Comparative Analysis of Fluorinated Anions for Polypyrrole Linear Actuator Electrolytes
Abstract
1. Introduction
2. Material and Methods
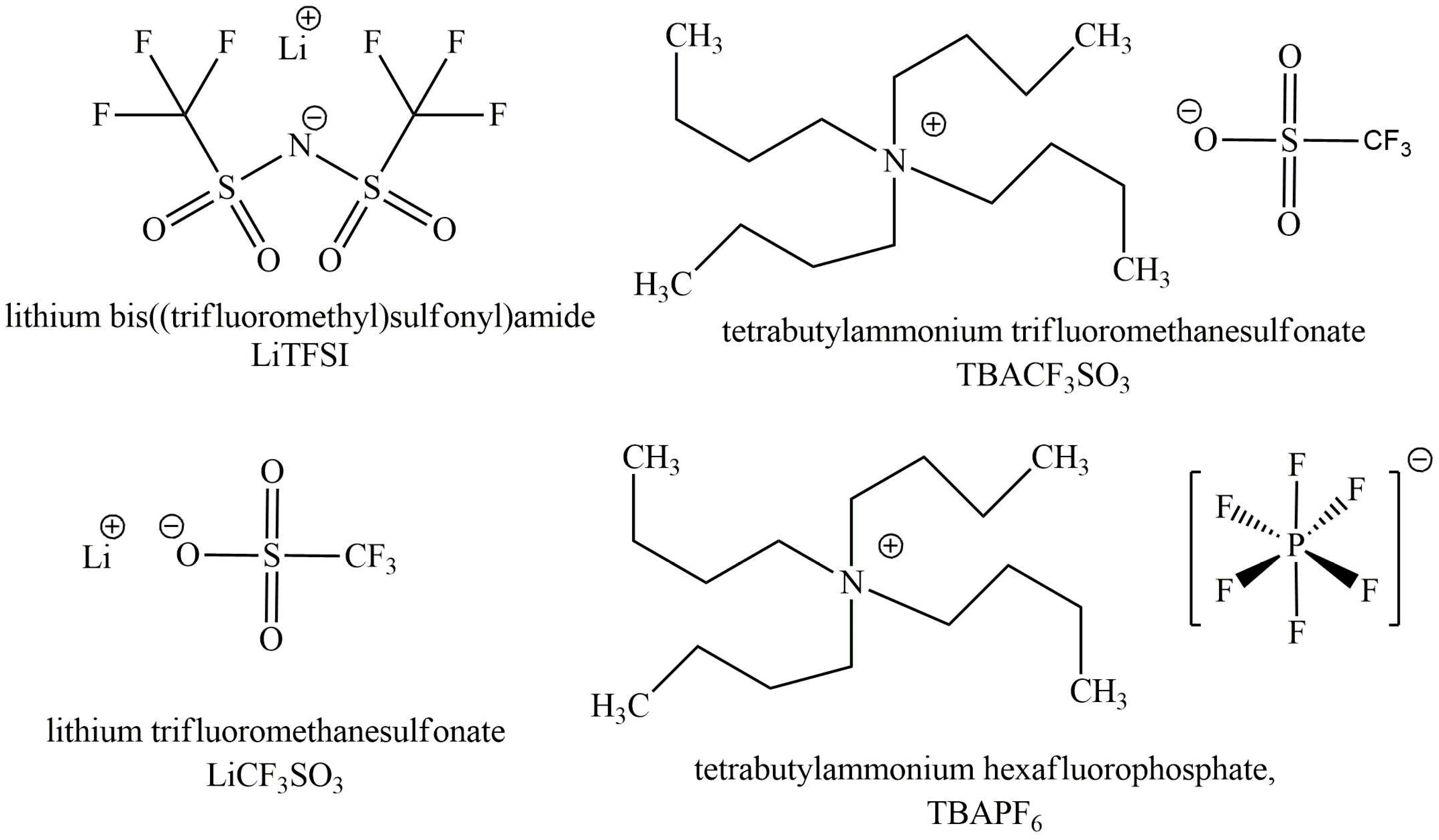
2.1. Materials
2.2. Electroformation of PPy/DBS Films
2.3. Isotonic Electro-Chemo-Mechanical Deformation (ECMD) Measurements
2.4. Characterization of PPy/DBS Samples after Actuation
3. Results and Discussion
3.1. Morphology
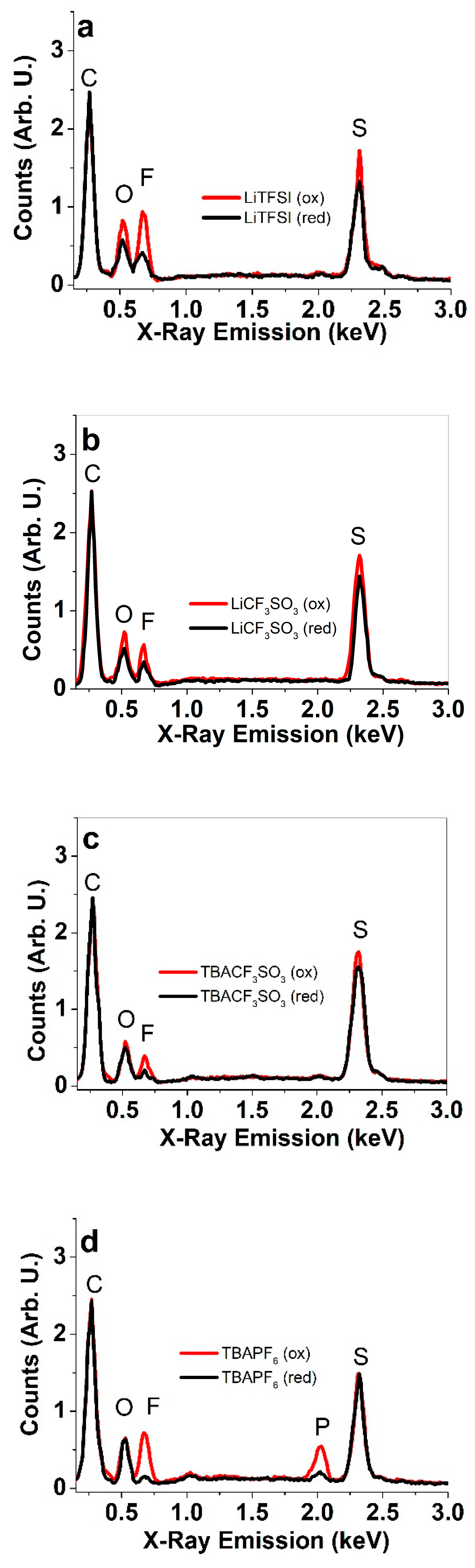
3.2. Elemental Composition
3.3. Actuation and Electroactivity
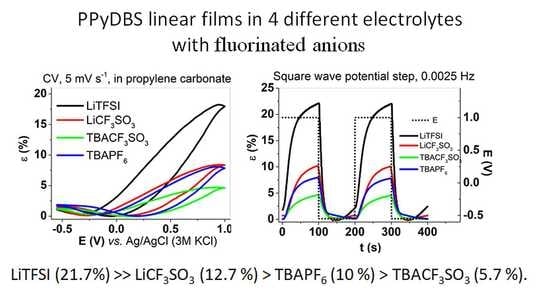
3.3.1. Cyclic Voltammetry
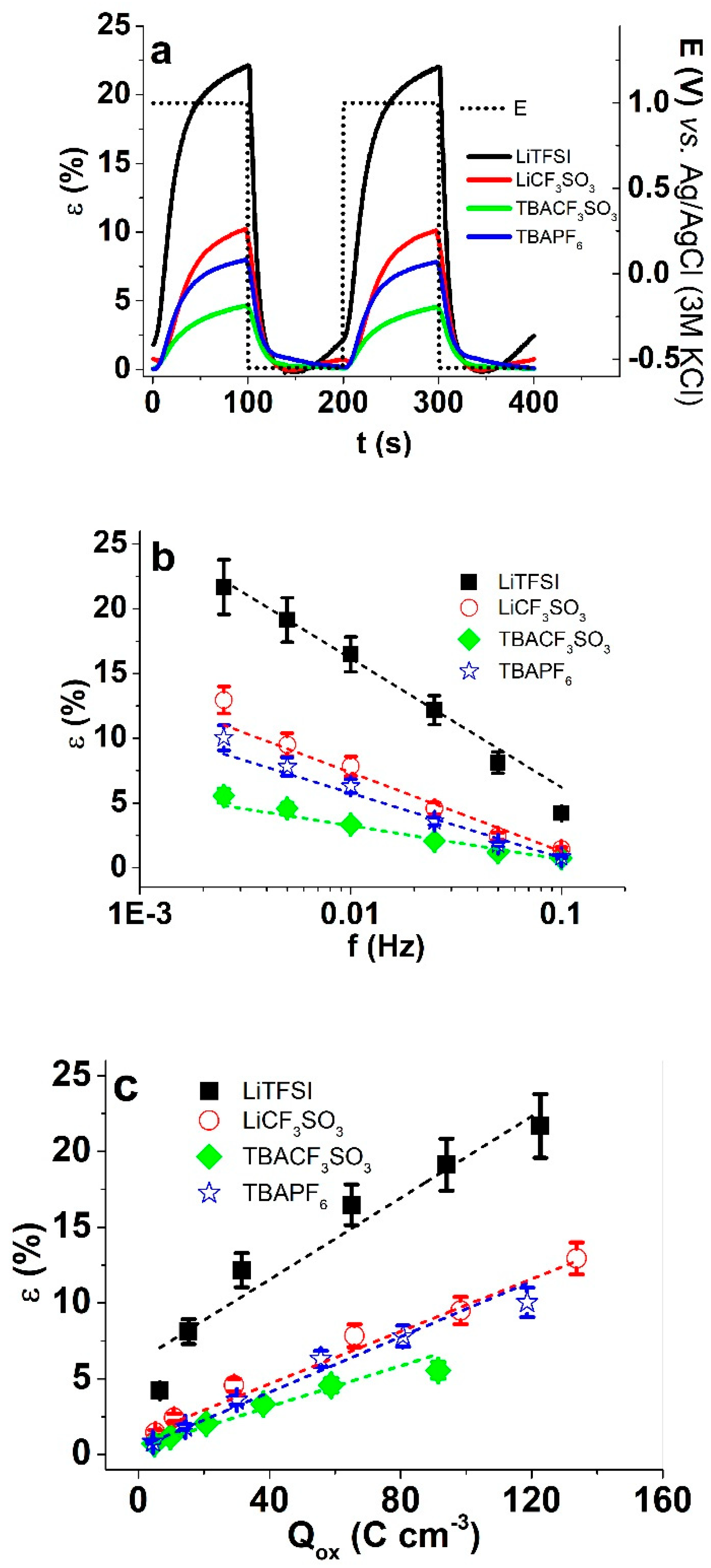
3.3.2. Square Wave Potentials Steps
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yan, B.; Wu, Y.; Guo, L.; Yan, B.; Wu, Y.; Guo, L. Recent Advances on Polypyrrole Electroactuators. Polymers 2017, 9, 446. [Google Scholar] [CrossRef]
- Baughman, R.H. Conducting polymer artificial muscles. Synth. Met. 1996, 78, 339–353. [Google Scholar] [CrossRef]
- Jager, E.W.H.; Smela, E.; Ingana, O.; Inganäs, O. Microfabricating Conjugated Polymer Actuators. Science 2000, 290, 111–114. [Google Scholar] [CrossRef]
- Mutlu, R.; Alici, G.; Li, W. An effective methodology to solve inverse kinematics of electroactive polymer actuators modelled as active and soft robotic structures. Mech. Mach. Theory 2013, 67, 94–110. [Google Scholar] [CrossRef]
- Smela, E. Conjugated polymer actuators for biomedical applications. Adv. Mater. 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Gelmi, A.; Ljunggren, M.K.; Rafat, M.; Jager, E.W.H. Influence of conductive polymer doping on the viability of cardiac progenitor cells. J. Mater. Chem. B 2014, 2, 3860. [Google Scholar] [CrossRef]
- Yow, S.-Z.; Lim, T.H.; Yim, E.K.F.; Lim, C.T.; Leong, K.W. A 3D Electroactive Polypyrrole-Collagen Fibrous Scaffold for Tissue Engineering. Polymers 2011, 3, 527–544. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, L.; Li, L.; Cheng, W.; Xu, L.; Ping, X.; Pan, L.; Shi, Y. Conducting Polymers and Their Applications in Diabetes Management. Sensors 2016, 16, 1787. [Google Scholar] [CrossRef] [PubMed]
- Maziz, A.; Concas, A.; Khaldi, A.; Stålhand, J.; Persson, N.-K.; Jager, E.W.H. Knitting and weaving artificial muscles. Sci. Adv. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Hara, S.; Zama, T.; Takashima, W.; Kaneto, K. Artificial Muscles Based on Polypyrrole Actuators with Large Strain and Stress Induced Electrically. Polym. J. 2004, 36, 151–161. [Google Scholar] [CrossRef]
- Otero, T.F.; Boyano, I. Comparative study of conducting polymers by the ESCR model. J. Phys. Chem. B 2003, 107, 6730–6738. [Google Scholar] [CrossRef]
- Chen, X.; Inganäs, O. Three-Step Redox in Polythiophenes: Evidence from Electrochemistry at an Ultramicroelectrode. J. Phys. Chem. 1996, 100, 15202–15206. [Google Scholar] [CrossRef]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers—Persistent Models and New concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef] [PubMed]
- West, B.J.; Otero, T.F.; Shapiro, B.; Smela, E. Chronoamperometric study of conformational relaxation in PPy(DBS). J. Phys. Chem. B 2009, 113, 1277–1293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kiefer, R.; Martinez, J.G.; Kesküla, A.; Anbarjafari, G.; Aabloo, A.; Otero, T.F. Polymeric actuators: Solvents tune reaction-driven cation to reaction-driven anion actuation. Sens. Actuators B Chem. 2016, 233, 461–469. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Structural Electrochemistry: Conductivities and Ionic Content from Rising Reduced Polypyrrole Films. Adv. Funct. Mater. 2014, 24, 1259–1264. [Google Scholar] [CrossRef]
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Zama, T.; Sewa, S.; Takashima, W.; Kaneto, K. Highly Stretchable and Powerful Polypyrrole Linear Actuators. Chem. Lett. 2003, 32, 576–577. [Google Scholar] [CrossRef]
- Zondaka, Z.; Harjo, M.; Khan, A.; Khanh, T.T.; Tamm, T.; Kiefer, R. Optimal phosphotungstinate concentration for polypyrrole linear actuation and energy storage. Multifunct. Mater 2018, 1, 14003. [Google Scholar] [CrossRef]
- Kiefer, R.; Chu, S.Y.; Kilmartin, P.A.; Bowmaker, G.A.; Cooney, R.P.; Travas-Sejdic, J. Mixed-ion linear actuation behavior of polypyrrole. Electrochim. Acta 2007, 52, 2386–2391. [Google Scholar] [CrossRef]
- Takashima, W.; Hashimoto, H.; Tominaga, K.; Tanaka, A.; Pandey, S.S.; Kaneto, K. Solvation effect on the ion exchange in polypyrrole film doped with sulfonated polyaniline. Thin Solid Films 2010, 519, 1093–1099. [Google Scholar] [CrossRef]
- Aydemir, N.; Kilmartin, P.A.; Travas-Sejdic, J.; Kesküla, A.; Peikolainen, A.-L.; Parcell, J.; Harjo, M.; Aabloo, A.; Kiefer, R. Electrolyte and solvent effects in PPy/DBS linear actuators. Sens. Actuators B Chem. 2015, 216, 24–32. [Google Scholar] [CrossRef]
- Kiefer, R.; Kesküla, A.; Martinez, J.G.; Anbarjafari, G.; Torop, J.; Otero, T.F. Interpenetrated triple polymeric layer as electrochemomechanical actuator: Solvent influence and diffusion coefficient of counterions. Electrochim. Acta 2017, 230, 461–469. [Google Scholar] [CrossRef]
- Kivilo, A.; Zondaka, Z.; Kesküla, A.; Rasti, P.; Tamm, T.; Kiefer, R. Electro-chemo-mechanical deformation properties of polypyrrole/dodecylbenzenesulfate linear actuators in aqueous and organic electrolyte. RSC Adv. 2016, 6, 69–75. [Google Scholar] [CrossRef]
- Mecerreyes, D.; Alvaro, V.; Cantero, I.; Bengoetxea, M.; Calvo, P.A.; Grande, H.; Rodriguez, J.; Pomposo, J.A. Low Surface Energy Conducting Polypyrrole Doped with a Fluorinated Counterion. Adv. Mater. 2002, 14, 749–752. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Araújo, J.M.M.; Martinho, S.; Alves, F.; Nunes, S.; Matias, A.; Duarte, C.M.M.; Rebelo, L.P.N.; Marrucho, I.M. Fluorinated Ionic Liquids: Properties and Applications. ACS Sustain. Chem. Eng. 2013, 1, 427–439. [Google Scholar] [CrossRef]
- Ansari Khalkhali, R.; Price, W.E.; Wallace, G.G. Quartz crystal microbalance studies of the effect of solution temperature on the ion-exchange properties of polypyrrole conducting electroactive polymers. React. Funct. Polym. 2003, 56, 141–146. [Google Scholar] [CrossRef]
- Tamm, J.; Raudsepp, T.; Marandi, M.; Tamm, T. Electrochemical properties of the polypyrrole films doped with benzenesulfonate. Synth. Met. 2007, 157, 66–73. [Google Scholar] [CrossRef]
- Ue, M. Mobility and Ionic Association of Lithium and Quaternary Ammonium Salts in Propylene Carbonate and /-Butyrolactone. J. Electrochem. Soc. 1994, 141, 3336–3342. [Google Scholar] [CrossRef]
- Ikezawa, Y.; Ariga, T. In situ FTIR spectra at the Cu electrode/propylene carbonate solution interface. Electrochim. Acta 2007, 52, 2710–2715. [Google Scholar] [CrossRef]
- Yeager, H.L.; Fedyk, J.D.; Parker, R.J. Spectroscopic Studies of Ionic Solvation in Propylene Carbonate. J. Phys. Chem. 1973, 77, 2407–2410. [Google Scholar] [CrossRef]
- Chaban, V. Solvation of the fluorine containing anions and their lithium salts in propylene carbonate and dimethoxyethane. J. Mol. Model. 2015, 21, 2–9. [Google Scholar] [CrossRef]
- Gade, V.K.; Shirale, D.J.; Gaikwad, P.D.; Kakde, K.P.; Savale, P.A.; Kharat, H.J.; Shirsat, M.D. Synthesis and Characterization of Ppy-PVS, Ppy-pTS, and Ppy-DBS Composite Films. Int. J. Polym. Mater. 2007, 56, 107–114. [Google Scholar] [CrossRef]
- Ue, M. Ionic Radius of (CF3SO2)3C- and Applicability of Stokes Law to Its Propylene Carbonate Solution. J. Electrochem. Soc. 1996, 143, L270–L272. [Google Scholar] [CrossRef]
- Valero, L.; Otero, T.F.; Martinez, J.G.; Martínez, J.G. Exchanged Cations and Water during Reactions in Polypyrrole Macroions from Artificial Muscles. ChemPhysChem 2014, 15, 293–301. [Google Scholar] [CrossRef]
- Khanh, T.T.; Kesküla, A.; Zondaka, Z.; Harjo, M.; Kivilo, A.; Khorram, M.S.; Tamm, T.; Kiefer, R. Role of polymerization temperature on the performance of polypyrrole/dodecylbenzenesulphonate linear actuators. Synth. Met. 2019, 247, 53–58. [Google Scholar] [CrossRef]
- Arnaud, R.; Benrabah, D.; Sanchez, J.-Y. Theoretical Study of CF3SO3Li, (CF3SO2)2NLi, and (CF3SO2)2CHLi Ion Pairs. J. Phys. Chem. 2002, 100, 10882–10891. [Google Scholar] [CrossRef]
- Zondaka, Z.; Valner, R.; Tamm, T.; Aabloo, A.; Kiefer, R. Carbide-derived carbon in polypyrrole changing the elastic modulus with a huge impact on actuation. RSC Adv. 2016, 6, 26380–26385. [Google Scholar] [CrossRef]
- Jo, C.; Pugal, D.; Oh, I.K.; Kim, K.J.; Asaka, K. Recent advances in ionic polymer-metal composite actuators and their modeling and applications. Prog. Polym. Sci. 2013, 38, 1037–1066. [Google Scholar] [CrossRef]
- Chen, I.W.P.; Yang, M.C.; Yang, C.H.; Zhong, D.X.; Hsu, M.C.; Chen, Y. Newton Output Blocking Force under Low-Voltage Stimulation for Carbon Nanotube-Electroactive Polymer Composite Artificial Muscles. ACS Appl. Mater. Interfaces 2017, 9, 5550–5555. [Google Scholar] [CrossRef]
- Terasawa, N.; Asaka, K. High-Performance PEDOT:PSS/Single-Walled Carbon Nanotube/Ionic Liquid Actuators Combining Electrostatic Double-Layer and Faradaic Capacitors. Langmuir 2016, 32, 7210–7218. [Google Scholar] [CrossRef]
- Martinez, J.G.; Otero, T.F.; Jager, E.W.H. Effect of the electrolyte concentration and substrate on conducting polymer actuators. Langmuir 2014, 30, 3894–3904. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Activation energy for polypyrrole oxidation: Film thickness influence. J. Solid State Electrochem. 2011, 15, 1169–1178. [Google Scholar] [CrossRef]


| Ions | Ion Radius [nm] [29] | λ0 [29] [S cm2 mol−1] | Solvation Number in PC |
|---|---|---|---|
| Li+ | 0.076 | 8.43 | 3−4 [30] |
| TBA+ | 0.415 | 9.09 | 0 [31] |
| TFSI− | 0.326 | 14.4 | low |
| CF3SO3− | 0.27 | 16.89 | low |
| PF6− | 0.254 | 17.86 | low |
| PPy/DBS Films | Conductivity [S cm−1] | Thickness [µm] |
|---|---|---|
| After polymerization, not actuated | 11 ± 1 | 19 ± 1.3 |
| Actuated in LiTFSI-PC | 17 ± 1.2 | 20 ± 1.7 |
| Actuated in LiCF3SO3-PC | 16 ± 1.1 | 21 ± 1.5 |
| Actuated in TBACF3SO3-PC | 10 ± 0.8 | 20 ± 1.1 |
| Actuated in TBAPF6-PC | 15 ± 1.3 | 21 ± 1.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuyen, N.Q.; Zondaka, Z.; Harjo, M.; Torop, J.; Tamm, T.; Kiefer, R. Comparative Analysis of Fluorinated Anions for Polypyrrole Linear Actuator Electrolytes. Polymers 2019, 11, 849. https://doi.org/10.3390/polym11050849
Khuyen NQ, Zondaka Z, Harjo M, Torop J, Tamm T, Kiefer R. Comparative Analysis of Fluorinated Anions for Polypyrrole Linear Actuator Electrolytes. Polymers. 2019; 11(5):849. https://doi.org/10.3390/polym11050849
Chicago/Turabian StyleKhuyen, Nguyen Quang, Zane Zondaka, Madis Harjo, Janno Torop, Tarmo Tamm, and Rudolf Kiefer. 2019. "Comparative Analysis of Fluorinated Anions for Polypyrrole Linear Actuator Electrolytes" Polymers 11, no. 5: 849. https://doi.org/10.3390/polym11050849
APA StyleKhuyen, N. Q., Zondaka, Z., Harjo, M., Torop, J., Tamm, T., & Kiefer, R. (2019). Comparative Analysis of Fluorinated Anions for Polypyrrole Linear Actuator Electrolytes. Polymers, 11(5), 849. https://doi.org/10.3390/polym11050849