Development of Diallylimidazolium Methoxyacetate/DMSO (DMF/DMA) Solvents for Improving Cellulose Dissolution and Fabricating Porous Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cellulose Dissolution
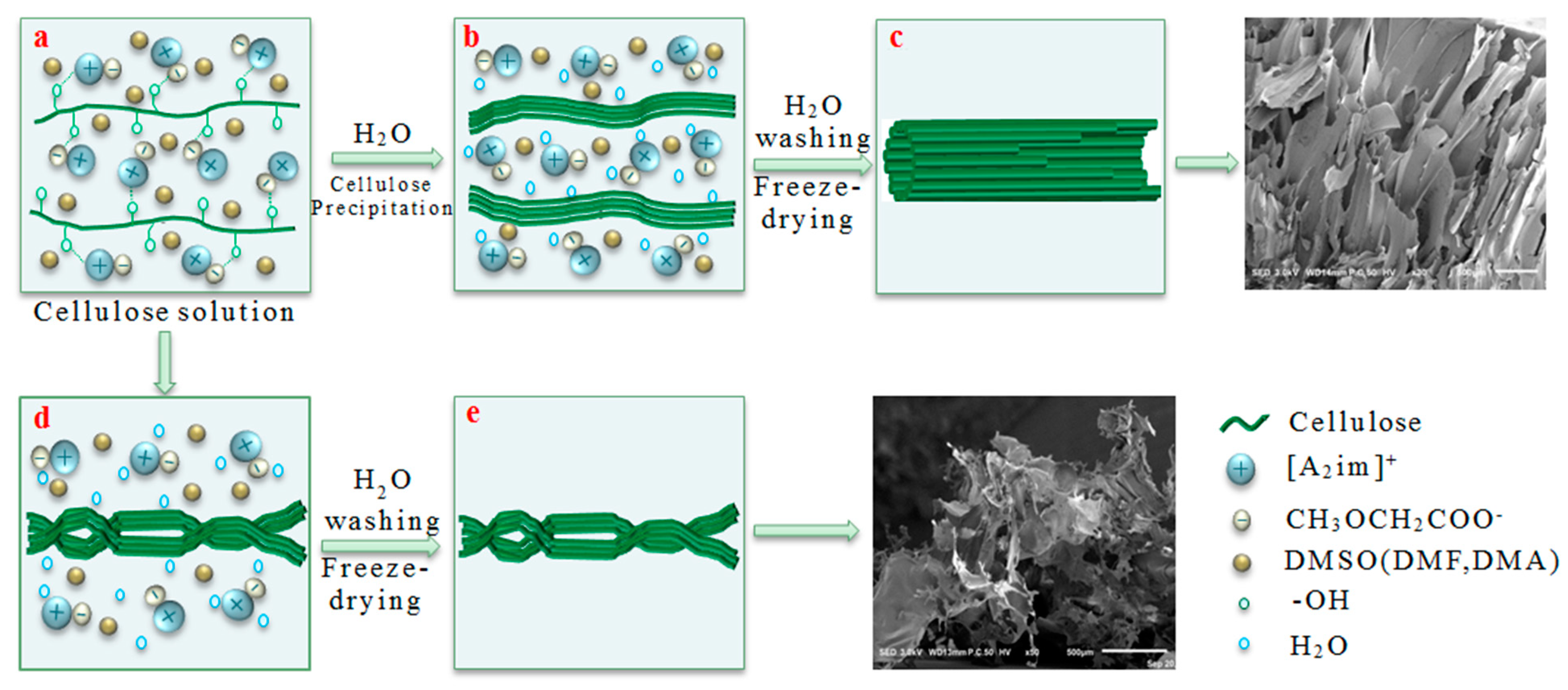
2.3. Preparation of Porous Cellulose Materials
2.4. Preparation and Characterization of Regenerated Cellulose Film
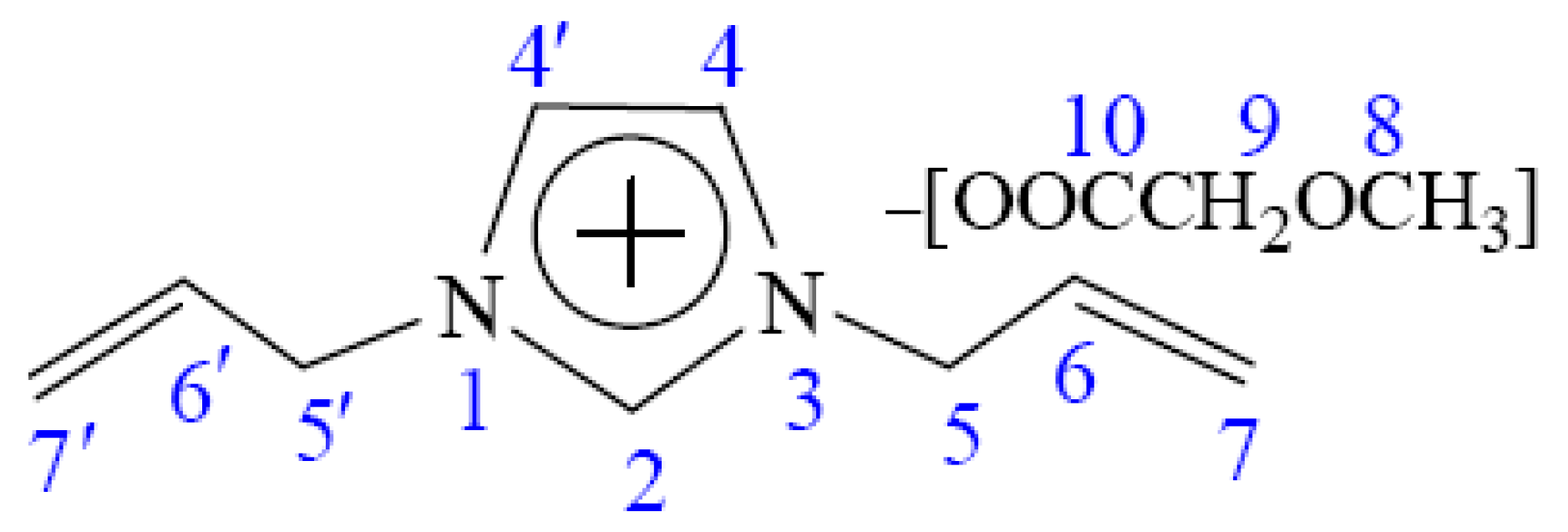
2.5. 13C NMR Spectra Measurements
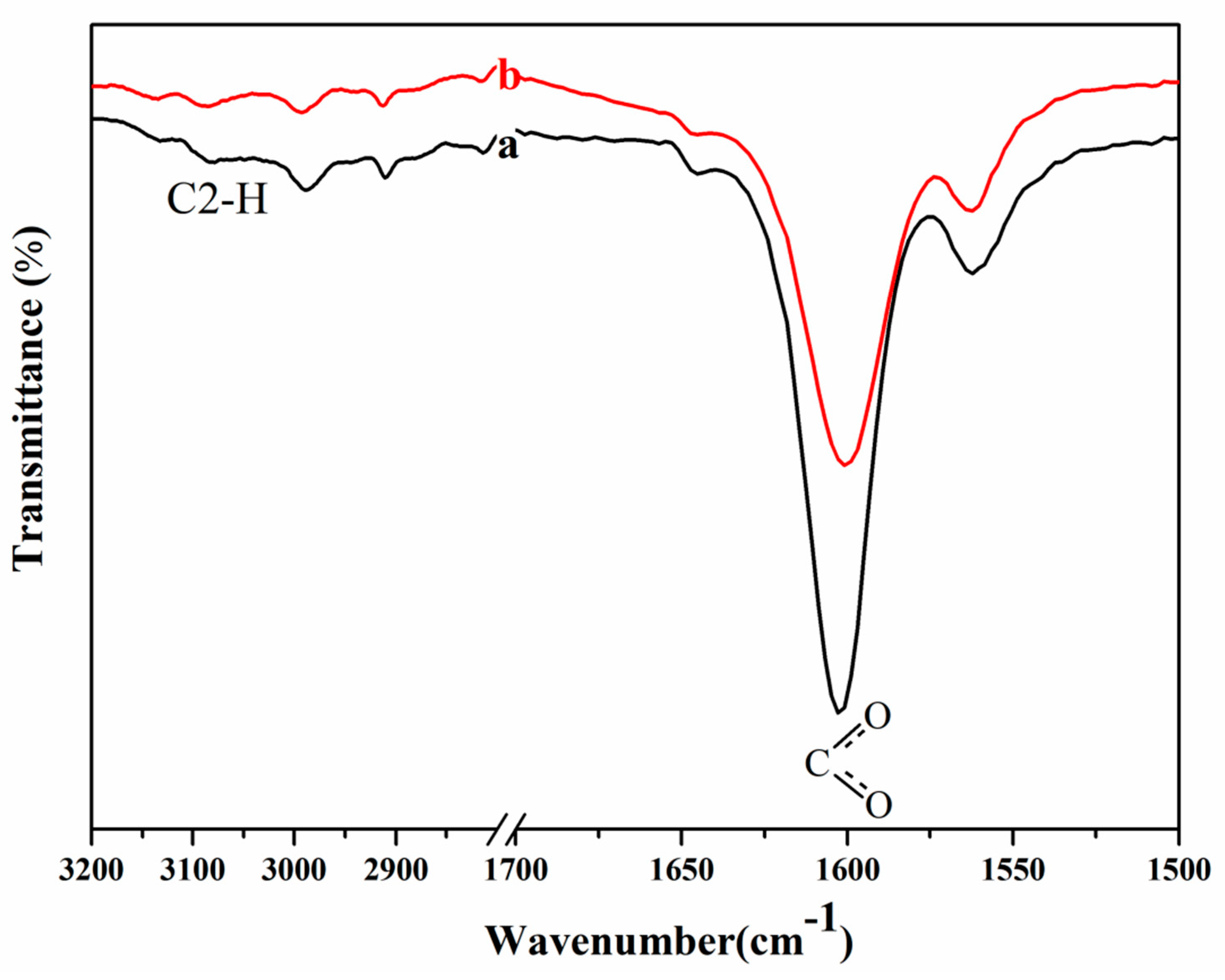
2.6. FTIR Spectra Measurements
3. Result and Discussion
3.1. Dependence of Cellulose Solubility on DMSO(DMF/DMA)/[A2im][CH3OCH2COO] Molar Ratio
3.2. 13C NMR and FTIR Analysis of Possible Dissolution Mechanism of Cellulose in [A2im][CH3OCH2COO]/DMSO(DMF/DMA) Solvent
3.3. Morphology and Formation Mechanism of the Porous Cellulose Materials
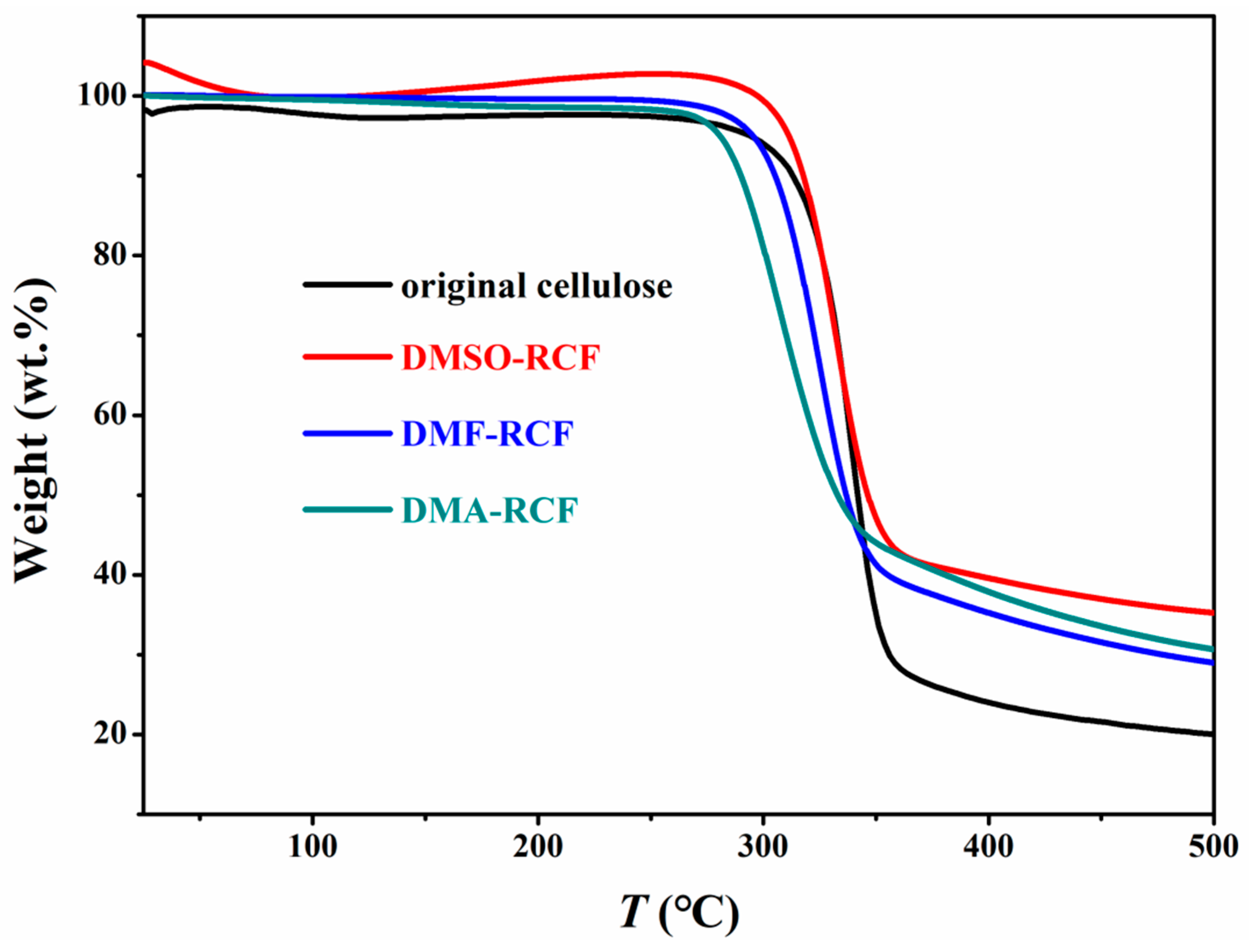
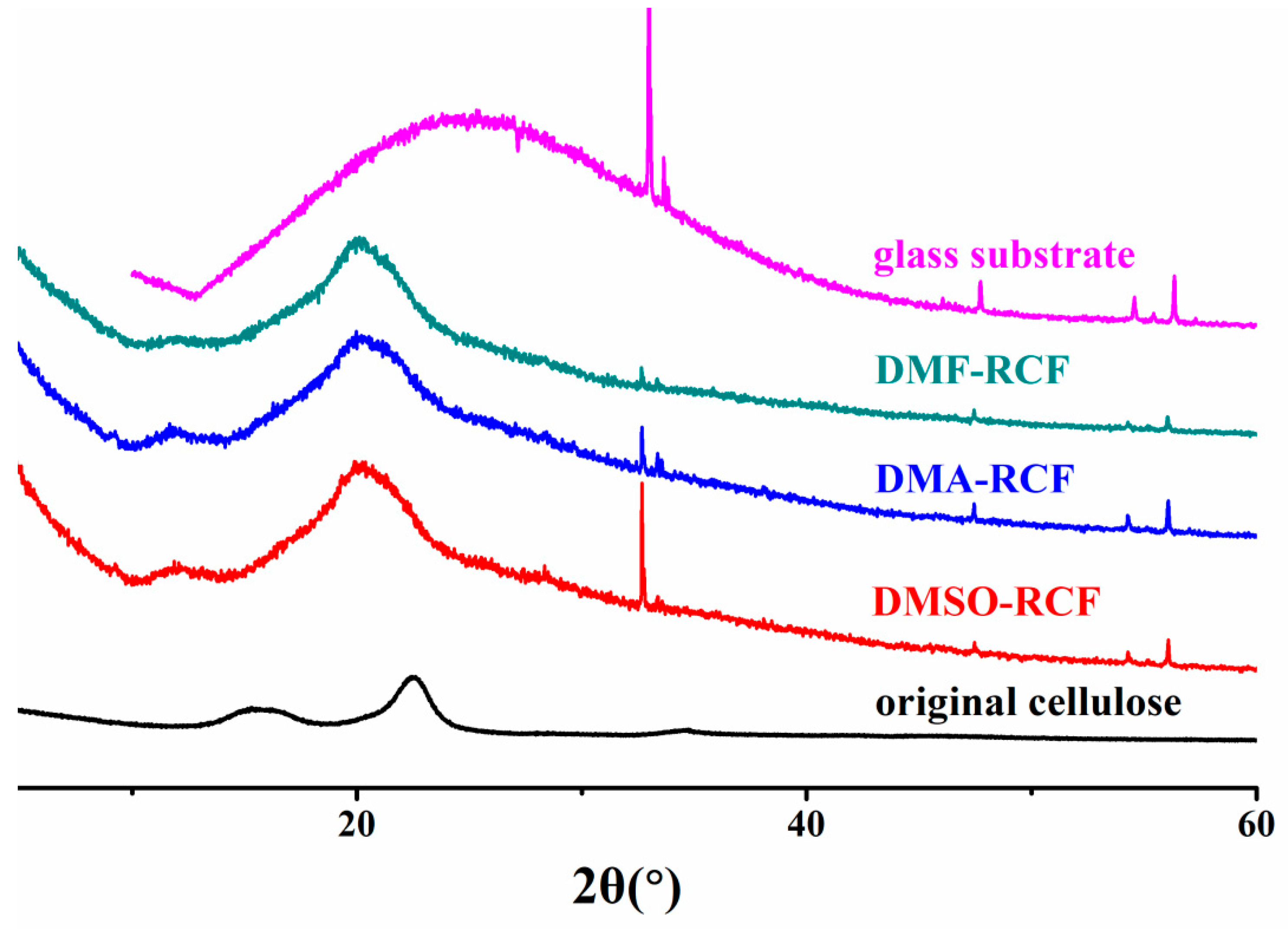
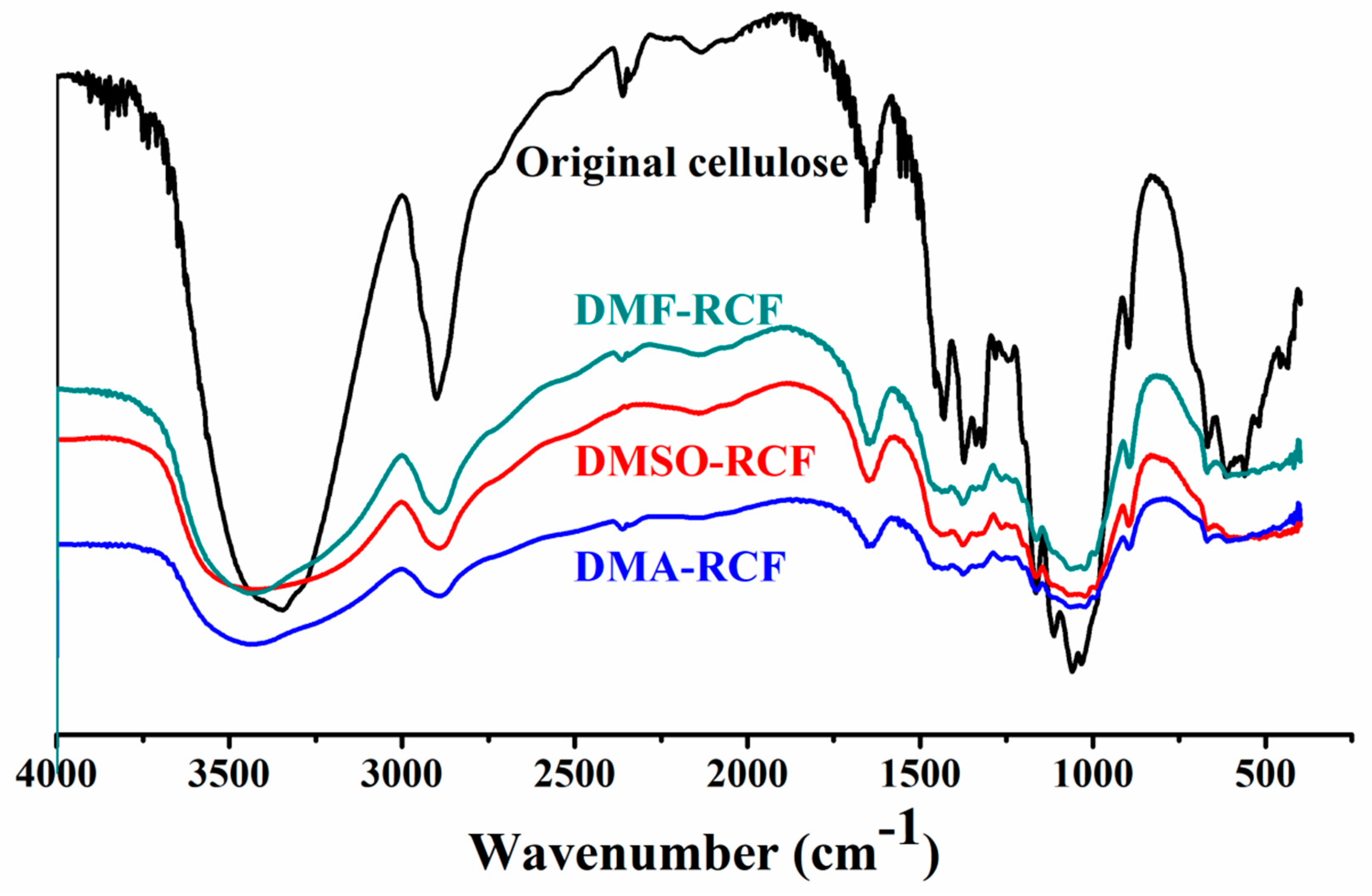
3.4. Thermostability and Chemical Structure of the Regenerated Cellulose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arioli, T.; Peng, L.; Betzner, A.S.; Burn, J.; Wittke, W.; Herth, W.; Camilleri, C.; Höfte, H.; Plazinski, J.; Birch, R.; et al. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 1998, 279, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Wiredu, B. Acidic ionic liquid catalyzed liquefaction of cellulose in ethylene glycol; identification of a new cellulose derived cyclopentenone derivative. Ind. Eng. Chem. Res. 2015, 54, 824–831. [Google Scholar] [CrossRef]
- Wittmar, A.S.M.; Ulbricht, M. Ionic liquid-based route for the preparation of catalytically active cellulose–TiO2 porous films and spheres. Ind. Eng. Chem. Res. 2017, 56, 2967–2975. [Google Scholar] [CrossRef]
- Chundawat, S.P.S.; Bellesia, G.; Uppugundla, N.; da Costa Sousa, L.; Gao, D.; Cheh, A.M.; Agarwal, U.P.; Bianchetti, C.M.; Phillips, G.N., Jr.; Langan, P.; et al. Restructuring the crystalline cellulose hydrogen bond network enhances its depolymerization rate. J. Am. Chem. Soc. 2011, 133, 11163–11174. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Maeda, A.; Inoue, T.; Sato, T. Dynamic segment size of the cellulose chain in an ionic liquid. Macromolecules 2013, 46, 7118–7124. [Google Scholar] [CrossRef]
- Fink, H.P.; Weigel, P.; Purz, H.J.; Ganster, J. Structure formation of regenerated cellulose materials from NMMO-solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Chen, X.; Burger, C.; Fang, D.; Ruan, D.; Zhang, L.; Hsiao, B.S.; Chu, B. X-ray studies of regenerated cellulose fibers wet spun from cotton linter pulp in NaOH/thiourea aqueous solutions. Polymer 2006, 47, 2839–2848. [Google Scholar] [CrossRef]
- Hermanutz, F.; Gähr, F.; Uerdingen, E.; Meister, F.; Kosan, B. New developments in dissolving and processing of cellulose in ionic liquids. Macromol. Symp. 2008, 262, 23–27. [Google Scholar] [CrossRef]
- McCormick, C.L.; Dawsey, T.R. Preparation of cellulose derivatives via ring-opening reactions with cyclic reagents in lithium chloride/N.N-dimethylacetamide. Macromolecules 1990, 23, 3606–3610. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T. Unconventional methods in cellulose functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, Y.; Zhou, J.; Cai, J. Effects of coagulation conditions on the properties of regenerated cellulose films prepared in NaOH/urea aqueous solution. Ind. Eng. Chem. Res. 2005, 44, 522–529. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, D.; Wu, F.; Cao, R. Deep oxidative desulfurization of fuels based on [C4mimCl]CoCl2 ionic liquid oxone solutions at room temperature. Fuel 2017, 208, 508–513. [Google Scholar] [CrossRef]
- Wang, J.; Xue, Z.; Yan, C.; Li, Z.; Mu, T. Fine regulation of cellulose dissolution and regeneration by low pressure CO2 in DMSO/organic base: Dissolution behavior and mechanism. Phys. Chem. Chem. Phys. 2016, 18, 32772–32779. [Google Scholar] [CrossRef]
- Meng, Y.; Pang, Z.; Dong, C. Enhancing cellulose dissolution in ionic liquid by solid acid addition. Carbohydr. Polym. 2017, 163, 317–323. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Vitz, J.; Erdmenger, T.; Haensch, C.; Schubert, U.S. Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem. 2009, 11, 417–424. [Google Scholar] [CrossRef]
- Dupont, J.; de Souza, R.F.; Suarez, P.A.Z. Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 2002, 102, 3667–3692. [Google Scholar] [CrossRef]
- Chen, F.F.; Huang, K.; Zhou, Y.; Tian, Z.Q.; Zhu, X.; Tao, D.J.; Jiang, D.E.; Dai, S. Multi-molar absorption of co2 by the activation of carboxylate groups in amino acid ionic liquids. Angew. Chem. Int. Ed. 2016, 55, 7166–7170. [Google Scholar] [CrossRef]
- Tao, D.J.; Chen, F.F.; Tian, Z.Q.; Huang, K.; Mahurin, S.M.; Jiang, D.E.; Dai, S. Highly efficient carbon monoxide capture by carbanion-functionalized ionic liquids through c-site interactions. Angew. Chem. 2017, 129, 6947–6951. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Xu, A.; Chen, L.; Wang, J. Functionalized imidazalium carboxylates for enhancing practical applicability in cellulose processing. Macromolecules 2018, 51, 4158–4166. [Google Scholar] [CrossRef]
- Xu, A.; Wang, J.; Wang, H. Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems. Green Chem. 2010, 12, 268–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, A.; Lu, B.; Li, Z.; Wang, J. Dissolution of cellulose in 1-allyl-3-methylimizodalium carboxylates at room temperature: A structure–property relationship study. Carbohydr. Polym. 2015, 117, 666–672. [Google Scholar] [CrossRef]
- Sun, X.; Chi, Y.; Mu, T. Studies on staged precipitation of cellulose from an ionic liquid by compressed carbon dioxide. Green Chem. 2014, 16, 2736–2744. [Google Scholar] [CrossRef]
- Fukaya, Y.; Sugimoto, A.; Ohno, H. Superior solubility of polysaccharides in low viscosity, polar, and halogen-free 1,3-dialkylimidazolium formates. Biomacromolecules 2006, 7, 3295–3297. [Google Scholar] [CrossRef]
- Lu, B.; Xu, A.; Wang, J. Cation does matter: How cationic structure affects the dissolution of cellulose in ionic liquids. Green Chem. 2014, 16, 1326–1335. [Google Scholar] [CrossRef]
- Fukaya, Y.; Hayashi, K.; Wada, M.; Ohno, H. Cellulose dissolution with polar ionic liquids under mild conditions: Required factors for anions. Green Chem. 2008, 10, 44–46. [Google Scholar] [CrossRef]
- Hou, X.D.; Xu, J.; Li, N.; Zong, M.H. Effect of anion structures on cholinium ionic liquids pretreatment of rice straw and the subsequent enzymatic hydrolysis. Biotechnol. Bioeng. 2015, 112, 65–73. [Google Scholar] [CrossRef]
- Liu, Q.P.; Hou, X.D.; Li, N.; Zong, M.H. Ionic liquids from renewable biomaterials: Synthesis, characterization and application in the pretreatment of biomass. Green Chem. 2012, 14, 304–307. [Google Scholar] [CrossRef]
- Kostag, M.; Liebert, T.; El Seoud, O.A.; Heinze, T. Efficient cellulose solvent: Quaternary ammonium chlorides. Macromol. Rapid Comm. 2013, 34, 1580–1584. [Google Scholar] [CrossRef]
- Rinaldi, R. Instantaneous dissolution of cellulose in organic electrolyte solutions. Chem. Commun. 2011, 47, 511–513. [Google Scholar] [CrossRef]
- Xu, A.; Cao, L.; Wang, B. Facile cellulose dissolution without heating in [C4mim][CH3COO]/DMF solvent. Carbohydr. Polym. 2015, 125, 249–254. [Google Scholar] [CrossRef]
- Xu, A.; Guo, X.; Xu, R. Understanding the dissolution of cellulose in 1-butyl-3-methylimidazolium acetate+DMAc solvent. Int. J. Biol. Macromol. 2015, 81, 1000–1004. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, Y.; Zhao, Y.; Wang, J. Cellulose dissolution at ambient temperature: Role of preferential solvation of cations of ionic liquids by a cosolvent. Carbohydr. Polym. 2013, 92, 540–544. [Google Scholar] [CrossRef]
- Xu, A.R.; Guo, X.; Ma, J.Y. Properties of cellulose regenerated from powerful 1-butyl-3-methylimidazolium acetate/dimethyl sulfoxide solvent. J. Macromol. Sci. B 2016, 55, 559–565. [Google Scholar] [CrossRef]
- Xu, A.; Li, Q. Sustainable and low viscous 1-allyl-3-methylimidazolium acetate + PEG solvent for cellulose processing. Polymers 2017, 9, 54. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhao, Y.; Zhang, H.; Wang, J. Molecular origin for the difficulty in separation of 5-hydroxymethylfurfural from imidazolium based ionic liquids. ACS Sustain. Chem. Eng. 2016, 4, 6712–6721. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohyd. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, J.; Zhang, J.; Li, H.; Zhang, Y.; He, J. Room temperature ionic liquids (RTILs): A new and versatile platform for cellulose processing and derivatization. Chem. Eng. J. 2009, 147, 13–21. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Zhang, L.; Ruan, D.; Zhou, J. Structure and properties of regenerated cellulose films prepared from cotton linters in NaOH/urea aqueous solution. Ind. Eng. Chem. Res. 2001, 40, 5923–5928. [Google Scholar] [CrossRef]
- Nathan, K.-Z.; Cantero-Tubilla, B.; Wilson., D.B. Characterization of cellulose crystallinity after enzymatic treatment using Fourier transform infrared spectroscopy (FTIR). Cellulose 2018, 25, 37–48. [Google Scholar]
- Tayeb, A.H.; Sadeghifar, H.; Hubbe, M.A.; Rojas, O.J. Lipoxygenase-mediated peroxidation of model plant extractives. Ind. Crop. Prod. 2017, 104, 253–262. [Google Scholar] [CrossRef]
- Higgins, H.G.; Stewart, C.M.; Harrington, K.J. Infrared spectra of cellulose and related polysaccharides. J. Polym. Sci. 1961, 51, 59–84. [Google Scholar] [CrossRef]
- Kataoka, Y.; Kondo, T. FT-IR microscopic analysis of changing cellulose crystalline structure during wood cell wall formation. Macromolecules 1998, 31, 760–764. [Google Scholar] [CrossRef]
- Tashiro, K.; Hongo, T.; Shirataki, H.; Yamane, C.; Ii, T. Influence of water on structure and mechanical properties of regenerated cellulose studied by an organized combination of infrared spectra, x-ray diffraction, and dynamic viscoelastic data measured as functions of temperature and humidity. Macromolecules 2001, 34, 1274–1280. [Google Scholar]
- Qu, L.J.; Zhang, Y.; Wang, J.Q.; Chi, D.L. Properties of new natural fibers: Eulaliopsis binata fibers. J. Qingdao Univ. 2008, 23, 44–47. [Google Scholar]

| [A2im][CH3OCH2COO]/DMSO solvents | |||
| Solvents | RDMSO | Solubility | Weight percentage of cellulose |
| [A2im][CH3OCH2COO] (RDMSO = 0) | 0 | 16.2 | 13.9 |
| [A2im][CH3OCH2COO]/DMSO (RDMSO = 0.17) | 0.17 | 19.6 | 16.4 |
| [A2im][CH3OCH2COO]/DMSO (RDMSO = 0.33) | 0.33 | 22.2 | 18.2 |
| [A2im][CH3OCH2COO]/DMSO (RDMSO = 0.50) | 0.50 | 25.3 | 20.2 |
| [A2im][CH3OCH2COO]/DMSO (RDMSO = 1.01) | 1.01 | 26.1 | 20.7 |
| [A2im][CH3OCH2COO]/DMSO (RDMSO = 2.00) | 2.00 | 20.9 | 17.3 |
| [A2im][CH3OCH2COO]/DMSO (RDMSO = 3.02) | 3.02 | 15.6 | 13.5 |
| [A2im][CH3OCH2COO]/DMSO (RDMSO = 6.01) | 6.01 | 11.4 | 10.2 |
| DMSO(RDMSO = 1) | – a | 0 | 0 |
| [A2im][CH3OCH2COO]/DMF solvents | |||
| Solvents | RDMF | Solubility | Weight percentage of cellulose |
| [A2im][CH3OCH2COO] (RDMF = 0) | 0 | 16.2 | 13.9 |
| [A2im][CH3OCH2COO]/DMF (RDMF = 0.17) | 0.17 | 20.2 | 16.8 |
| [A2im][CH3OCH2COO]/DMF (RDMF = 0.33) | 0.33 | 22.0 | 18.0 |
| [A2im][CH3OCH2COO]/DMF (RDMF = 0.50) | 0.50 | 23.1 | 18.8 |
| [A2im][CH3OCH2COO]/DMF (RDMF = 1.05) | 1.05 | 21.1 | 17.4 |
| [A2im][CH3OCH2COO]/DMF (RDMF = 2.03) | 2.03 | 16.0 | 13.8 |
| [A2im][CH3OCH2COO]/DMF (RDMF = 3.03) | 3.03 | 8.9 | 8.2 |
| [A2im][CH3OCH2COO]/DMF (RDMF = 6.01) | 6.01 | 5.7 | 5.4 |
| DMF(RDMF = 1) | – a | 0 | 0 |
| [A2im][CH3OCH2COO]/DMA solvents | |||
| Solvents | RDMA | Solubility | Weight percentage of cellulose |
| [A2im][CH3OCH2COO] (RDMA = 0) | 0 | 16.2 | 13.9 |
| [A2im][CH3OCH2COO]/DMA (RDMA = 0.17) | 0.17 | 19.3 | 16.2 |
| [A2im][CH3OCH2COO]/DMA (RDMA = 0.33) | 0.33 | 21.4 | 17.6 |
| [A2im][CH3OCH2COO]/DMA (RDMA = 0.50) | 0.50 | 21.8 | 17.9 |
| [A2im][CH3OCH2COO]/DMA (RDMA = 1.00) | 1.00 | 17.7 | 15.0 |
| [A2im][CH3OCH2COO]/DMA (RDMA = 2.02) | 2.02 | 13.8 | 12.1 |
| [A2im][CH3OCH2COO]/DMA (RDMA = 3.00) | 3.00 | 6.1 | 5.7 |
| [A2im][CH3OCH2COO]/DMA (RDMA = 6.03) | 6.03 | 2.8 | 2.7 |
| DMAc(RDMA = 1) | – a | 0 | 0 |
| [A2im][CH3OCH2COO]/DMSO/Cellulose Solution | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cellulose Concentration (%) | δ (ppm) | |||||||
| C2 | C4,4′ | C5,5′ | C6,6′ | C7,7′ | C8 | C9 | C10 | |
| 0 | 137.80 | 122.93 | 57.46 | 132.48 | 119.51 | 50.57 | 72.99 | 172.81 |
| 8 | 137.52 | 122.90 | 57.57 | 132.35 | 119.72 | 50.70 | 72.77 | 173.25 |
| Δδ | −0.28 | −0.03 | 0.11 | −0.13 | 0.21 | 0.13 | −0.22 | 0.44 |
| [A2im][CH3OCH2COO]/DMF/cellulose solution | ||||||||
| 0 | 138.98 | 123.83 | 58.19 | 133.41 | 120.24 | 51.52 | 73.93 | 173.91 |
| 8 | 138.67 | 123.78 | 58.26 | 133.27 | 120.39 | 51.62 | 73.69 | 174.29 |
| Δδ | −0.31 | −0.05 | 0.07 | −0.14 | 0.15 | 0.10 | −0.24 | 0.38 |
| [A2im][CH3OCH2COO]/DMA/cellulose solution | ||||||||
| 0 | 137.77 | 122.57 | 56.89 | 132.23 | 118.95 | 50.18 | 72.64 | 172.53 |
| 8 | 137.37 | 122.47 | 56.93 | 132.02 | 119.06 | 50.26 | 72.34 | 172.90 |
| Δδ | −0.40 | −0.10 | 0.04 | −0.21 | 0.11 | 0.08 | −0.30 | 0.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, A.; Chen, L.; Wang, Y.; Liu, R.; Niu, W. Development of Diallylimidazolium Methoxyacetate/DMSO (DMF/DMA) Solvents for Improving Cellulose Dissolution and Fabricating Porous Material. Polymers 2019, 11, 845. https://doi.org/10.3390/polym11050845
Xu A, Chen L, Wang Y, Liu R, Niu W. Development of Diallylimidazolium Methoxyacetate/DMSO (DMF/DMA) Solvents for Improving Cellulose Dissolution and Fabricating Porous Material. Polymers. 2019; 11(5):845. https://doi.org/10.3390/polym11050845
Chicago/Turabian StyleXu, Airong, Lin Chen, Yongxin Wang, Rukuan Liu, and Wentian Niu. 2019. "Development of Diallylimidazolium Methoxyacetate/DMSO (DMF/DMA) Solvents for Improving Cellulose Dissolution and Fabricating Porous Material" Polymers 11, no. 5: 845. https://doi.org/10.3390/polym11050845
APA StyleXu, A., Chen, L., Wang, Y., Liu, R., & Niu, W. (2019). Development of Diallylimidazolium Methoxyacetate/DMSO (DMF/DMA) Solvents for Improving Cellulose Dissolution and Fabricating Porous Material. Polymers, 11(5), 845. https://doi.org/10.3390/polym11050845




