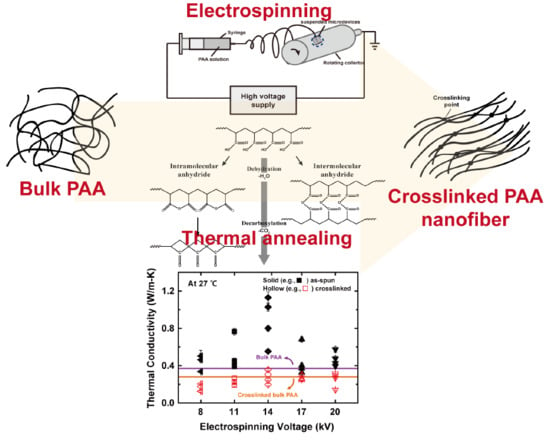
Crosslinking Effect on Thermal Conductivity of Electrospun Poly(acrylic acid) Nanofibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
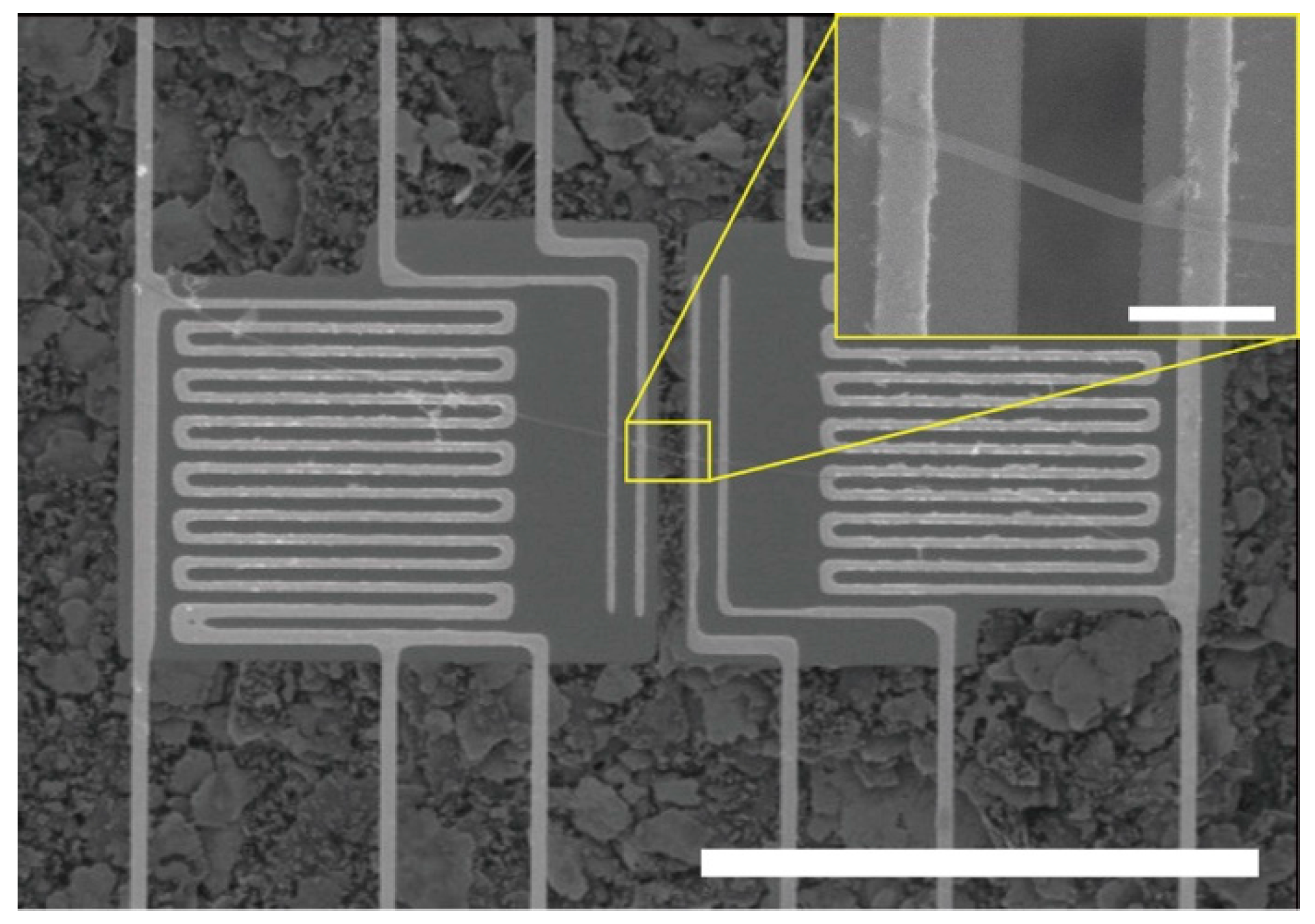
2.2. Assembly of the PAA NFs on Suspended Microdevices
2.3. Thermal Conductivity Measurement
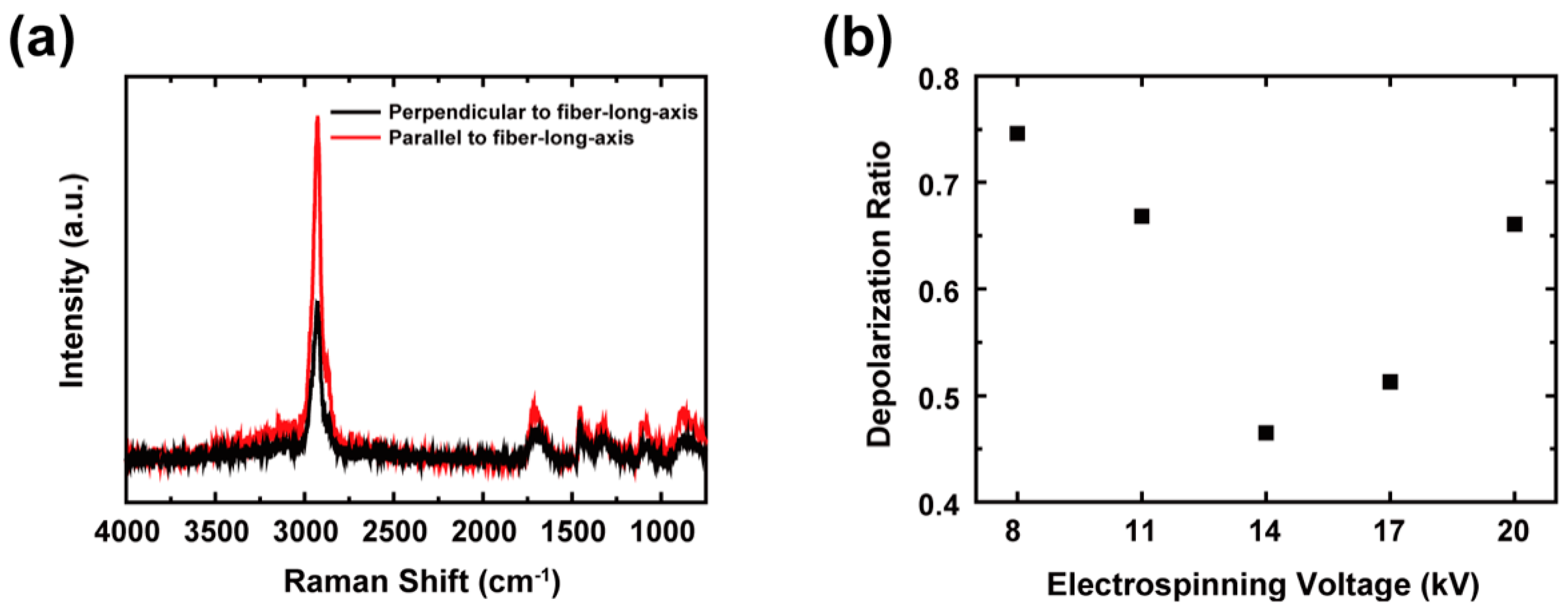
2.4. Characterizations
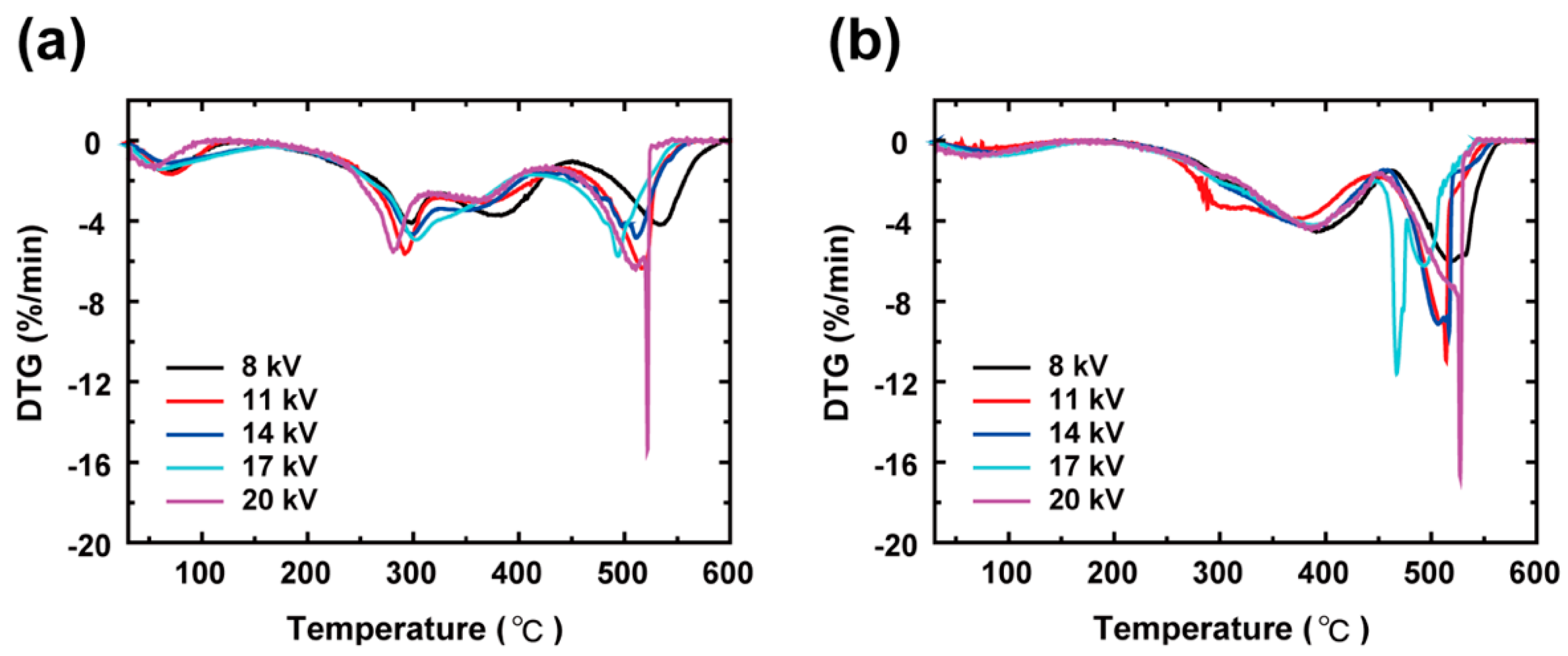
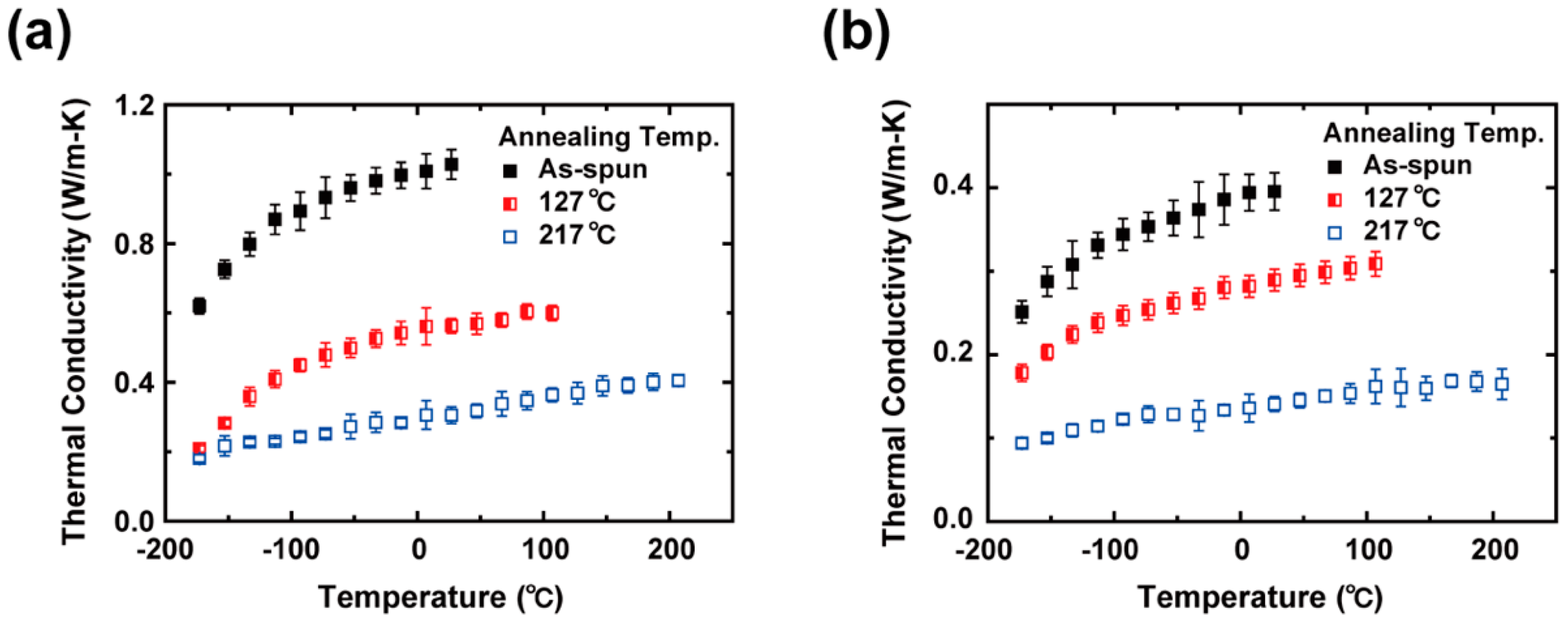
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2015, 59, 41–85. [Google Scholar] [CrossRef]
- Choy, C.L.; Wong, Y.W.; Yang, G.W.; Kanamoto, T. Elastic modulus and thermal conductivity of ultradrawn polyethylene. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 3359–3367. [Google Scholar] [CrossRef]
- Shen, S.; Henry, A.; Tong, J.; Zheng, R.; Chen, G. Polyethylene nanofibres with very high thermal conductivities. Nat. Nanotechnol. 2010, 5, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.; Chen, G. High Thermal Conductivity of Single Polyethylene Chains Using Molecular Dynamics Simulations. Phys. Lett. 2008, 101, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wingert, M.C.; Strzalka, J.; Wang, H.-H.; Sun, T.; Wang, J.; Chen, R.; Jiang, Z. Structure-induced enhancement of thermal conductivities in electrospun polymer nanofibers. Nanoscale 2014, 6, 8283–8291. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, Q.; Mayo, A.; Ni, Z.; Yi, H.; Chen, Y.; Mu, R.; Bellan, L.M.; Li, D. Thermal conductivity of electrospun polyethylene nanofibers. Nanoscale 2015, 7, 16899–16908. [Google Scholar] [CrossRef]
- Zeng, X.; Xiong, Y.; Fu, Q.; Sun, R.; Xu, J.; Xu, D.; Wong, C.-P. Structure-induced variation of thermal conductivity in epoxy resin fibers. Nanoscale 2017, 9, 10585–10589. [Google Scholar] [CrossRef]
- Park, Y.; You, M.; Shin, J.; Ha, S.; Kim, D.; Heo, M.H.; Nah, J.; Kim, Y.A.; Seol, J.H. Thermal conductivity enhancement in electrospun poly(vinyl alcohol) and poly(vinyl alcohol)/cellulose nanocrystal composite nanofibers. Sci. Rep. 2019, 9, 3026. [Google Scholar] [CrossRef]
- Knappe, W.; Yamamoto, O. Effects of crosslinking and chain degradation on the thermal conductivity of polymers. Kolloid-Zeitschrift und Zeitschrift für Polymere 1970, 240, 775–783. [Google Scholar] [CrossRef]
- Yamamoto, O. Thermal Conductivity of Cross-linked Polymers. Polym. J. 1971, 2, 509–517. [Google Scholar] [CrossRef][Green Version]
- Tonpheng, B.; Yu, J.; Andersson, O. Thermal Conductivity, Heat Capacity, and Cross-Linking of Polyisoprene/Single-Wall Carbon Nanotube Composites under High Pressure. Macromolecules 2009, 42, 9295–9301. [Google Scholar] [CrossRef]
- Kikugawa, G.; Desai, T.G.; Keblinski, P.; Ohara, T. Effect of crosslink formation on heat conduction in amorphous polymers. J. Appl. Phys. 2013, 114, 34302. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, M.; Liu, C.; Li, X.; Tang, D. Thermal conductivity of cross-linked polyethylene from molecular dynamics simulation. J. Appl. Phys. 2017, 122, 035104. [Google Scholar] [CrossRef]
- Xie, X.; Yang, K.; Li, D.; Tsai, T.-H.; Shin, J.; Braun, P.V.; Cahill, D.G. High and low thermal conductivity of amorphous macromolecules. Phys. B 2017, 95, 035406. [Google Scholar] [CrossRef]
- Ruesch, M.; Bai, Z.; Zhang, L.; Liu, L. Tuning thermal conductivity of crystalline polymer nanofibers by interchain hydrogen bonding. RSC Adv. 2015, 5, 87981–87986. [Google Scholar]
- Kim, G.-H.; Lee, D.; Shanker, A.; Shao, L.; Kwon, M.S.; Gidley, D.; Kim, J.; Pipe, K.P. High thermal conductivity in amorphous polymer blends by engineered interchain interactions. Nat. Mater. 2015, 14, 295–300. [Google Scholar] [CrossRef]
- Ni, B.; Watanabe, T.; Phillpot, S.R. Thermal transport in polyethylene and at polyethylene–diamond interfaces investigated using molecular dynamics simulation. J. Phys. Condens. Matter 2009, 21, 084219. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Park, C.; Hong, S.M.; Koo, C.M. Thermal conduction behaviors of chemically cross-linked high-density polyethylenes. Thermochim. Acta 2014, 583, 67–71. [Google Scholar] [CrossRef]
- Xie, X.; Li, D.; Tsai, T.-H.; Liu, J.; Braun, P.V.; Cahill, D.G. Thermal Conductivity, Heat Capacity, and Elastic Constants of Water-Soluble Polymers and Polymer Blends. Macromolecules 2016, 49, 972–978. [Google Scholar] [CrossRef]
- Jang, W.; Majumdar, A.; Shi, L.; Li, D.; Yu, C.; Kim, D.; Yao, Z.; Kim, P. Measuring Thermal and Thermoelectric Properties of One-Dimensional Nanostructures Using a Microfabricated Device. J. Heat Transf. 2003, 125, 881–888. [Google Scholar]
- Mavrokefalos, A.; Lin, Q.; Beekman, M.; Seol, J.H.; Lee, Y.J.; Kong, H.; Pettes, M.T.; Johnson, D.C.; Shi, L. In-plane thermal and thermoelectric properties of misfit-layered [(PbSe) 0.99] x (WSe2) x superlattice thin films. Appl. Phys. Lett. 2010, 96, 181908. [Google Scholar] [CrossRef]
- Walczak, W.J.; Hoagland, D.A.; Hsu, S.L. Analysis of polyelectrolyte chain conformation of polarized Raman-spectroscopy. Macromolecules 1992, 25, 7317–7323. [Google Scholar] [CrossRef]
- Walczak, W.J.; Hoagland, D.A.; Hsu, S.L. Spectroscopic Evaluation of Models for Polyelectrolyte Chain Conformation in Dilute Solution. Macromolecules 1996, 29, 7514–7520. [Google Scholar] [CrossRef]
- McNeill, I.; Sadeghi, S. Thermal stability and degradation mechanisms of poly(acrylic acid) and its salts: Part 1—Poly(acrylic acid). Degrad. Stab. 1990, 29, 233–246. [Google Scholar] [CrossRef]
- McGaugh, M.C.; Kottle, S. The thermal degradation of poly(acrylic acid). J. Polym. Sci. Part B Polym. Lett. 1967, 5, 817–820. [Google Scholar] [CrossRef]
- Eisenberg, A.; Yokoyama, T.; Sambalido, E. Dehydration kinetics and glass transition of poly(acrylic acid). J. Polym. Sci. Part A-1 Polym. Chem. 1969, 7, 1717–1728. [Google Scholar] [CrossRef]
- Chee, K.K. Dependence of glass transition temperature on chain flexibility and intermolecular interactions in polymers. J. Appl. Sci. 1991, 43, 1205–1208. [Google Scholar] [CrossRef]
- Richard-Lacroix, M.; Pellerin, C. Orientation and Structure of Single Electrospun Nanofibers of Poly(ethylene terephthalate) by Confocal Raman Spectroscopy. Macromolecules 2012, 45, 1946–1953. [Google Scholar] [CrossRef]
- Dong, J.; Ozaki, Y.; Nakashima, K. Infrared, Raman, and Near-Infrared Spectroscopic Evidence for the Coexistence of Various Hydrogen-Bond Forms in Poly(acrylic acid). Macromolecules 1997, 30, 1111–1117. [Google Scholar] [CrossRef]
- Dubinsky, S.; Grader, G.S.; Shter, G.E.; Silverstein, M.S. Thermal degradation of poly(acrylic acid) containing copper nitrate. Degrad. Stab. 2004, 86, 171–178. [Google Scholar] [CrossRef]
- Chen, S.-A.A.; Lee, H.-T.T. Structure and Properties of Poly(acrylic acid)-Doped Polyaniline. Macromolecules 1995, 28, 2858–2866. [Google Scholar] [CrossRef]
- Dong, J.; Ozaki, Y.; Nakashima, K. FTIR studies of conformational energies of poly(acrylic acid) in cast films. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 507–515. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, T. Role of Chain Morphology and Stiffness in Thermal Conductivity of Amorphous Polymers. J. Phys. Chem. B 2016, 120, 803–812. [Google Scholar] [CrossRef]
- Shanker, A.; Li, C.; Kim, G.-H.; Gidley, D.; Pipe, K.P.; Kim, J. High thermal conductivity in electrostatically engineered amorphous polymers. Sci. Adv. 2017, 3, 3. [Google Scholar] [CrossRef]
- Dashora, P.; Gupta, G. On the temperature dependence of the thermal conductivity of linear amorphous polymers. Polymer 1996, 37, 231–234. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Q.; Zhang, Y.; Zhou, L.; Yang, J.; Ni, Z. A rapid and simple method to draw polyethylene nanofibers with enhanced thermal conductivity. Appl. Phys. Lett. 2016, 109, 033101. [Google Scholar] [CrossRef]
- Arinstein, A.; Burman, M.; Gendelman, O.; Zussman, E. Effect of supramolecular structure on polymer nanofibre elasticity. Nat. Nanotechnol. 2007, 2, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Pai, C.-L.; Boyce, M.C.; Rutledge, G.C. Mechanical properties of individual electrospun PA 6(3)T fibers and their variation with fiber diameter. Polymer 2011, 52, 2295–2301. [Google Scholar] [CrossRef]
- Lim, C.T.; Tan, E.P.S.; Ng, S.Y. Effects of crystalline morphology on the tensile properties of electrospun polymer nanofibers. Appl. Phys. Lett. 2008, 92, 141908. [Google Scholar] [CrossRef]
- Papkov, D.; Zou, Y.; Andalib, M.N.; Goponenko, A.; Cheng, S.Z.D.; Dzenis, Y.A. Simultaneously Strong and Tough Ultrafine Continuous Nanofibers. ACS Nano 2013, 7, 3324–3331. [Google Scholar] [CrossRef]
- Richard-Lacroix, M.; Pellerin, C. Orientation and Partial Disentanglement in Individual Electrospun Fibers: Diameter Dependence and Correlation with Mechanical Properties. Macromolecules 2015, 48, 4511–4519. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, X.; Wang, L.; Huang, Y. Electrospinning of ethyl-cyanoethyl cellulose/tetrahydrofuran solutions. J. Appl. Polym. Sci. 2004, 91, 242–246. [Google Scholar] [CrossRef]
- Ero-Phillips, O.; Jenkins, M.; Stamboulis, A. Tailoring Crystallinity of Electrospun Plla Fibres by Control of Electrospinning Parameters. Polymers 2012, 4, 1331–1348. [Google Scholar] [CrossRef]


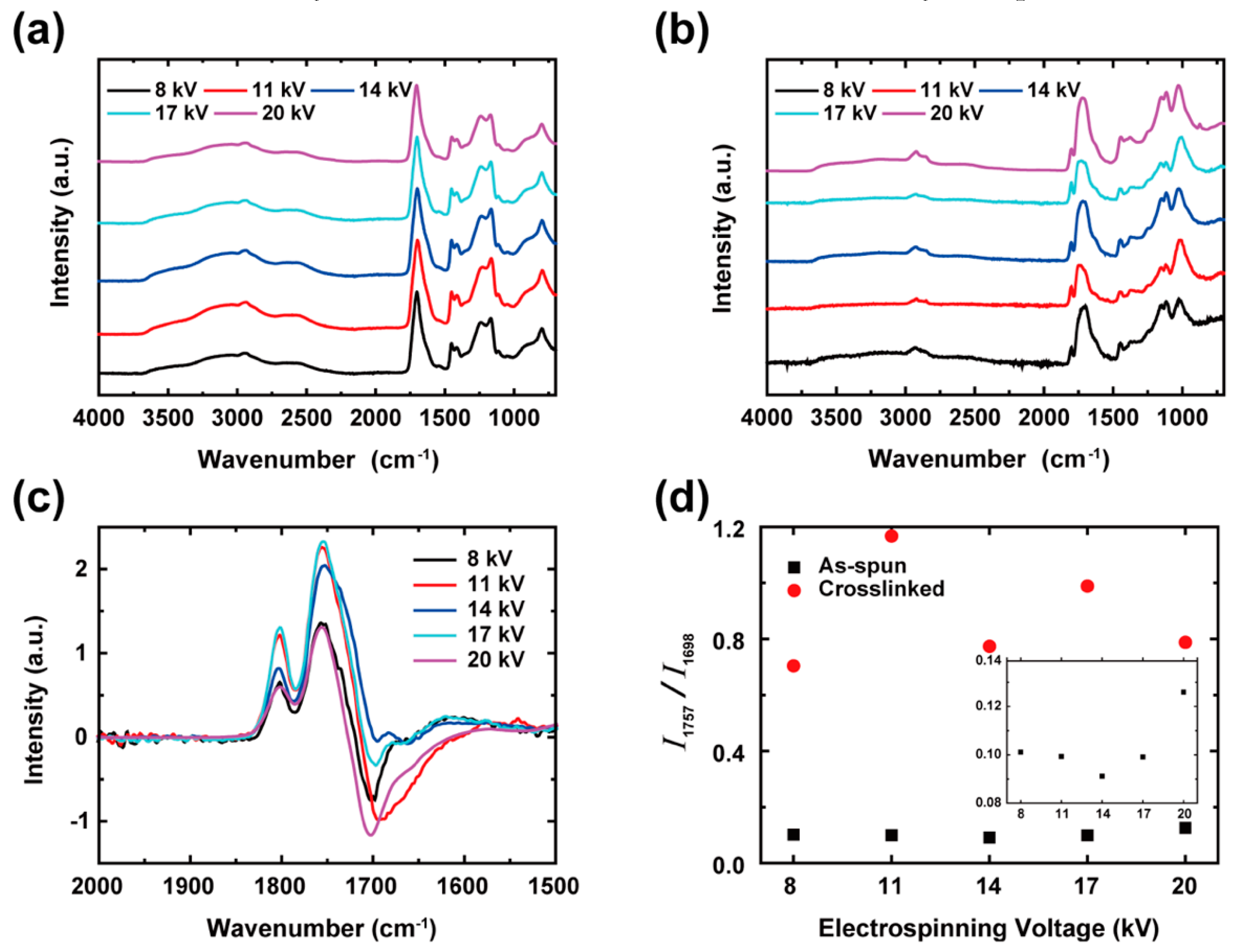

| Band (cm−1) | Assignments | Remark |
|---|---|---|
| 800 | OH–O out-of-plane deformation | Strong interchain H-bonds |
| 1045 | C–O–C stretching | Non-conjugated cyclic anhydride |
| 1115 | Ketone | Anhydride |
| 1165 | C–O stretching | |
| 1200–1315 (shoulder) | C–O stretching | |
| 1390–1450 (shoulder) | C–O stretching coupled with O–H in-plane bending | |
| 1452 | CH2 deformation | |
| 1700 (strong) | C=O stretching | |
| 1802 | C–O–C stretching | Anhydride |
| 2500–2700 (shoulder) | Overtones and combinations of bands near 1200–1315 and in-plane deformation of C–O–H in 1390–1450 cm−1 | |
| 2800–3100 | CH or CH2 stretching | Overlapped; show up after crosslinking |
| 3000 (broad) | O–H stretching | Intra- and inter-chain H-bonds |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Lee, S.; Ha, S.S.; Alunda, B.; Noh, D.Y.; Lee, Y.J.; Kim, S.; Seol, J.H. Crosslinking Effect on Thermal Conductivity of Electrospun Poly(acrylic acid) Nanofibers. Polymers 2019, 11, 858. https://doi.org/10.3390/polym11050858
Park Y, Lee S, Ha SS, Alunda B, Noh DY, Lee YJ, Kim S, Seol JH. Crosslinking Effect on Thermal Conductivity of Electrospun Poly(acrylic acid) Nanofibers. Polymers. 2019; 11(5):858. https://doi.org/10.3390/polym11050858
Chicago/Turabian StylePark, Yeongcheol, Suyeong Lee, Sung Soo Ha, Bernard Alunda, Do Young Noh, Yong Joong Lee, Sangwon Kim, and Jae Hun Seol. 2019. "Crosslinking Effect on Thermal Conductivity of Electrospun Poly(acrylic acid) Nanofibers" Polymers 11, no. 5: 858. https://doi.org/10.3390/polym11050858
APA StylePark, Y., Lee, S., Ha, S. S., Alunda, B., Noh, D. Y., Lee, Y. J., Kim, S., & Seol, J. H. (2019). Crosslinking Effect on Thermal Conductivity of Electrospun Poly(acrylic acid) Nanofibers. Polymers, 11(5), 858. https://doi.org/10.3390/polym11050858