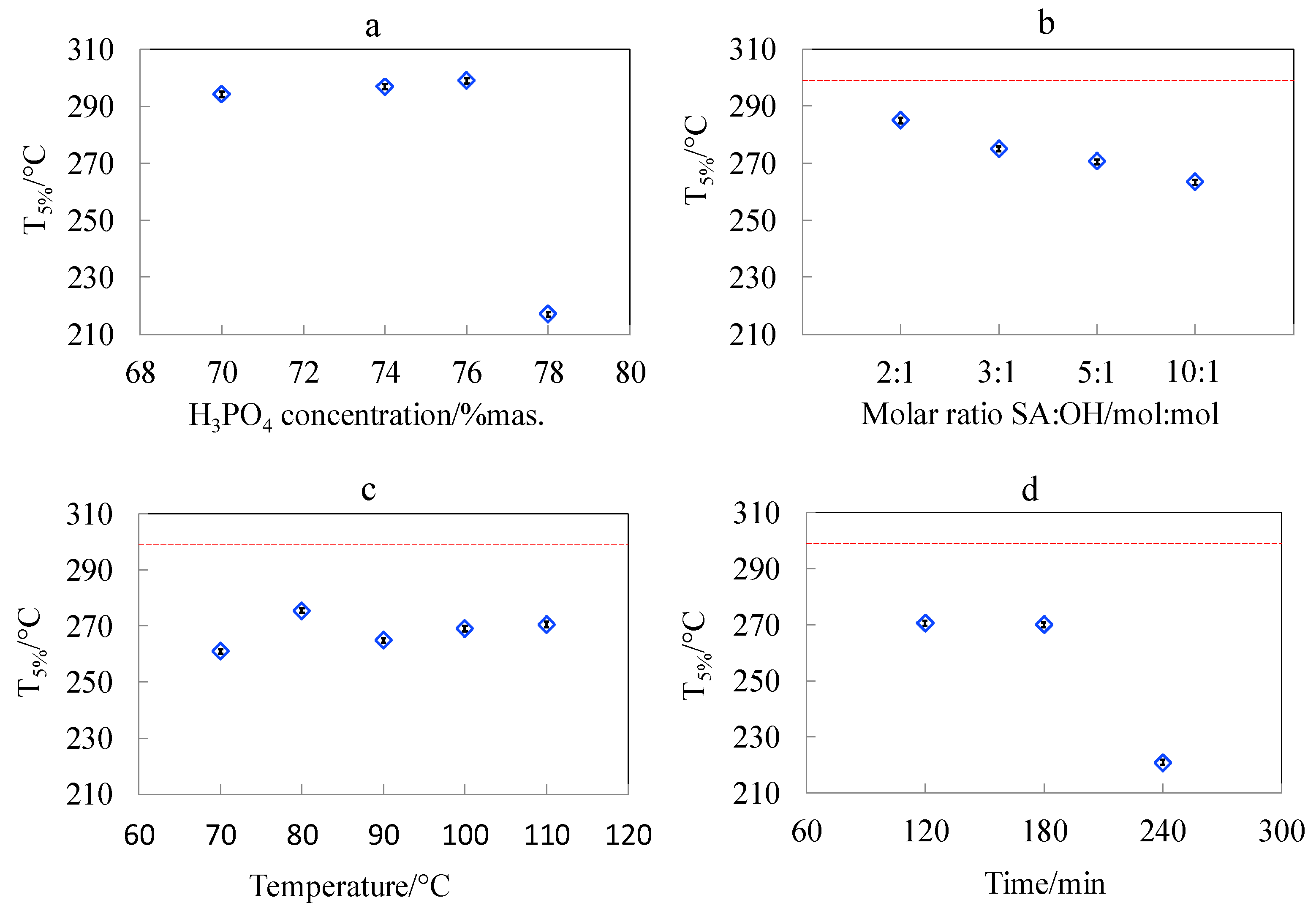
3.6. Studies of Pyrolytic and Thermo-Oxidative Degradation of CNCs Obtained by Hydrolysis and Surface Esterification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kargarzadeh, H.; Ahmad, Á.I.; Ahmad, I.; Mariano, M.; Gopakumar Á Thomas, D.S. Advances in Cellulose Nanomaterials; Springer: Dordrecht, The Netherlands, 2018; Volume 25, ISBN 1057001817235. [Google Scholar]
- Leung, A.C.W.; Hrapovic, S.; Lam, E.; Liu, Y.; Male, K.B.; Mahmoud, K.A.; Luong, J.H.T. Characteristics and properties of carboxylated cellulose nanocrystals prepared from a novel one-step procedure. Small 2011, 7, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Leszczyńska, A.; Radzik, P.; Haraźna, K.; Pielichowski, K. Thermal stability of cellulose nanocrystals prepared by succinic anhydride assisted hydrolysis. Thermochim. Acta 2018, 663, 145–156. [Google Scholar] [CrossRef]
- Lahiji, R.R.; Xu, X.; Reifenberger, R.; Raman, A.; Rudie, A.; Moon, R.J. Atomic force microscopy characterization of cellulose nanocrystals. Langmuir 2010, 26, 4480–4488. [Google Scholar] [CrossRef] [PubMed]
- Siaueira, G.; Bras, J.; Dufresne, A. Cellulose whiskers versus microfibrils: Influence of the nature of the nanoparticle and its surface functionalization on the thermal and mechanical properties of nanocomposites. Biomacromolecules 2009, 10, 425–432. [Google Scholar]
- Fan, J.S.; Li, Y.H. Maximizing the yield of nanocrystalline cellulose from cotton pulp fiber. Carbohydr. Polym. 2012, 88, 1184–1188. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, S.; Zhang, N.; Zhang, J. Preparation and characterization of nanocrystalline cellulose via low-intensity ultrasonic-assisted sulfuric acid hydrolysis. Cellulose 2014, 21, 335–346. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D. Effect of conditions on the properties behavior of wood cellulose nanocrystals suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Trache, D.; Haaflt, M.; Hussin, M.H.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef]
- Leite, L.S.F.; Battirola, L.C.; da Silva, L.C.E.; Gonalves, M.C. Morphological investigation of cellulose acetate/cellulose nanocrystal composites obtained by melt extrusion. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef]
- Zhang, P.P.; Tong, D.S.; Lin, C.X.; Yang, H.M.; Zhong, Z.K.; Yu, W.H.; Wang, H.; Zhou, C.H. Effects of acid treatments on bamboo cellulose nanocrystals. Asia-Pac. J. Chem. Eng. 2014, 9, 686–695. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Mu, X.; Gong, W.; Lv, D.; Hong, Y.; Si, C.; Li, B. Preparation and characterization of thermally stable cellulose nanocrystals via a sustainable approach of FeCl3-catalyzed formic acid hydrolysis. Cellulose 2016, 23, 2389–2407. [Google Scholar] [CrossRef]
- Filpponen, I.; Sadeghifar, H.; Argyropoulos, D.S. Photoresponsive Cellulose Nanocrystals. Nanomater. Nanotechnol. 2011, 1, 7. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Filpponen, I.; Clarke, S.P.; Brougham, D.F.; Argyropoulos, D.S. Production of cellulose nanocrystals using hydrobromic acid and click reactions on their surface. J. Mater. Sci. 2011, 46, 7344–7355. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrm, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, L.; Lu, Q.; Wang, S.; Chen, X.; Huang, B. Preparation of cellulose nanocrystals and carboxylated cellulose nanocrystals from borer powder of bamboo. Cellulose 2014, 21, 1611–1618. [Google Scholar] [CrossRef]
- Montanari, S.; Roumani, M.; Heux, L.; Vignon, M.R. Topochemistry of Carboxylated Cellulose Nanocrystals Resulting from TEMPO-Mediated Oxidation. Macromolecules 2005, 38, 1665–1671. [Google Scholar] [CrossRef]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple Approach to Prepare Carboxycellulose Nanofibers from Untreated Biomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhao, L.; Jiang, X.; Wang, H.; Gao, W. Cellulose nanocrystals modified with a triazine derivative and their reinforcement of poly(lactic acid)-based bionanocomposites. Cellulose 2018, 25, 2965–2976. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, L.; Jiang, X.; Wang, H.; Gao, W. Synthesis of triazine derivative and its application in the modification of cellulose nanocrystals. BioResources 2017, 12, 7427–7438. [Google Scholar]
- Yue, L.; Maiorana, A.; Khelifa, F.; Patel, A.; Raquez, J.-M.; Bonnaud, L.; Gross, R.; Dubois, P.; Manas-Zloczower, I. Surface-modified cellulose nanocrystals for biobased epoxy nanocomposites. Polymer 2018, 134, 155–162. [Google Scholar] [CrossRef]
- Panchal, P.; Ogunsona, E.; Mekonnen, T. Trends in Advanced Functional Material Applications of Nanocellulose. Processes 2018, 7, 10. [Google Scholar] [CrossRef]
- Tang, J.; Song, Y.; Tanvir, S.; Anderson, W.A.; Berry, R.M.; Tam, K.C. Polyrhodanine Coated Cellulose Nanocrystals: A Sustainable Antimicrobial Agent. ACS Sustain. Chem. Eng. 2015, 3, 1801–1809. [Google Scholar] [CrossRef]
- Drogat, N.; Granet, R.; Sol, V.; Memmi, A.; Saad, N.; Klein Koerkamp, C.; Bressollier, P.; Krausz, P. Antimicrobial silver nanoparticles generated on cellulose nanocrystals. J. Nanopart. Res. 2011, 13, 1557–1562. [Google Scholar] [CrossRef]
- Sharma, P.R.; Varma, A.J. Functional nanoparticles obtained from cellulose: Engineering the shape and size of 6-carboxycellulose. Chem. Commun. 2013, 49, 8818–8820. [Google Scholar] [CrossRef]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Nonappa; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced Materials through Assembly of Nanocelluloses. Adv. Mater. 2018, 30, 1703779. [Google Scholar] [CrossRef]
- Liu, P.; Borrell, P.F.; Božič, M.; Kokol, V.; Oksman, K.; Mathew, A.P. Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+ and Fe3+ from industrial effluents. J. Hazard. Mater. 2015, 294, 177–185. [Google Scholar] [CrossRef]
- Menon, M.P.; Selvakumar, R.; Suresh, P.; Ramakrishna, S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef]
- Jiang, M.; Ma, J.; Wu, M.; Liu, R.; Liang, L.; Xin, F. Progress of succinic acid production from renewable resources: Metabolic and fermentative strategies. Bioresour. Technol. 2017, 245, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Namazi, H.; Fathi, F.; Heydari, A. Nanoparticles Based on Modified Polysaccharides. In The Delivery of Nanoparticles; IntechOpen: London, UK, 2012; pp. 149–185. ISBN 978-953-51-0615-9. [Google Scholar]
- Sehaqui, H.; Kulasinski, K.; Pfenninger, N.; Zimmermann, T.; Tingaut, P. Highly Carboxylated Cellulose Nanofibers via Succinic Anhydride Esterification of Wheat Fibers and Facile Mechanical Disintegration. Biomacromolecules 2017, 18, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.O.; Tabary, N.; Martel, B.; Gandini, A.; Belgacem, N.; Bras, J. Effect of different carboxylic acids in cyclodextrin functionalization of cellulose nanocrystals for prolonged release of carvacrol. Mater. Sci. Eng. C 2016, 69, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tong, S.; Ge, M.; Wu, L.; Zuo, J.; Cao, C.; Song, W. Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. J. Environ. Sci. (China) 2013, 25, 933–943. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S. Efficient Removal of UO22+ from Water Using Carboxycellulose Nanofibers Prepared by the Nitro-Oxidation Method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Antoine, R.; Hsiao, B.S. Efficient Removal of Arsenic Using Zinc Oxide Nanocrystal-Decorated Regenerated Microfibrillated Cellulose Scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 6140–6151. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Zhan, C.; Sharma, S.K.; Geng, L.; Hsiao, B.S. Lead removal from water using carboxycellulose nanofibers prepared by nitro-oxidation method. Cellulose 2018, 25, 1961–1973. [Google Scholar] [CrossRef]
- Khoshkava, V.; Kamal, M.R. Effect of surface energy on dispersion and mechanical properties of polymer/nanocrystalline cellulose nanocomposites. Biomacromolecules 2013, 14, 3155–3163. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. Alkenylation of cellulose nanocrystals (CNC) and their applications. Polymer 2016, 101, 338–346. [Google Scholar] [CrossRef]
- Mariano, M.; Chirat, C.; El Kissi, N.; Dufresne, A. Impact of cellulose nanocrystal aspect ratio on crystallization and reinforcement of poly(butylene adipate-co-terephthalate). J. Polym. Sci. Part B Polym. Phys. 2016, 54, 2284–2297. [Google Scholar] [CrossRef]
- Espinosa, S.C.; Kuhnt, T.; Foster, E.J.; Weder, C. Isolation of Thermally Stable Cellulose Nanocrystals by Phosphoric Acid Hydrolysis. Biomacromolecules 2013, 14, 1223–1230. [Google Scholar] [CrossRef]
- Liu, C.; Li, B.; Du, H.; Lv, D.; Zhang, Y.; Yu, G.; Mu, X.; Peng, H. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr. Polym. 2016, 151, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, G.; Lenes, M.; Eriksen, Ø.; Gregersen, Ø. Surface chemical modification of microfibrillated cellulose: Improvement of barrier properties for packaging applications. Cellulose 2011, 18, 127–134. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A Mater. Energy Sustain. 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanomaterials: Size and surface influence on the thermal and rheological behavior. Polimeros 2018, 28, 93–102. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Fraschini, C.; Chauve, G.; Le Berre, J.-F.; Ellis, S.; Methot, M.; O’Connor, B.; Bouchard, J. Critical discussion of light scattering and microscopy techniques for CNC particle sizing. Nord. Pulp Pap. Res. J. 2014, 29, 031–040. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Santana, R.M.C. Structural differences between wood species: Evidence from chemical composition, FTIR spectroscopy, and thermogravimetric analysis. J. Appl. Polym. Sci. 2012, 126, E337–E344. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.; Zattera, A.; Poletto, M.; Ornaghi, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials (Basel) 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [PubMed]
- Spinella, S.; Maiorana, A.; Qian, Q.; Dawson, N.J.; Hepworth, V.; McCallum, S.A.; Ganesh, M.; Singer, K.D.; Gross, R.A. Concurrent Cellulose Hydrolysis and Esterification to Prepare a Surface-Modified Cellulose Nanocrystal Decorated with Carboxylic Acid Moieties. ACS Sustain. Chem. Eng. 2016, 4, 1538–1550. [Google Scholar] [CrossRef]
- Fengel, D. Influence of Water on the OH Valency Range in Deconvoluted FTIR Spectra of Cellulose. Holzforschung 1993, 47, 103–108. [Google Scholar] [CrossRef]
- Gierlinger, N.; Schwanninger, M.; Reinecke, A.; Burgert, I. Molecular changes during tensile deformation of single wood fibers followed by Raman microscopy. Biomacromolecules 2006, 7, 2077–2081. [Google Scholar] [CrossRef] [PubMed]
- Moronkola, B.A.; Moronkola, D.O.; Iwoye, A. Studies on the isolation, structural and functional properties of Starch Succinate of Cocoyam (Colocasia antiquorum). Der Chem. Sin. 2011, 2, 228–244. [Google Scholar]
- Mannan, T.M.; Soares, J.B.P.; Berry, R.M.; Hamad, W.Y. In-situ production of polyethylene/cellulose nanocrystal composites. Can. J. Chem. Eng. 2016, 94, 2107–2113. [Google Scholar] [CrossRef]
- Melo, J.C.P.; Silva Filho, E.C.; Santana, S.A.A.; Airoldi, C. Synthesized cellulose/succinic anhydride as an ion exchanger. Calorimetry of divalent cations in aqueous suspension. Thermochim. Acta 2011, 524, 29–34. [Google Scholar] [CrossRef]
- Watts, J.F.; Wolstenholme, J. An Introduction to Surface Analysis by XPS and AES; John and Wiley and Sons: Hoboken, NJ, USA, 2003; ISBN 9780470867938. [Google Scholar]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. V. Data for 14 organic compounds over the 50–2000 eV range. Surf. Interface Anal. 1994, 21, 165–176. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D.; SurfaceSpectra Ltd. The XPS of Polymers Database; SurfaceSpectra: Manchester, UK, 2000; ISBN 9780953784844. [Google Scholar]
- Andresen, M.; Johansson, L.S.; Tanem, B.S.; Stenius, P. Properties and characterization of hydrophobized microfibrillated cellulose. Cellulose 2006, 13, 665–677. [Google Scholar] [CrossRef]
- Liu, C.F.; Zhang, A.P.; Li, W.Y.; Sun, R.C. Chemical modification of cellulose with succinic anhydride in ionic liquid with or without catalysts. Ion. Liq. Appl. Perspect. 2011. [Google Scholar] [CrossRef]
- Shang, W.; Sheng, Z.; Shen, Y.; Ai, B.; Zheng, L.; Yang, J.; Xu, Z. Study on oil absorbency of succinic anhydride modified banana cellulose in ionic liquid. Carbohydr. Polym. 2016, 141, 135–142. [Google Scholar] [CrossRef]
- Li, W.Y.; Jin, A.X.; Liu, C.F.; Sun, R.C.; Zhang, A.P.; Kennedy, J.F. Homogeneous modification of cellulose with succinic anhydride in ionic liquid using 4-dimethylaminopyridine as a catalyst. Carbohydr. Polym. 2009, 78, 389–395. [Google Scholar] [CrossRef]
- Yin, X.; Yu, C.; Zhang, X.; Yang, J.; Lin, Q.; Wang, J.; Zhu, Q. Comparison of succinylation methods for bacterial cellulose and adsorption capacities of bacterial cellulose derivatives for Cu2+ ion. Polym. Bull. 2011, 67, 401–412. [Google Scholar] [CrossRef]
- Yuan, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Surface Acylation of Cellulose Whiskers by Drying Aqueous Emulsion. Biomacromolecules 2006, 7, 696–700. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Battista, O.A.; Coppick, S.; Howsmon, J.A.; Morehead, F.F.; Sisson, W.A. Level-Off Degree of Polymerization. Ind. Eng. Chem. 1956, 48, 333–335. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohydr. Polym. 2015, 122, 60–68. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Wang, N.; Ding, E.; Cheng, R. Thermal degradation behaviors of spherical cellulose nanocrystals with sulfate groups. Polymer 2007, 48, 3486–3493. [Google Scholar] [CrossRef]
- Trinh, B.M.; Mekonnen, T. Hydrophobic esterification of cellulose nanocrystals for epoxy reinforcement. Polymer 2018, 155, 64–74. [Google Scholar] [CrossRef]
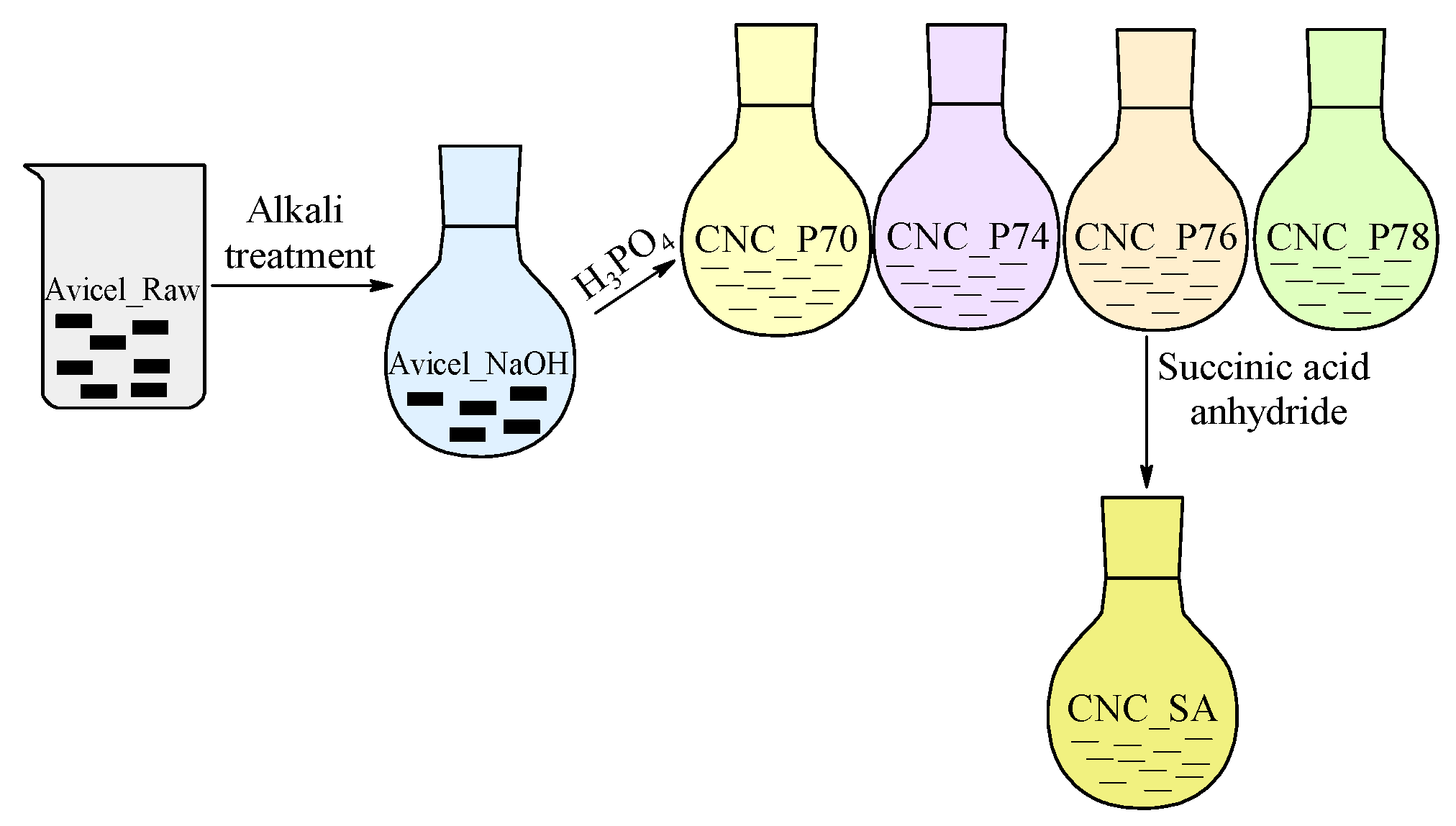
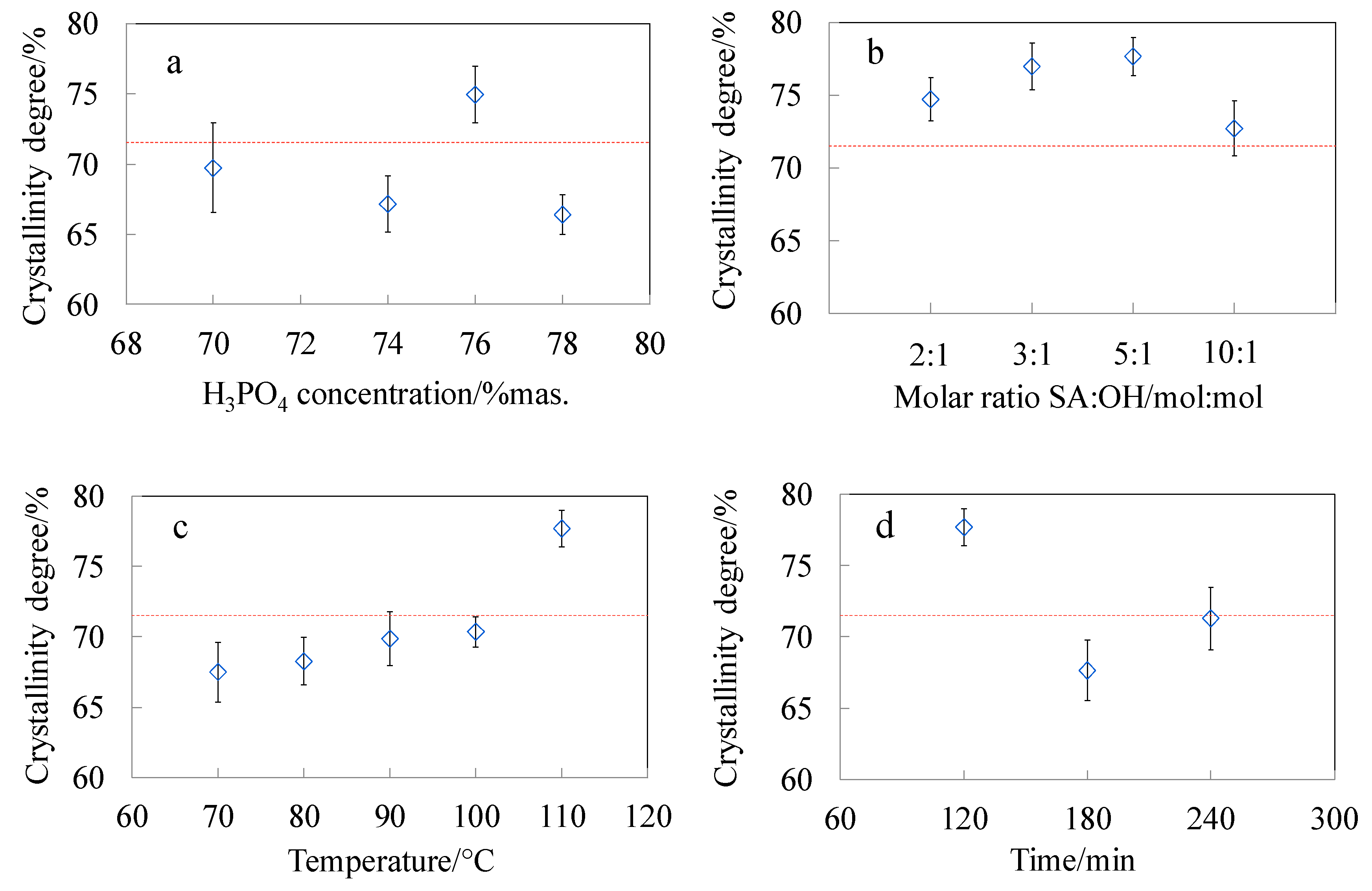
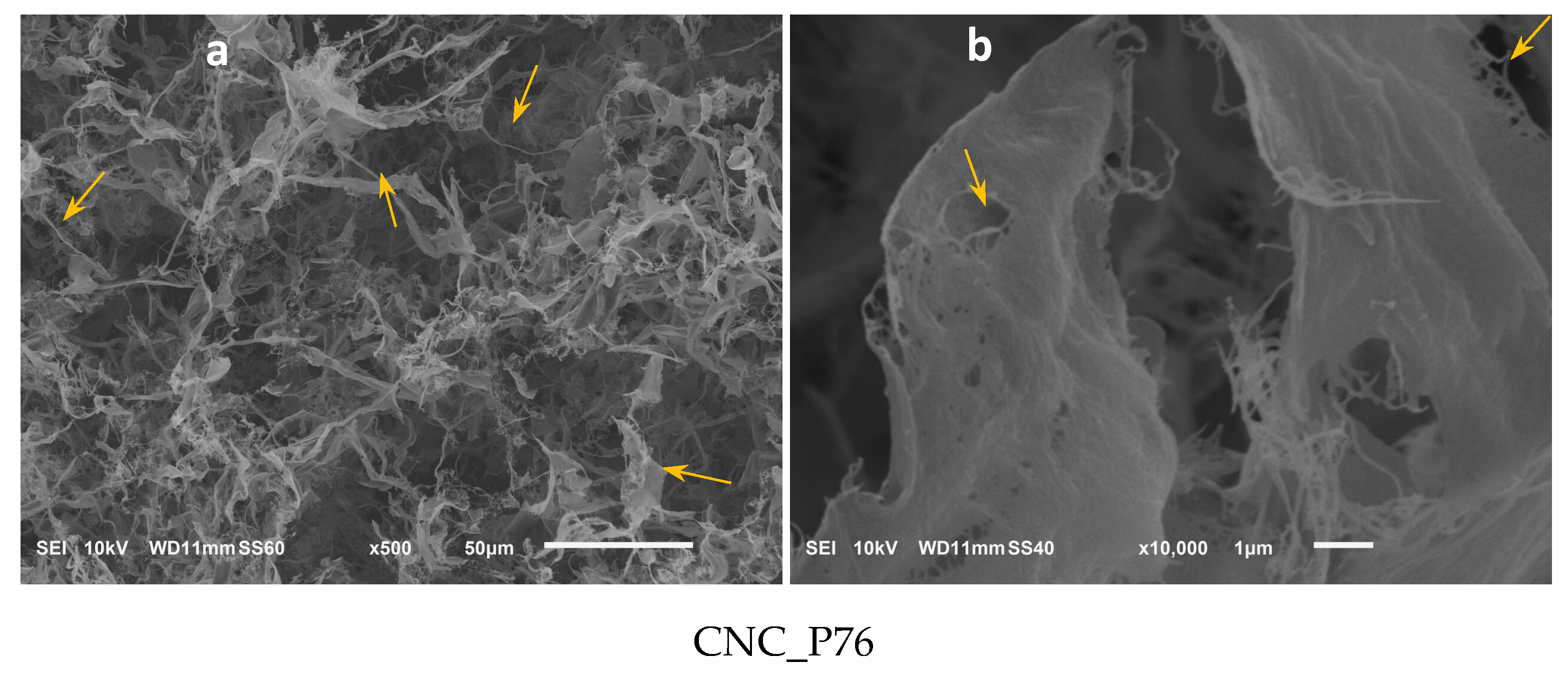
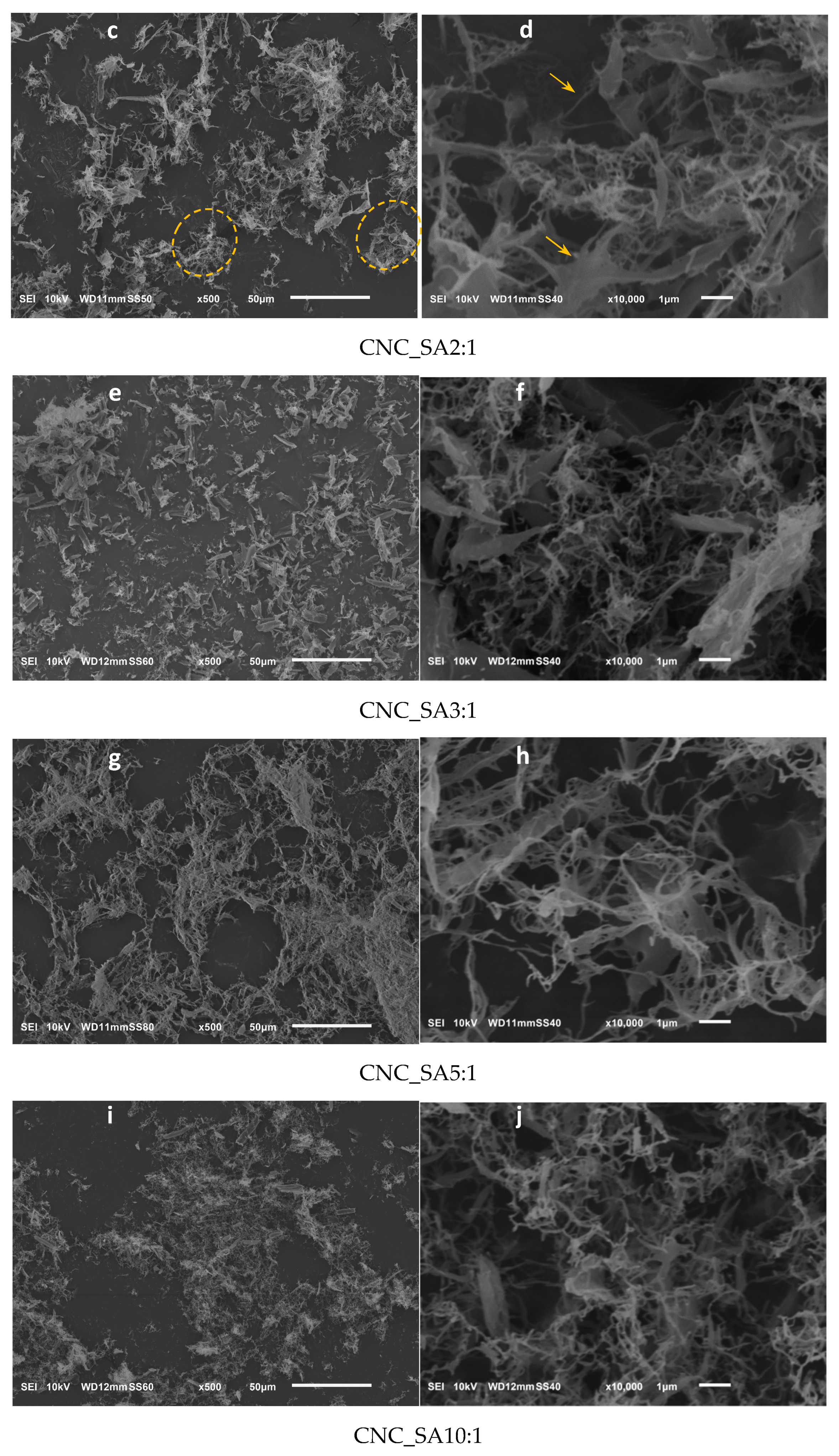
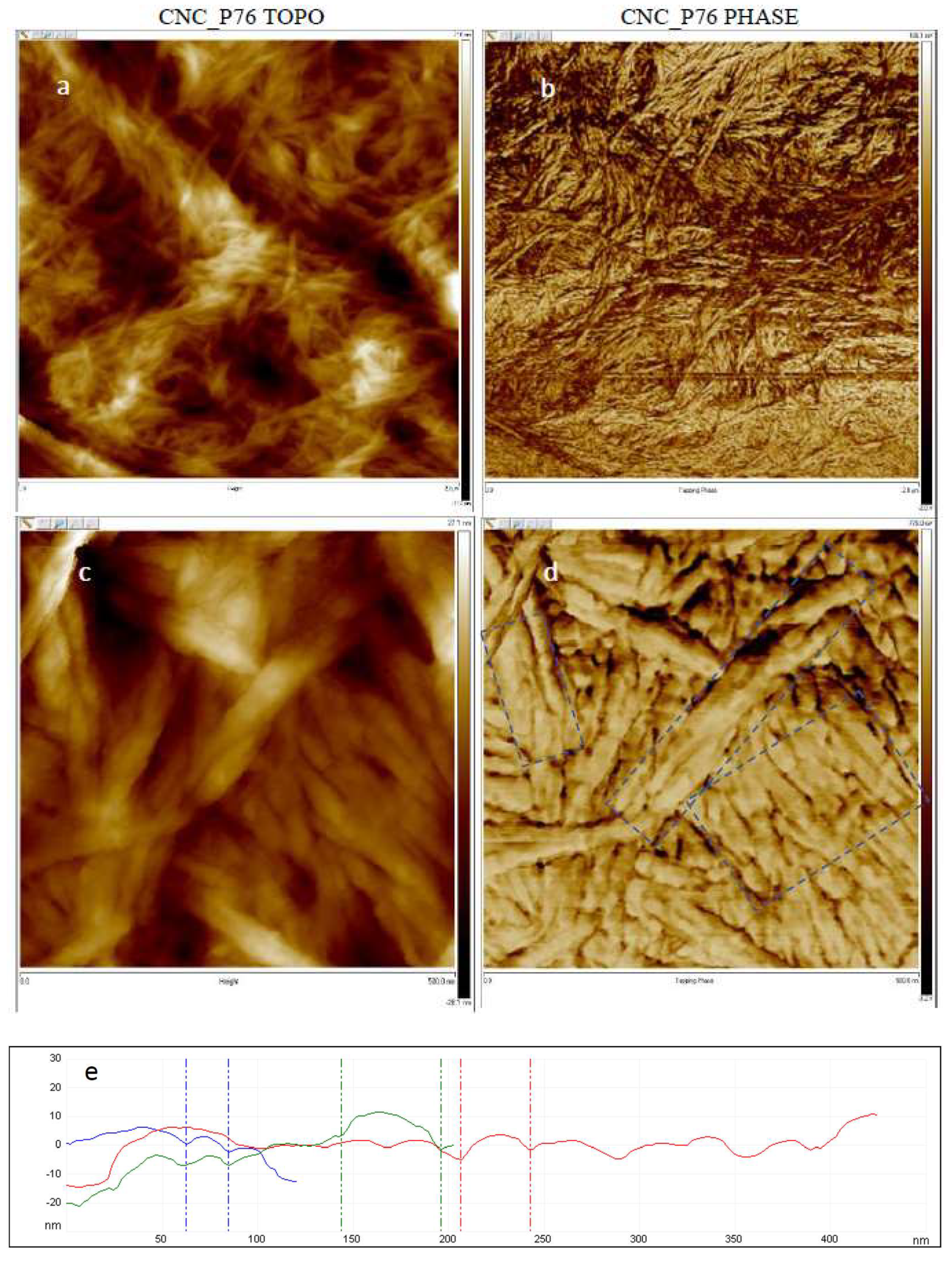
| Sample Denotation | Hydrolysis Catalyst | Concentration of Acid Solution, wt. % | Time, min | Temperature, °C |
| Avicel _Raw | - | - | - | - |
| CNC_P70 | H3PO4 | 70.0 | 90 | 100 |
| CNC_P74 | 74.0 | |||
| CNC_P76 | 76.0 | |||
| CNC_P78 | 78.0 | |||
| Sample Denotation | Modifier | Molar Ratio of SA:OH | Time, min | Temperature, °C |
| CNC_SA2:1 | Succinic anhydride | 2:1 | 120 | 110 |
| CNC_SA3:1 | 3:1 | |||
| CNC_SA5:1 | 5:1 | |||
| CNC_SA10:1 | 10:1 | |||
| CNC_ 70 °C | Succinic anhydride | 5:1 | 120 | 70 |
| CNC_80 °C | 80 | |||
| CNC_90 °C | 90 | |||
| CNC_100 °C | 100 | |||
| CNC_110 °C | 110 | |||
| CNC_60 min | Succinic anhydride | 5:1 | 60 | 110 |
| CNC_120 min | 120 | |||
| CNC_180 min | 180 | |||
| CNC_240 min | 240 |
| Sample | O [%] | C [%] | C/O [%] | Resolved C 1s Peak | Surface Substitution Degree (SSD) | |||
|---|---|---|---|---|---|---|---|---|
| C–HX [%] | C–O [%] | C=O/O–C–O [%] | O–C=O [%] | |||||
| Avicel_NaOH | 43.0 | 57.0 | 1.3 | 3.9 | 41.2 | 9.9 | 1.9 | 0.12 |
| CNC_P76 | 42.0 | 58.0 | 1.4 | 6.3 | 41.1 | 9.1 | 1.4 | 0.08 |
| CNC_SA2:1 | 41.5 | 56.6 | 1.4 | 6.4 | 40.9 | 8.4 | 1.9 | 0.12 |
| CNC_SA3:1 | 41.9 | 56.6 | 1.4 | 6.7 | 40.2 | 8.9 | 1.6 | 0.10 |
| CNC_SA5:1 | 43.0 | 56.5 | 1.3 | 3.8 | 39.9 | 9.2 | 3.7 | 0.23 |
| CNC_SA10:1 | 42.6 | 56.7 | 1.3 | 4.4 | 39.7 | 8.0 | 5.1 | 0.32 |
| Sample | Pyrolytic Degradation | Thermooxidative Degradation | ||||||
|---|---|---|---|---|---|---|---|---|
| T5%, °C ± 0.4 °C | Tonset, °C ± 2 °C | Tmax, °C ± 2 °C | Char % * at 600 °C | T5%, °C ± 0.4 °C | Tonset, °C ± 2 °C | Tmax, °C ± 2 °C | Char % * at 600 °C | |
| Avicel-Raw | 300.7 | 314 | 335 | 8.13 | 293.0 | 303 | 320 | 2.10 |
| Avicel-NaOH | 281.6 | 335 | 363 | 10.72 | 290.0 | 320 | 333 | 1.84 |
| CNC_P70 | 285.6 | 338 | 365 | 10.44 | 294.2 | 315 | 327 | 2.15 |
| CNC_P74 | 288.9 | 336 | 357 | 7.30 | 297.0 | 315 | 327 | 3.46 |
| CNC_P76 | 285.6 | 332 | 354 | 4.60 | 299.0 | 314 | 327 | 2.84 |
| CNC_P78 | 233.6 | 300 | 338 | 14.18 | 217.0 | 247 | 308 | 14.57 |
| CNC_SA2:1 | 286.4 | 340 | 367 | 7.23 | 285.1 | 316 | 330 | 8.70 |
| CNC_SA3:1 | 262.4 | 334 | 367 | 6.87 | 275.1 | 311 | 328 | 4.90 |
| CNC_SA5:1 | 260.1 | 342 | 373 | 6.57 | 270.6 | 311 | 326 | 2.80 |
| CNC_SA10:1 | 258.1 | 327 | 358 | 5.56 | 263.4 | 304 | 322 | 2.98 |
| CNC_ 70 °C | 260.4 | 331 | 364 | 7.50 | 261.0 | 312 | 325 | 2.05 |
| CNC_ 80 °C | 277.1 | 340 | 364 | 5.04 | 275.6 | 314 | 328 | 4.46 |
| CNC_ 90 °C | 269.1 | 333 | 361 | 7.26 | 264.9 | 309 | 324 | 3.04 |
| CNC_ 100 °C | 282.9 | 340 | 371 | 6.86 | 269.1 | 316 | 330 | 3.78 |
| CNC_ 110 °C | 260.1 | 342 | 373 | 6.57 | 270.6 | 311 | 326 | 2.80 |
| CNC_ 120 min | 260.1 | 342 | 373 | 6.57 | 270.6 | 311 | 326 | 2.80 |
| CNC_ 180 min | 260.9 | 334 | 365 | 6.37 | 270.1 | 306 | 321 | 1.44 |
| CNC_ 240 min | 212.1 | 330 | 356 | 7.68 | 220.9 | 300 | 322 | 0.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leszczyńska, A.; Radzik, P.; Szefer, E.; Mičušík, M.; Omastová, M.; Pielichowski, K. Surface Modification of Cellulose Nanocrystals with Succinic Anhydride. Polymers 2019, 11, 866. https://doi.org/10.3390/polym11050866
Leszczyńska A, Radzik P, Szefer E, Mičušík M, Omastová M, Pielichowski K. Surface Modification of Cellulose Nanocrystals with Succinic Anhydride. Polymers. 2019; 11(5):866. https://doi.org/10.3390/polym11050866
Chicago/Turabian StyleLeszczyńska, Agnieszka, Paulina Radzik, Ewa Szefer, Matej Mičušík, Mária Omastová, and Krzysztof Pielichowski. 2019. "Surface Modification of Cellulose Nanocrystals with Succinic Anhydride" Polymers 11, no. 5: 866. https://doi.org/10.3390/polym11050866
APA StyleLeszczyńska, A., Radzik, P., Szefer, E., Mičušík, M., Omastová, M., & Pielichowski, K. (2019). Surface Modification of Cellulose Nanocrystals with Succinic Anhydride. Polymers, 11(5), 866. https://doi.org/10.3390/polym11050866