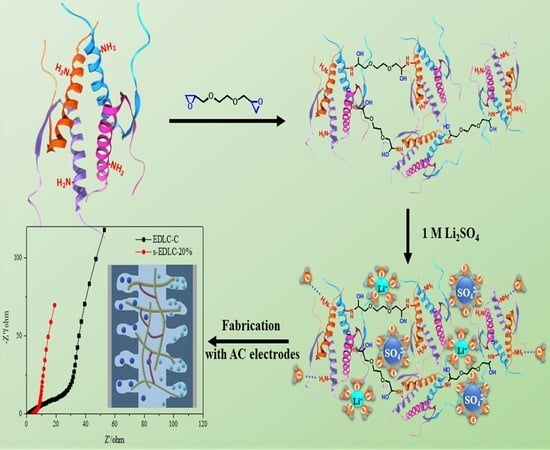
A Crosslinked Soybean Protein Isolate Gel Polymer Electrolyte Based on Neutral Aqueous Electrolyte for a High-Energy-Density Supercapacitor
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of SPI Membranes and GPEs
2.3. Fabrication of the EDLC Single Cells with the GPE-x
2.4. Characterization
2.4.1. FTIR
2.4.2. XRD
2.4.3. Ionic Conductivity
2.4.4. Electrochemical Performances of EDLC Single Cells
3. Results and Discussion
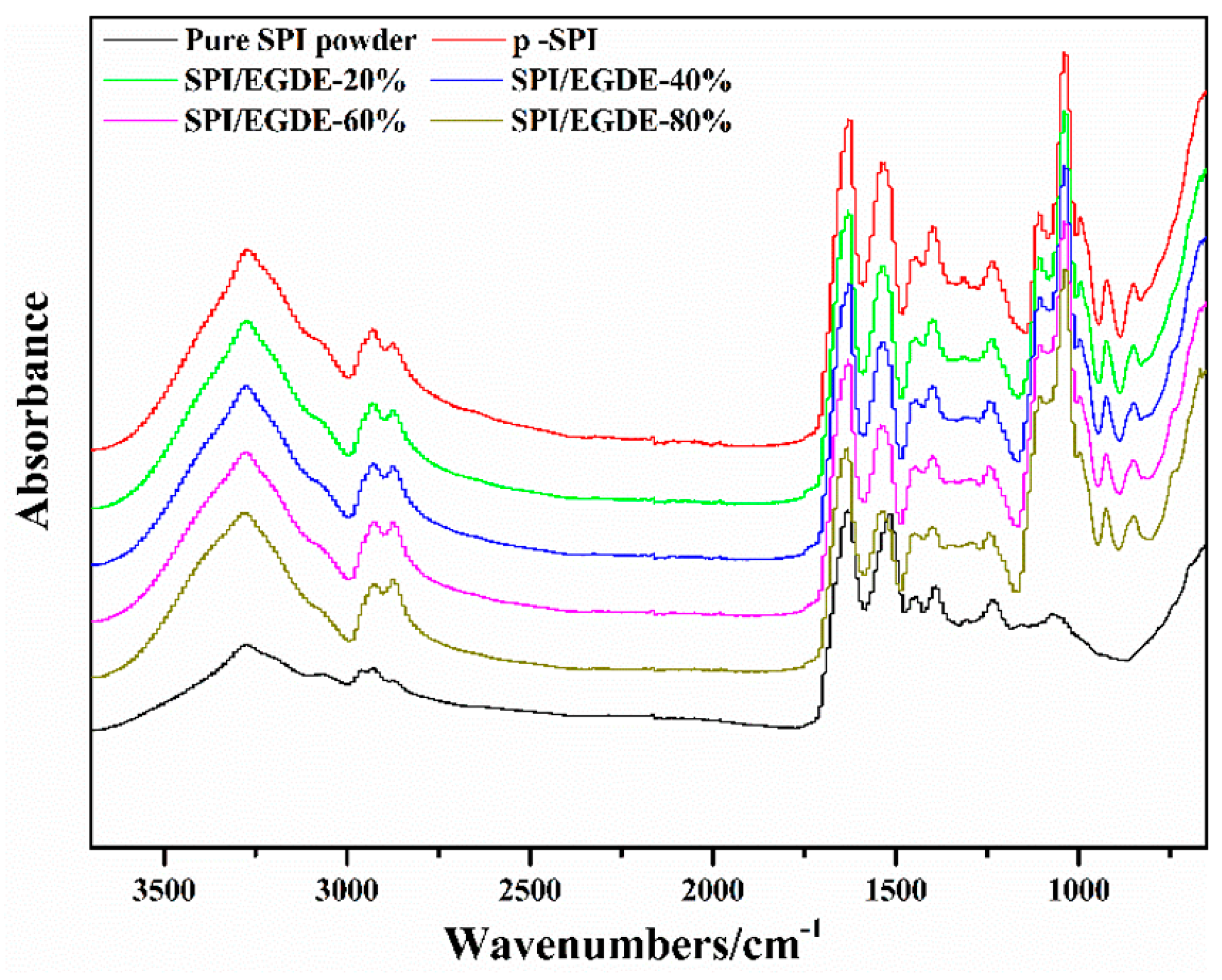
3.1. ATR-FTIR Spectra of SPI Membranes
3.2. XRD of SPI Membranes
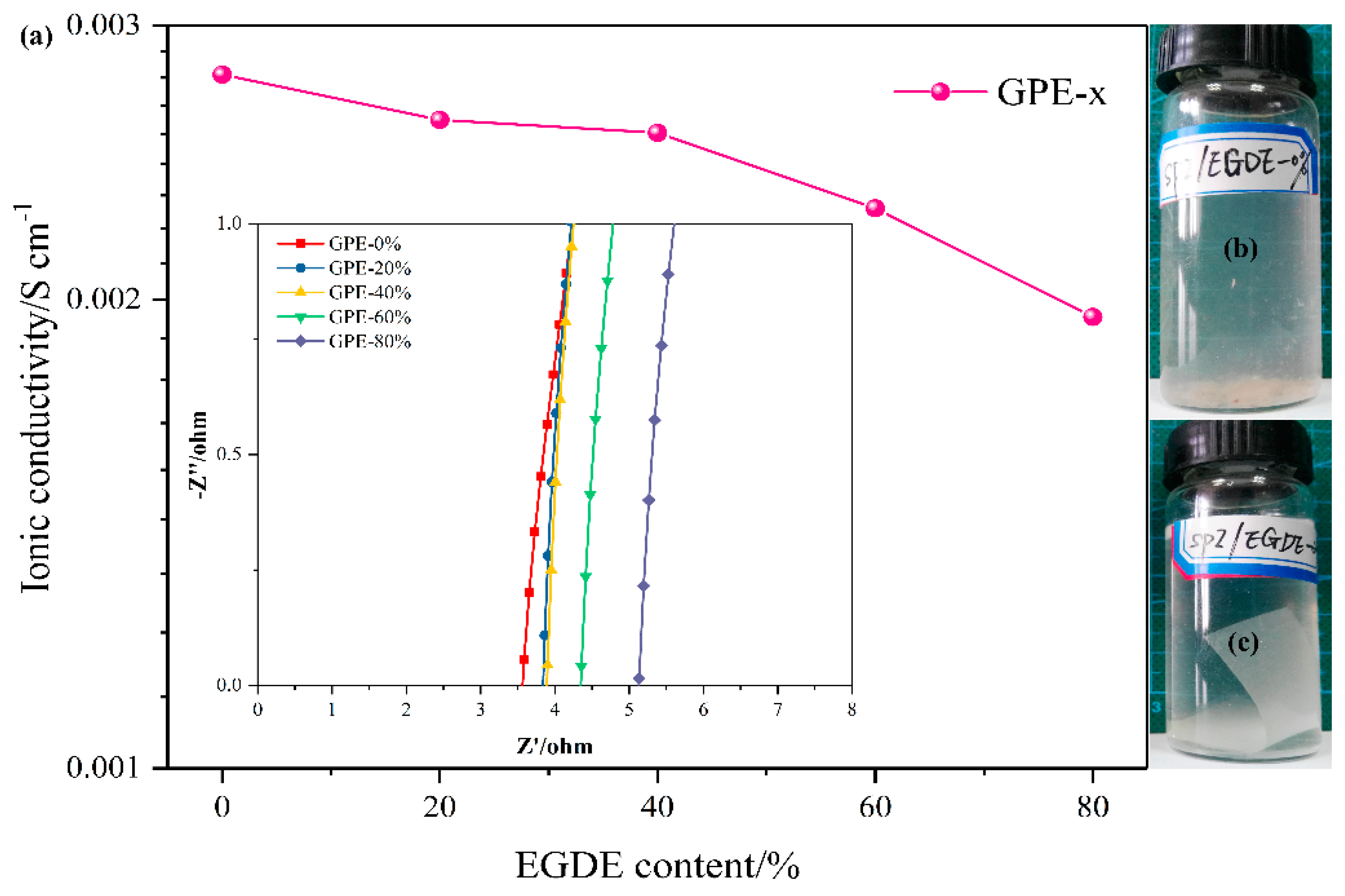
3.3. Ionic Conductivities of GPE
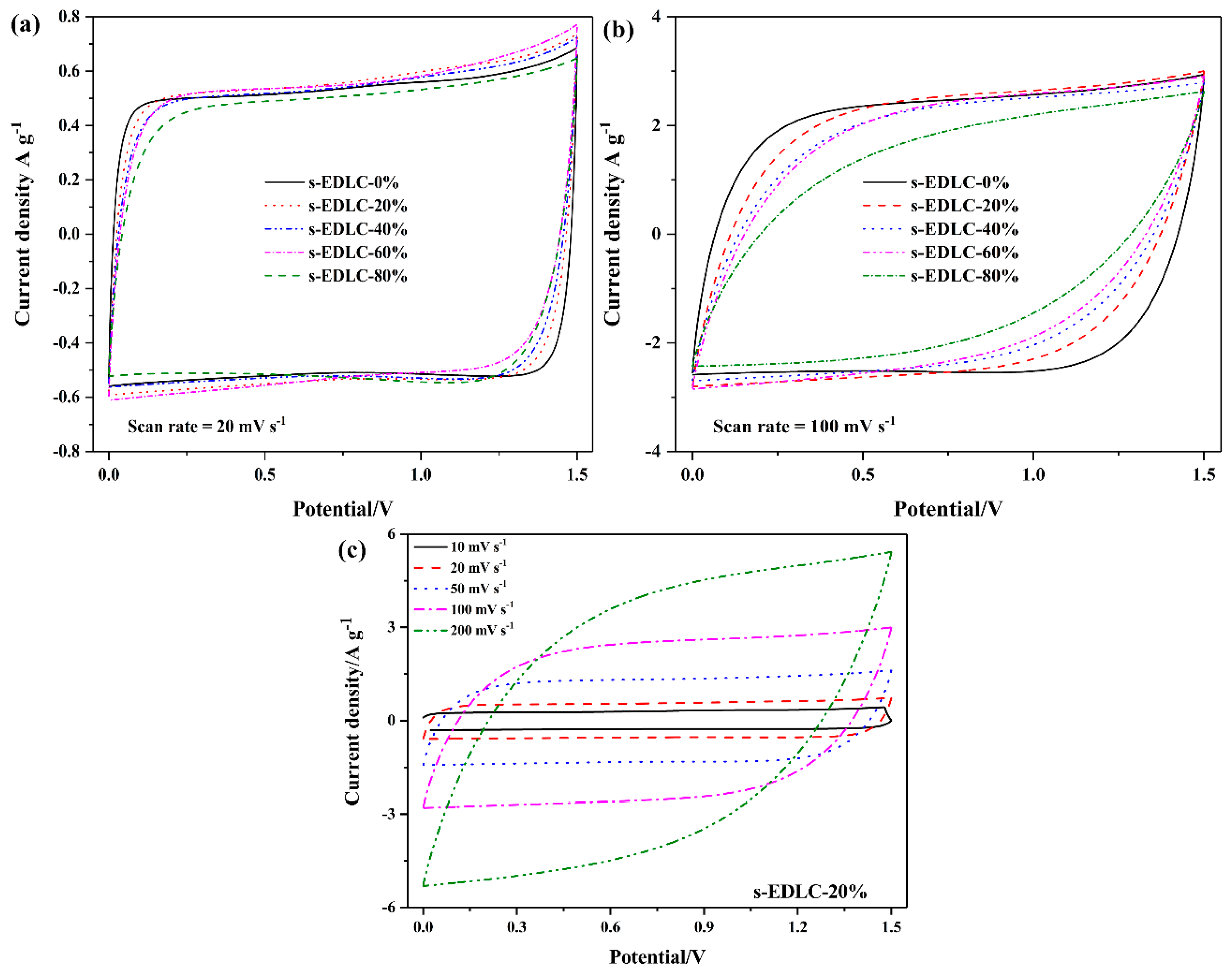
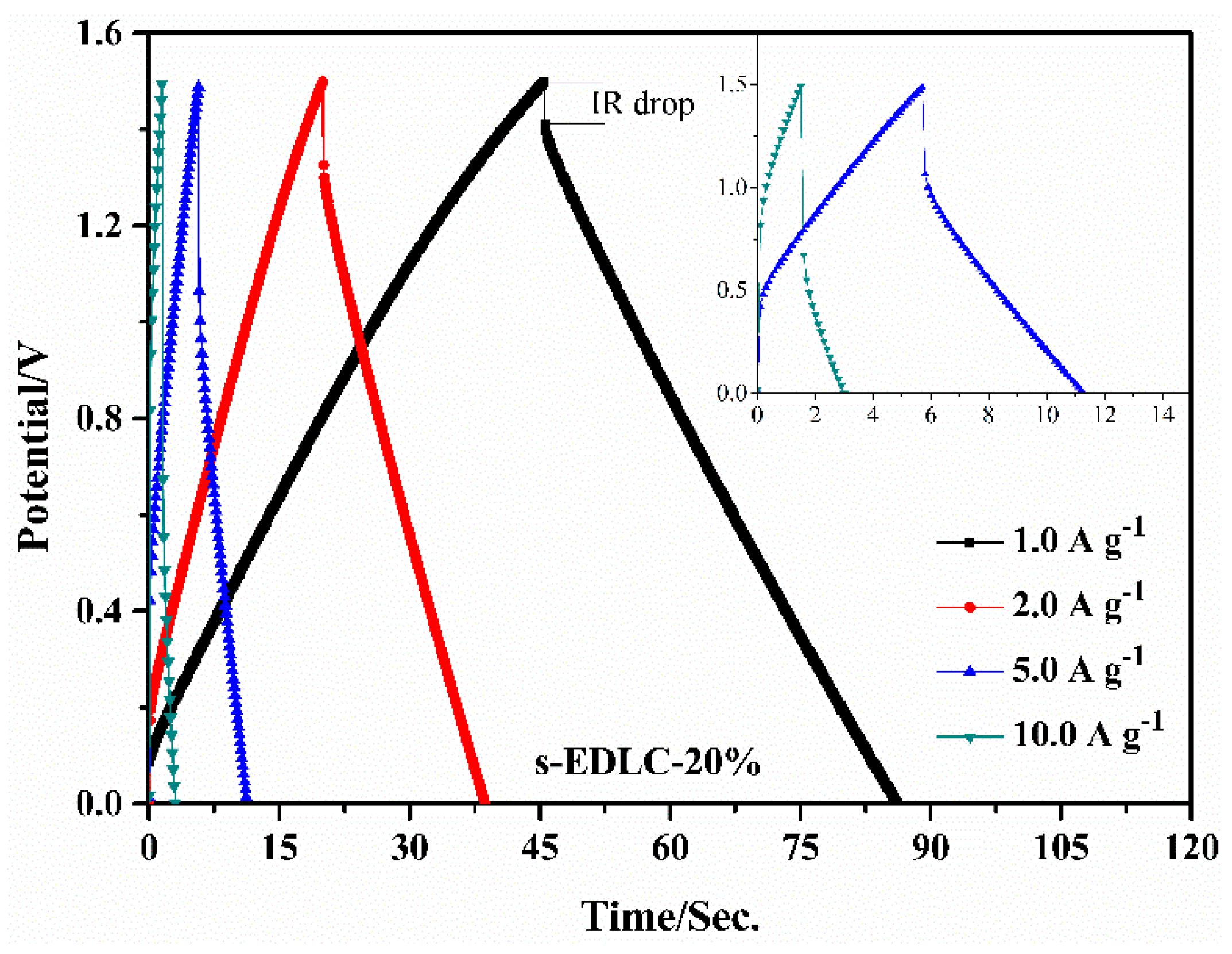
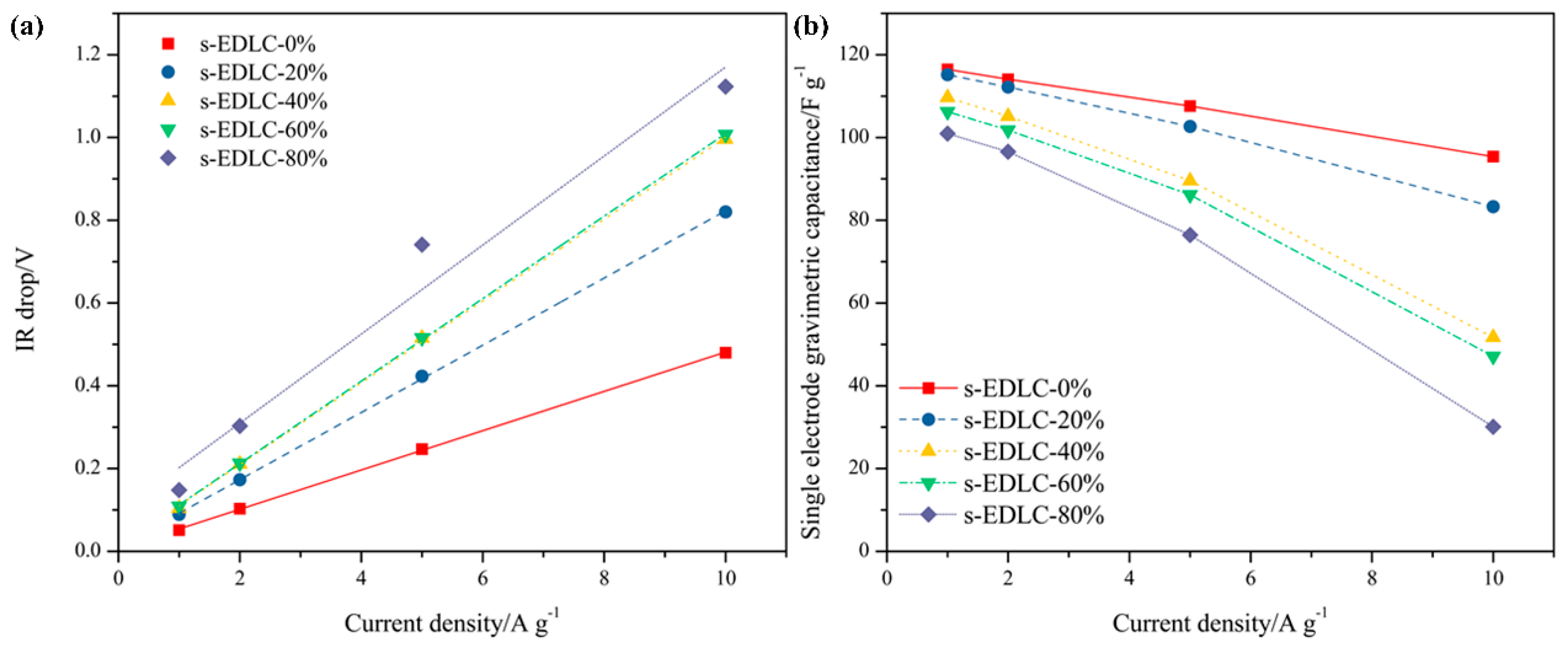
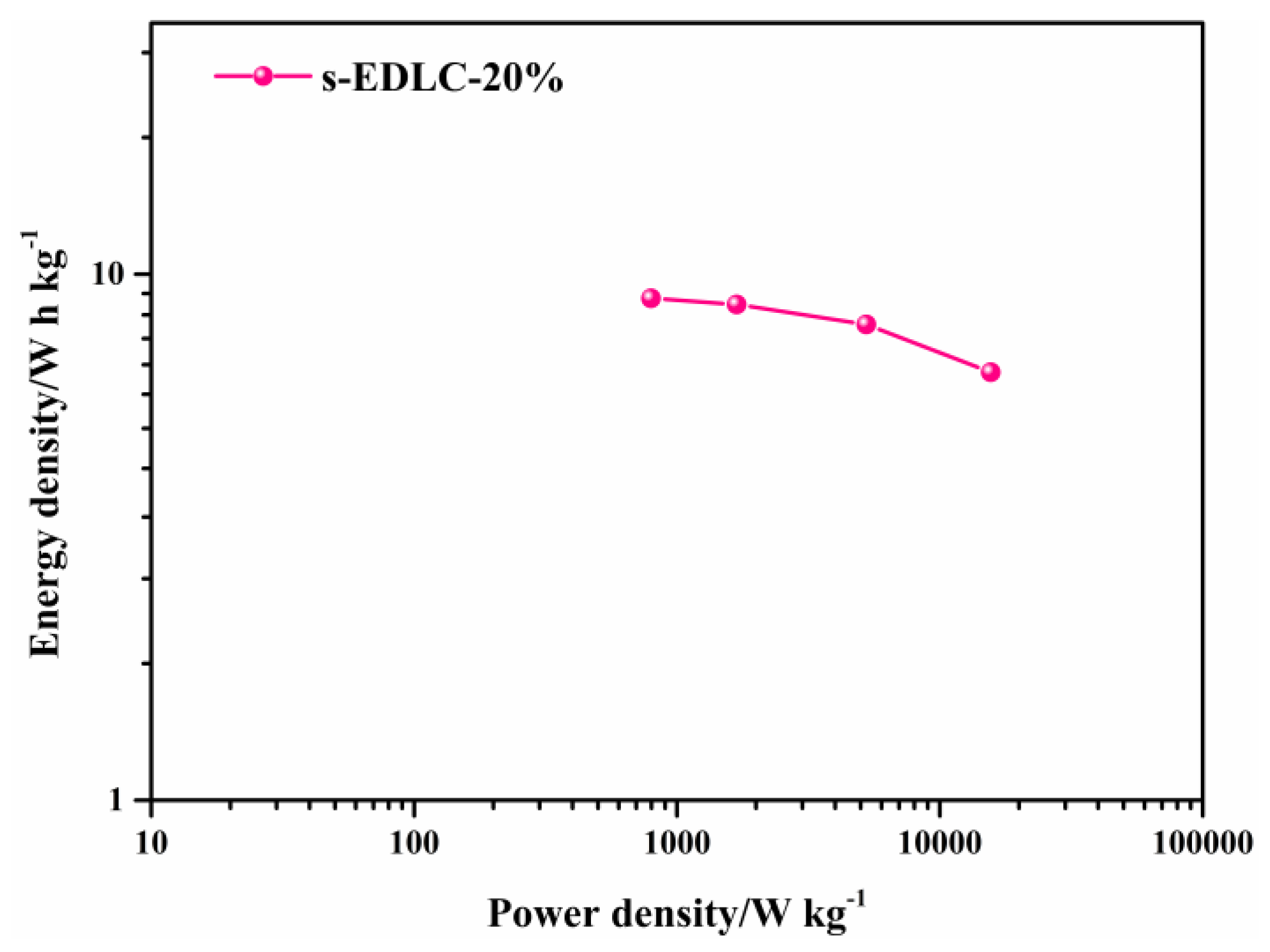
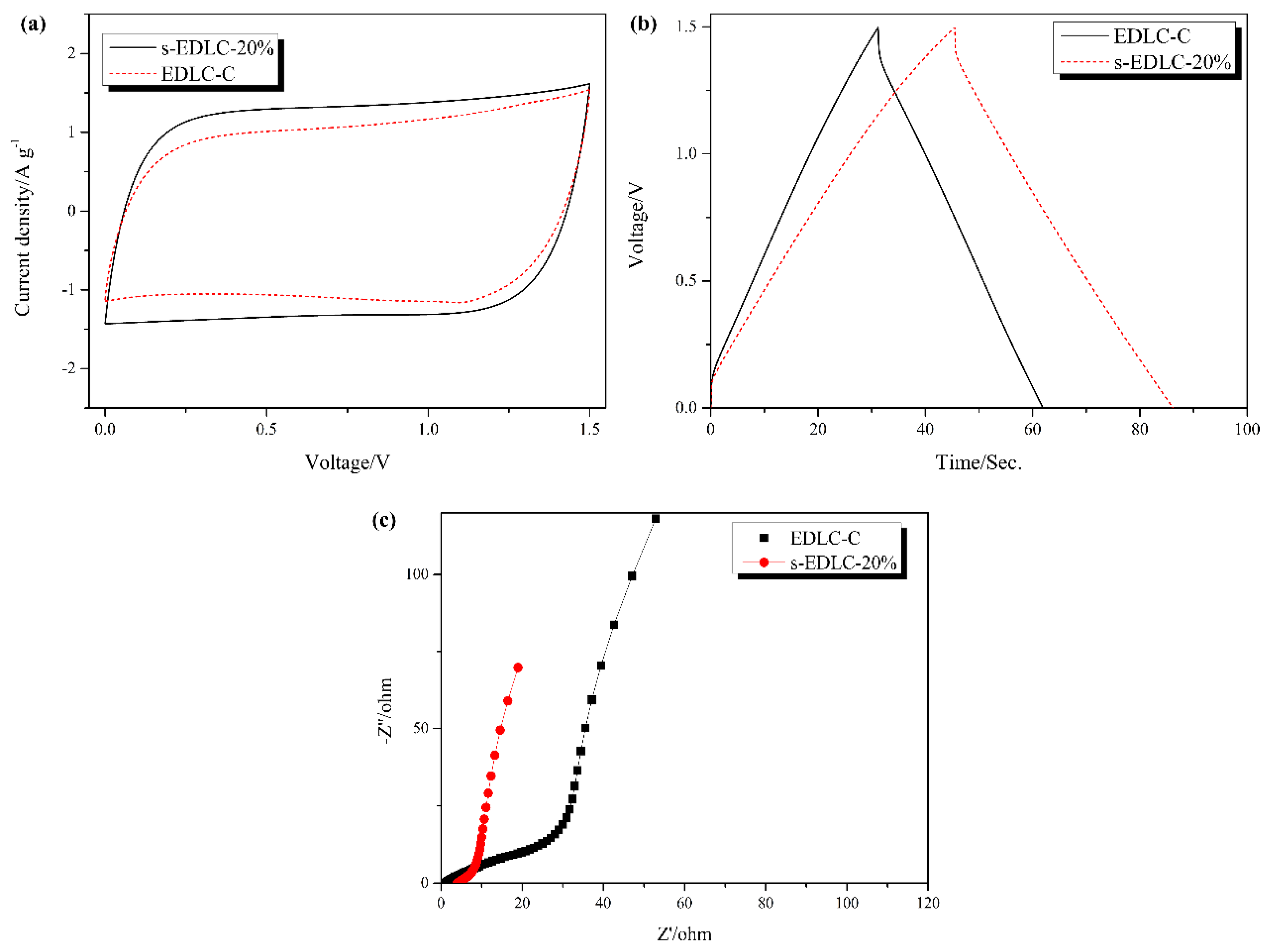
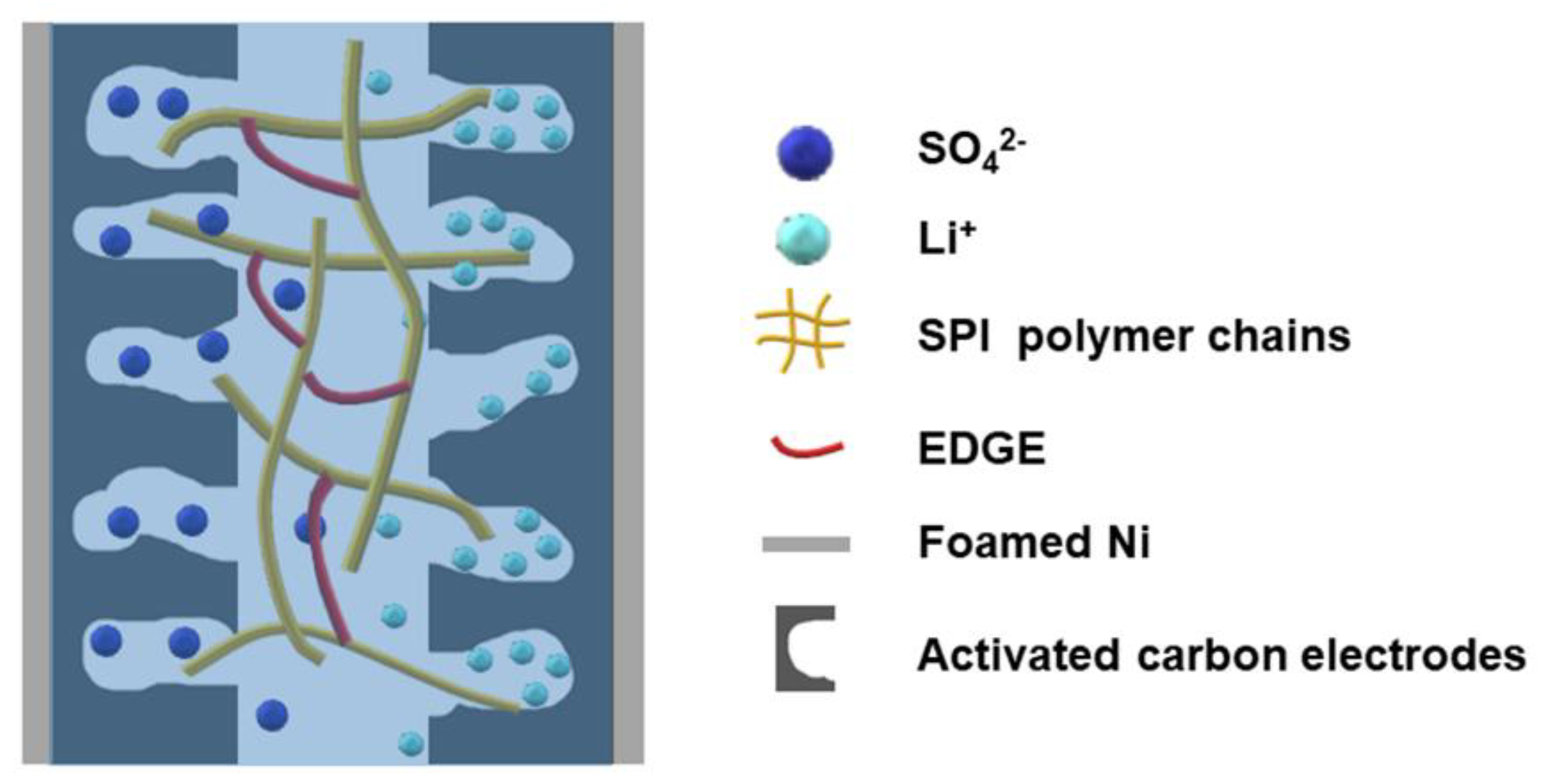
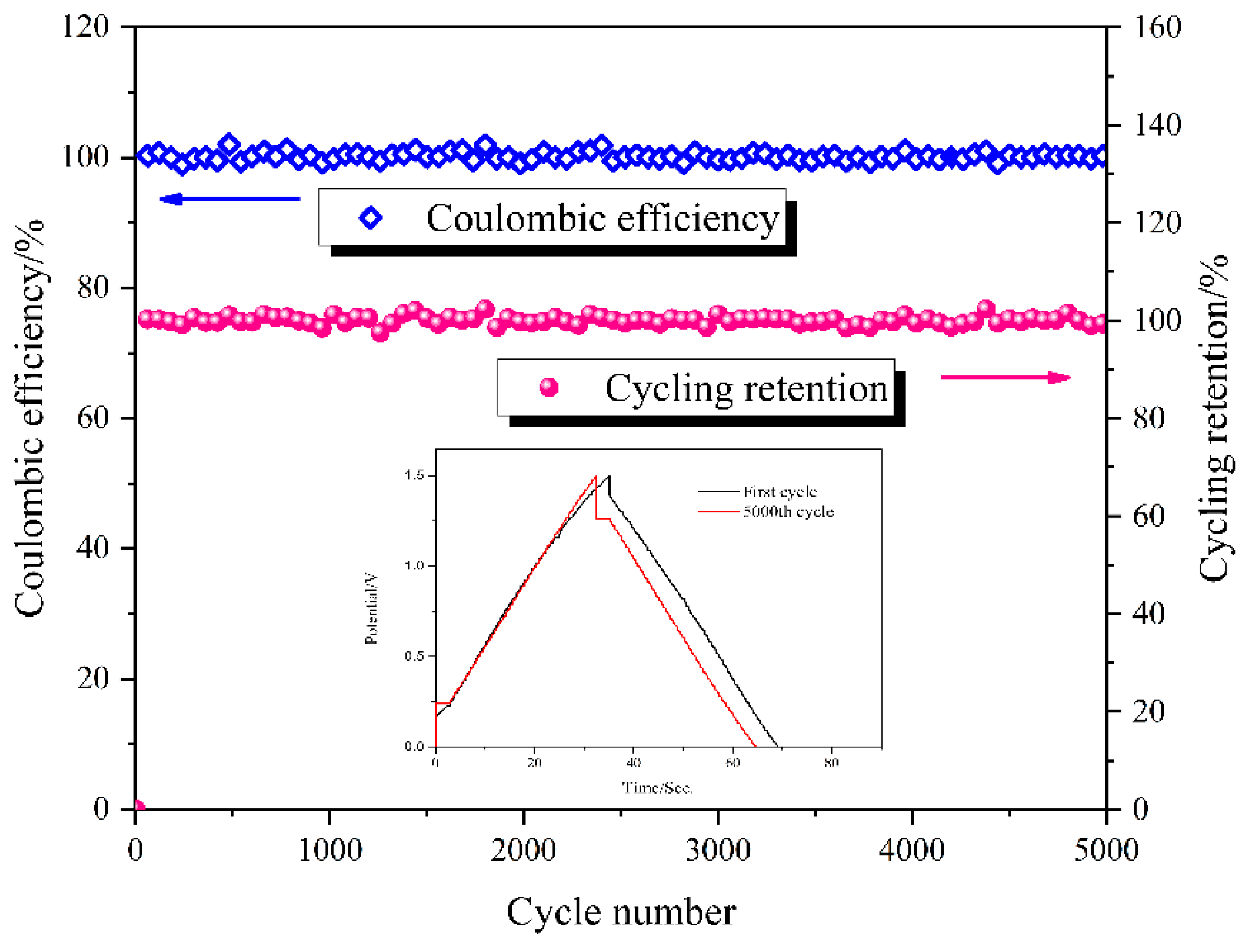
3.4. Electrochemical Properties of the Solid-State Supercapacitors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Peng, L.; Wu, C.; Xie, Y. Two dimensional nanomaterials for flexible supercapacitors. Chem. Soc. Rev. 2014, 43, 3303–3323. [Google Scholar] [CrossRef]
- Peng, X.; Liu, H.; Yin, Q.; Wu, J.; Chen, P.; Zhang, G.; Liu, G.; Wu, C.; Xie, Y. A zwitterionic gel electrolyte for efficient solid-state supercapacitors. Nat. Commun. 2016, 7, 11782. [Google Scholar] [CrossRef]
- Nath, N.C.D.; Jeon, I.Y.; Ju, M.J.; Ansari, S.A.; Baek, J.B.; Lee, J.J. Edge-carboxylated graphene nanoplatelets as efficient electrode materials for electrochemical supercapacitors. Carbon 2019, 142, 89–98. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Li, Y.; Dai, J.; Song, J.; Yao, Y.; Gong, Y.; Kierzewski, I.; Xie, J.; Hu, L. All-wood, low tortuosity, aqueous, biodegradable supercapacitors with ultra-high capacitance. Energy Environ. Sci. 2017, 10, 538–545. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Q.; Lin, C.; Wei, T.; Fan, Z. Interconnected frameworks with a sandwiched porous carbon layer/graphene hybrids for supercapacitors with high gravimetric and volumetric performances. Adv. Energy Mater. 2014, 4, 1400500. [Google Scholar] [CrossRef]
- Rahmanifar, M.S.; Hemmati, M.; Noori, A.; El-Kady, M.F.; Mousavi, M.F.; Kaner, R.B. Asymmetric supercapacitors: An alternative to activated carbon negative electrodes based on earth abundant elements. Mater. Today Energy 2019, 12, 26–36. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, S.; Wu, J.; Shi, X.; Zhu, B.; Meng, X. Wearable high-performance supercapacitors based on silver-sputtered textiles with FeCo2S4–NiCo2S4 composite nanotube-built multitripod architectures as advanced flexible electrodes. Adv. Energy Mater. 2017, 7, 1601234. [Google Scholar] [CrossRef]
- Huang, C.W.; Wu, C.A.; Hou, S.S.; Kuo, P.L.; Hsieh, C.T.; Teng, H. Gel electrolyte derived from poly (ethylene glycol) blending poly (acrylonitrile) applicable to roll-to-roll assembly of electric double layer capacitors. Adv. Funct. Mater. 2012, 22, 4677–4685. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Q.; Wang, W.; Dong, H.; Xu, D.; Zou, G.; Duan, H.; Yu, D. Novel planar-structure electrochemical devices for highly flexible semitransparent power generation/storage sources. Nano Lett. 2013, 13, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Peng, J.; Cao, P.; Cai, X.; Li, J.; Zhai, M. A flexible ionic liquid gelled PVA-Li2SO4 polymer electrolyte for semi-solid-state supercapacitors. Adv. Mater. Interfaces 2015, 2, 1500267. [Google Scholar] [CrossRef]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, C.; Zhang, Q.; Chen, W.; Liu, S.; Wang, Q.; Li, Y.; Yu, H. High performance, flexible, solid-state supercapacitors based on a renewable and biodegradable mesoporous cellulose membrane. Adv. Energy Mater. 2017, 7, 1700739. [Google Scholar] [CrossRef]
- Na, R.; Huo, G.; Zhang, S.; Huo, P.; Du, Y.; Luan, J.; Zhu, K.; Wang, G. A novel poly (ethylene glycol)–grafted poly (arylene ether ketone) blend micro-porous polymer electrolyte for solid-state electric double layer capacitors formed by incorporating a chitosan-based LiClO4 gel electrolyte. J. Mater. Chem. A 2016, 4, 18116–18127. [Google Scholar] [CrossRef]
- Wang, X.; Chandrabose, R.S.; Jian, Z.; Xing, Z.; Ji, X. A 1.8 V aqueous supercapacitor with a bipolar assembly of ion-exchange membranes as the separator. J. Electrochem. Soc. 2016, 163, A1853–A1858. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; Jiang, L.; Wu, C.; Zhao, Q.; Liu, X.; Yi, L. The effects of electrolyte on the supercapacitive performance of activated calcium carbide-derived carbon. J. Power Sources 2013, 226, 202–209. [Google Scholar] [CrossRef]
- Yang, C.M.; Kim, Y.J.; Endo, M.; Kanoh, H.; Yudasaka, M.; Iijima, S.; Kaneko, K. Nanowindow-regulated specific capacitance of supercapacitor electrodes of single-wall carbon nanohorns. J. Am. Chem. Soc. 2007, 129, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Yoshikawa, K.; Nohara, S.; Furukawa, N.; Inoue, H.; Sugoh, N.; Iwasaki, H.; Iwakura, C. Electrochemical characteristics of new electric double layer capacitor with acidic polymer hydrogel electrolyte. J. Power Sources 2006, 159, 1464–1467. [Google Scholar] [CrossRef]
- Lee, K.T.; Wu, N.L. Manganese oxide electrochemical capacitor with potassium poly (acrylate) hydrogel electrolyte. J. Power Sources 2008, 179, 430–434. [Google Scholar] [CrossRef]
- Hu, J.; Xie, K.; Liu, X.; Guo, S.; Shen, C.; Liu, X.; Li, X.; Wang, J.; Wei, B. Dramatically enhanced ion conductivity of gel polymer electrolyte for supercapacitor via h-BN nanosheets doping. Electrochim. Acta 2017, 227, 455–461. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.; Zang, X.; Cao, Y.; He, Y.; Li, P.; Wang, K.; Wei, J.; Wu, D.; Zhu, H. Effect of different gel electrolytes on graphene-based solid-state supercapacitors. RSC Adv. 2014, 4, 36253–36256. [Google Scholar] [CrossRef]
- Wada, H.; Nohara, S.; Furukawa, N.; Inoue, H.; Sugoh, N.; Iwasaki, H.; Morita, M.; Iwakura, C. Electrochemical characteristics of electric double layer capacitor using sulfonated polypropylene separator impregnated with polymer hydrogel electrolyte. Electrochim. Acta 2004, 49, 4871–4875. [Google Scholar] [CrossRef]
- Yin, Y.; Zhou, J.; Mansour, A.N.; Zhou, X. Effect of NaI/I2 mediators on properties of PEO/LiAlO2 based all-solid-state supercapacitors. J. Power Sources 2011, 196, 5997–6002. [Google Scholar] [CrossRef]
- Solarajan, A.K.; Murugadoss, V.; Angaiah, S. Montmorillonite embedded electrospun PVdF–HFP nanocomposite membrane electrolyte for Li-ion capacitors. Appl. Mater. Today 2016, 5, 33–40. [Google Scholar] [CrossRef]
- Fu, X.; Jewel, Y.; Wang, Y. Decoupled ion transport in a protein-based solid ion conductor. J. Phys. Chem. Lett. 2016, 7, 4304–4310. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Tan, C.; Fang, Q.; Gao, L.; Sui, G.; Yang, X. High performance and biodegradable skeleton material based on soy protein isolate for gel polymer electrolyte. ACS Sustain. Chem. Eng. 2016, 4, 4498–4505. [Google Scholar] [CrossRef]
- Dias, T.P.; Grosso, C.R.; Andreuccetti, C.; Carvalho, R.A.D.; Galicia-García, T.; Martinez-Bustos, F. Effect of the addition of soy lecithin and Yucca schidigera extract on the properties of gelatin and glycerol based biodegradable films. Polímeros 2013, 23, 339–345. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, C.; Dong, Y.; Yan, Y.; Li, J.; Shi, S.Q.; Cai, L. Soy protein isolate-based films reinforced by surface modified cellulose nanocrystal. Ind. Crops Prod. 2016, 80, 207–213. [Google Scholar] [CrossRef]
- Zhao, Y.; He, M.; Zhao, L.; Wang, S.; Li, Y.; Gan, L.; Li, M.; Li, X.; Chang, P.R.; Anderson, D.P.; et al. Epichlorohydrin-crosslinked hydroxyethyl cellulose/soy protein isolate composite films as biocompatible and biodegradable implants for tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 2781–2795. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Physical and mechanical properties of compression molded and solution casting soybean protein concentrate based films. Food Hydrocoll. 2014, 38, 193–204. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Zhu, Q.; Chen, F.; Zhao, X.; Ao, Q. Determination of the domain structure of the 7s and 11s globulins from soy proteins by XRD and FTIR. J. Sci. Food Agric. 2013, 93, 1687–1691. [Google Scholar] [CrossRef]
- Liu, D.; Fu, C.; Zhang, N.; Zhou, H.; Kuang, Y. Three-dimensional porous nitrogen doped graphene hydrogel for high energy density supercapacitors. Electrochim. Acta 2016, 213, 291–297. [Google Scholar] [CrossRef]
- Hashemi, M.; Rahmanifar, M.S.; El-Kady, M.F.; Noori, A.; Mousavi, M.F.; Kaner, R.B. The use of an electrocatalytic redox electrolyte for pushing the energy density boundary of a flexible polyaniline electrode to a new limit. Nano Energy 2018, 44, 489–498. [Google Scholar] [CrossRef]
- Na, R.; Huo, P.; Zhang, X.; Zhang, S.; Du, Y.; Zhu, K.; Lu, Y.; Zhang, M.; Luan, J.; Wang, G. A flexible solid-state supercapacitor based on a poly (aryl ether ketone)–poly (ethylene glycol) copolymer solid polymer electrolyte for high temperature applications. RSC Adv. 2016, 6, 65186–65195. [Google Scholar] [CrossRef]
- Huo, P.; Liu, Y.; Na, R.; Zhang, X.; Zhang, S.; Wang, G. Quaternary ammonium functionalized poly (arylene ether sulfone)/poly (vinylpyrrolidone) composite membranes for electrical double-layer capacitors with activated carbon electrodes. J. Membr. Sci. 2016, 505, 148–156. [Google Scholar] [CrossRef]
- Na, R.; Wang, X.; Lu, N.; Huo, G.; Lin, H.; Wang, G. Novel egg white gel polymer electrolyte and a green solid-state supercapacitor derived from the egg and rice waste. Electrochim. Acta 2018, 274, 316–325. [Google Scholar] [CrossRef]
- Huo, P.; Zhang, S.; Zhang, X.; Geng, Z.; Luan, J.; Wang, G. Quaternary ammonium functionalized poly (aryl ether sulfone) s as separators for supercapacitors based on activated carbon electrodes. J. Membr. Sci. 2015, 475, 562–570. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Zhang, F.; Zhang, T.; Huang, Y.; Chen, Y. A high-performance all-solid-state supercapacitor with graphene-doped carbon material electrodes and a graphene oxide-doped ion gel electrolyte. Carbon 2014, 72, 381–386. [Google Scholar] [CrossRef]

| Electrode Materials | Electrolytes | Specific Capacitance (Cs) | Energy Density (Ecell) | Operating Voltage | Reference |
|---|---|---|---|---|---|
| AC a | SPI/EGDE/Li2SO4 | 115.17 F·g−1 at 1.0 A·g−1 (RT h) | 9.00 W·h·kg−1 at 1.0 A·g−1 (RT) | 1.5 | This work |
| AC | mCe-membrane/KOH c | 120.6 F·g−1 at 0.5 A·g−1 (RT) | 4.37 W·h·kg−1 at 0.5 A·g−1 (RT) | 1.0 | [15] |
| AC | PAEK–40%PEG–LiClO4 d | 103.17 F·g−1 at 0.1 A·g−1 (120 °C) | 6.76 at 0.1 A·g−1 (120 °C) | 1.5 | [35] |
| AC | PAES-Q/PVP/KOH e | 140.85 F·g−1 at 0.1 A·g−1 (RT) | 4.81 W·h·kg−1 at 0.1 A·g−1 (RT) | 1.0 | [36] |
| RH-AC b | EW-GPE-2 (NaCl) f | 214.3 F·g−1 at 0.2 A·g−1 (RT) | 4.76 W·h·kg−1 at 0.2 A·g−1 (RT) | 0.8 | [37] |
| AC | PAES-Q-1.1 g | 92.79 F·g−1 at 0.1 A·g−1 (RT) | 2.61 W·h·kg−1 at 0.1 A· g−1 (RT) | 1.0 | [38] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, P.; Ni, S.; Hou, P.; Xun, Z.; Liu, Y.; Gu, J. A Crosslinked Soybean Protein Isolate Gel Polymer Electrolyte Based on Neutral Aqueous Electrolyte for a High-Energy-Density Supercapacitor. Polymers 2019, 11, 863. https://doi.org/10.3390/polym11050863
Huo P, Ni S, Hou P, Xun Z, Liu Y, Gu J. A Crosslinked Soybean Protein Isolate Gel Polymer Electrolyte Based on Neutral Aqueous Electrolyte for a High-Energy-Density Supercapacitor. Polymers. 2019; 11(5):863. https://doi.org/10.3390/polym11050863
Chicago/Turabian StyleHuo, Pengfei, Shoupeng Ni, Pu Hou, Zhiyu Xun, Yang Liu, and Jiyou Gu. 2019. "A Crosslinked Soybean Protein Isolate Gel Polymer Electrolyte Based on Neutral Aqueous Electrolyte for a High-Energy-Density Supercapacitor" Polymers 11, no. 5: 863. https://doi.org/10.3390/polym11050863
APA StyleHuo, P., Ni, S., Hou, P., Xun, Z., Liu, Y., & Gu, J. (2019). A Crosslinked Soybean Protein Isolate Gel Polymer Electrolyte Based on Neutral Aqueous Electrolyte for a High-Energy-Density Supercapacitor. Polymers, 11(5), 863. https://doi.org/10.3390/polym11050863