Facile Preparation of EVOH-Based Amphoteric Ion Exchange Membrane Using Radiation Grafting Technique: A Preliminary Investigation on Its Application for Vanadium Redox Flow Battery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
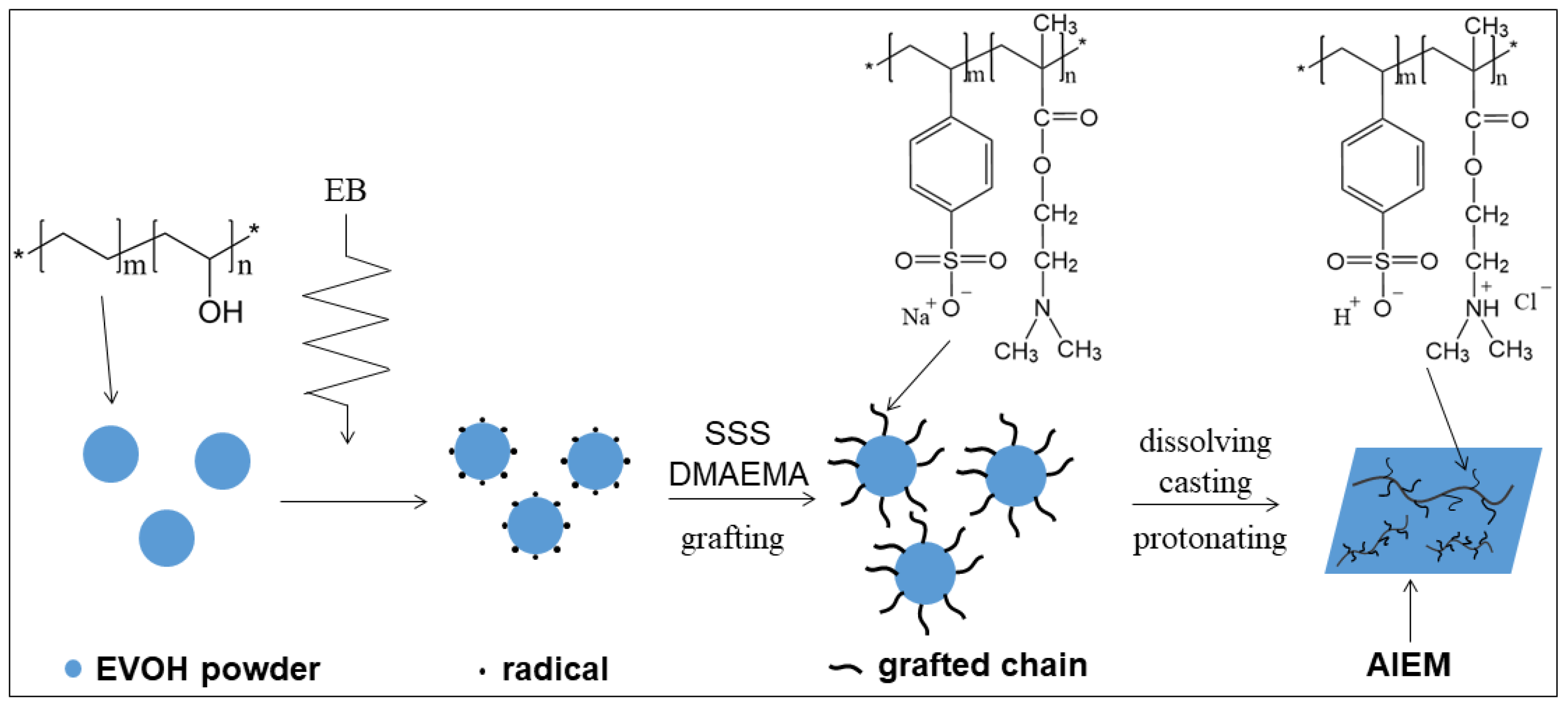
2.2. Preparation of AIEM
2.3. Characterization
2.3.1. Fourier Transform Infrared Spectrometry (FT-IR)
2.3.2. H-Nuclear Magnetic Resonance (1H-NMR)
2.3.3. X-ray Diffraction (XRD)
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. Element Analysis
2.3.6. Surface Morphology
2.3.7. Mechanical Properties
2.3.8. Water Uptake
2.3.9. Ion Exchange Capacity
2.3.10. Proton Conductivity
2.3.11. Permeability of V (IV) Ions Through the AIEM
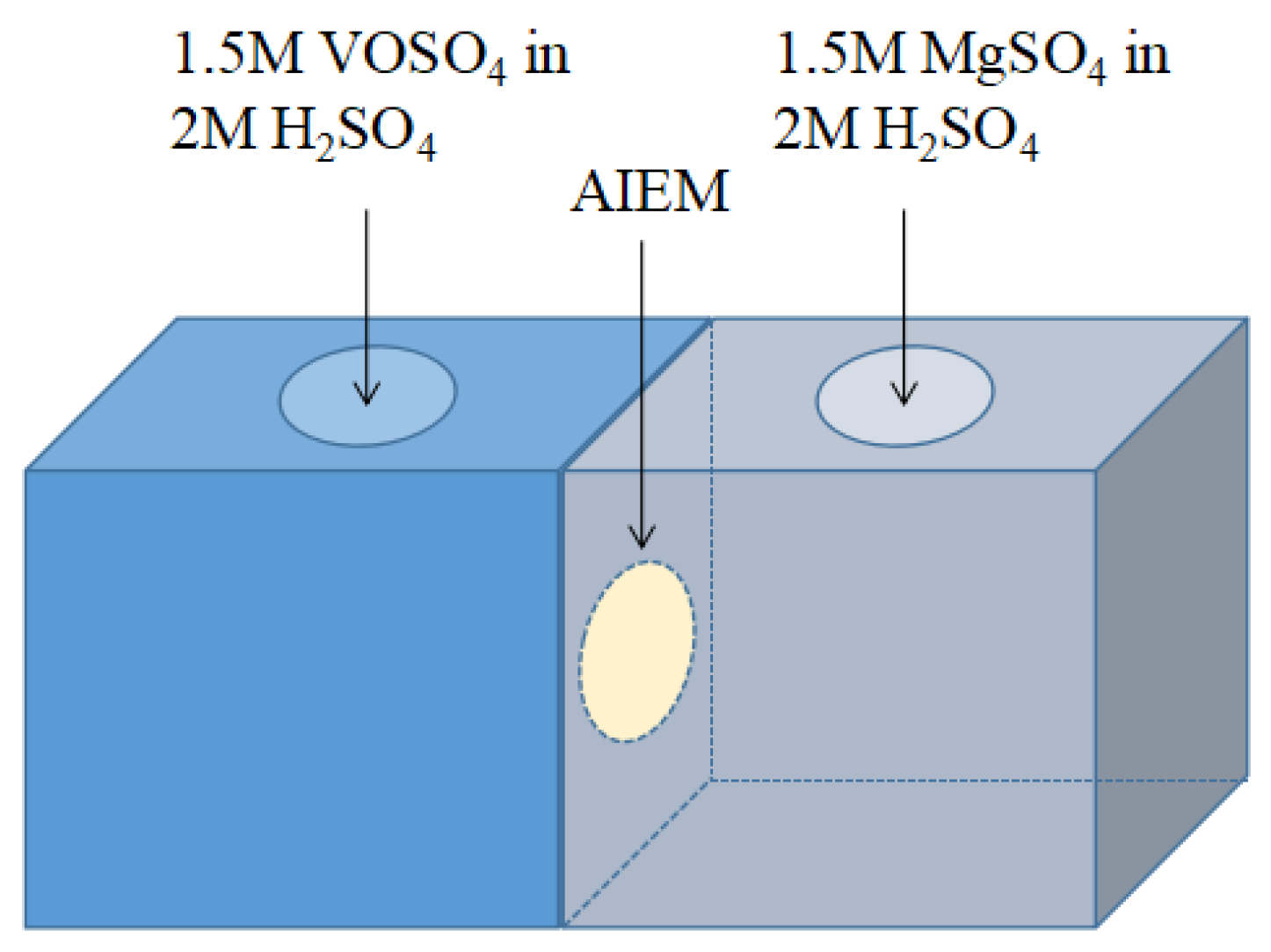
2.3.12. Open Circuit Voltage of the VRFB
3. Results
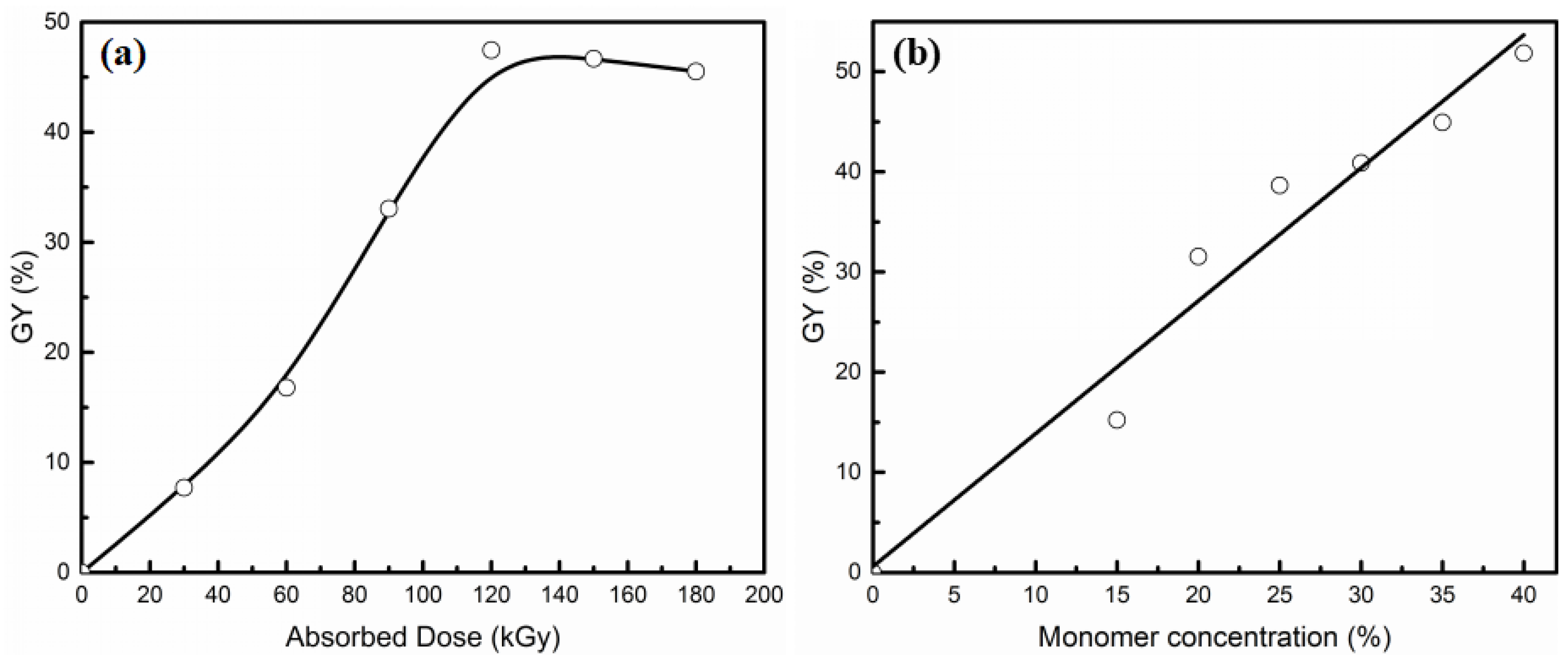
3.1. Radiation Grafting
3.2. Membrane Characterization
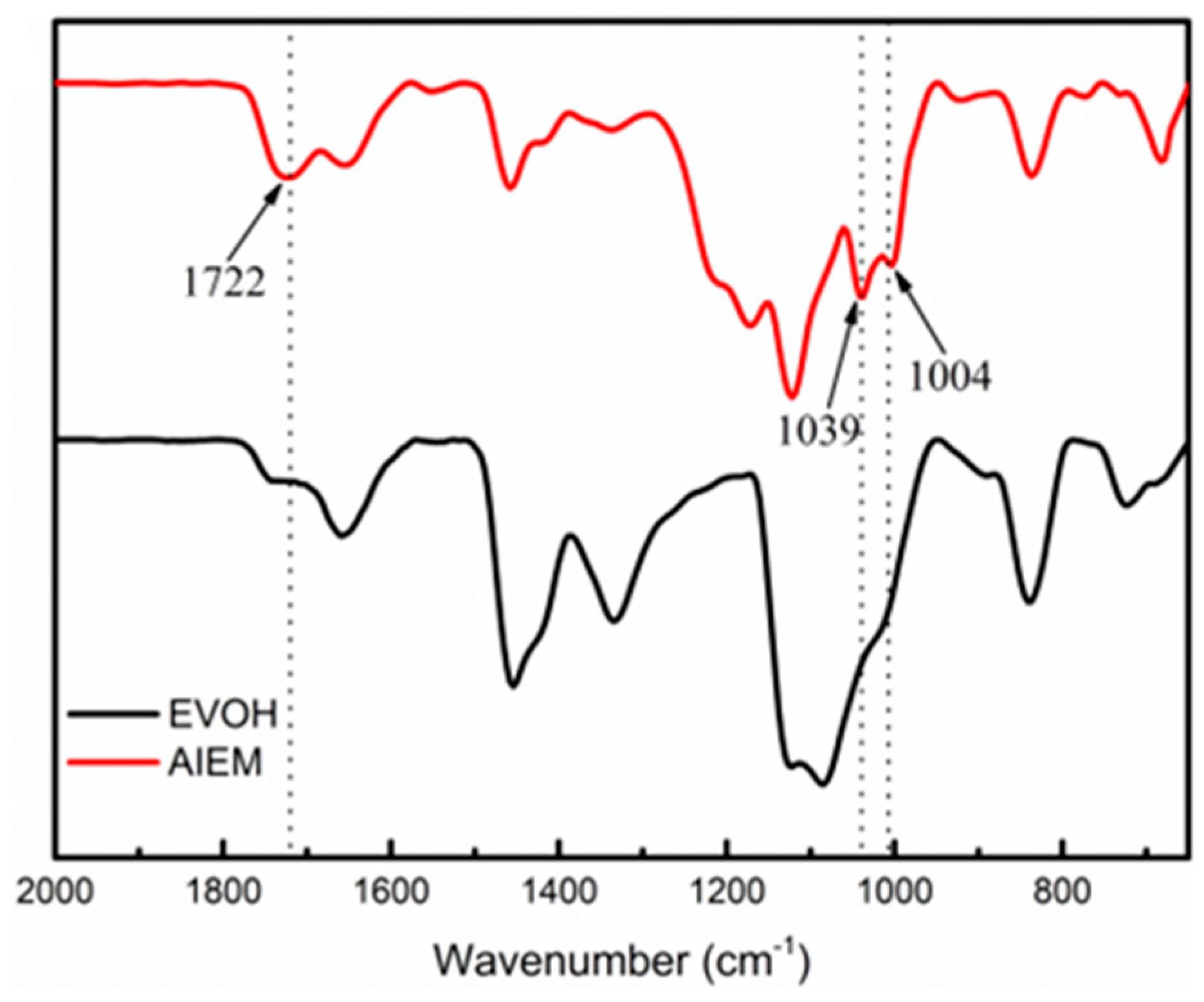
3.2.1. FT-IR Analysis
3.2.2. H-Nuclear Magnetic Resonance (1H-NMR)
3.2.3. X-ray Diffraction
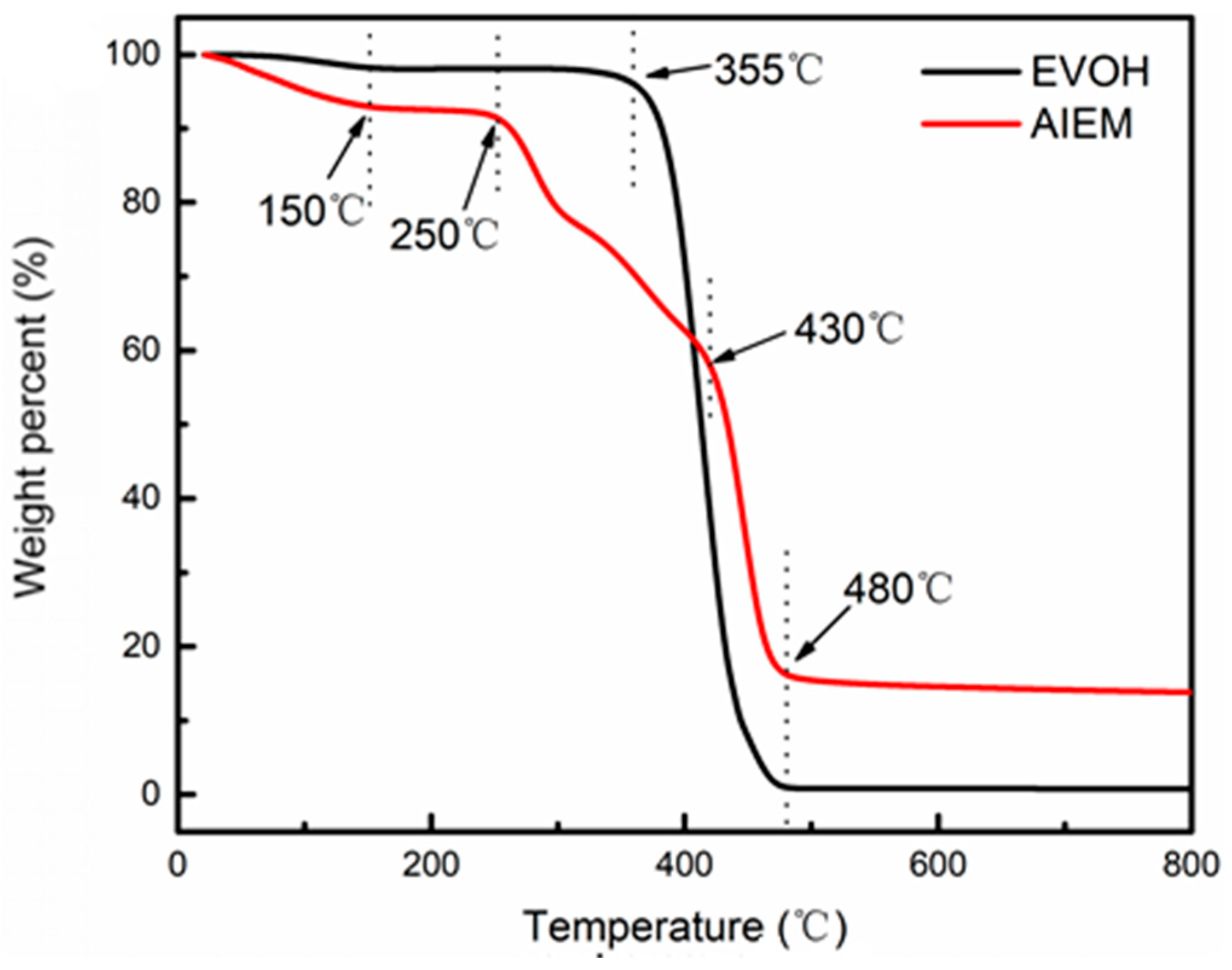
3.2.4. TGA Measurement
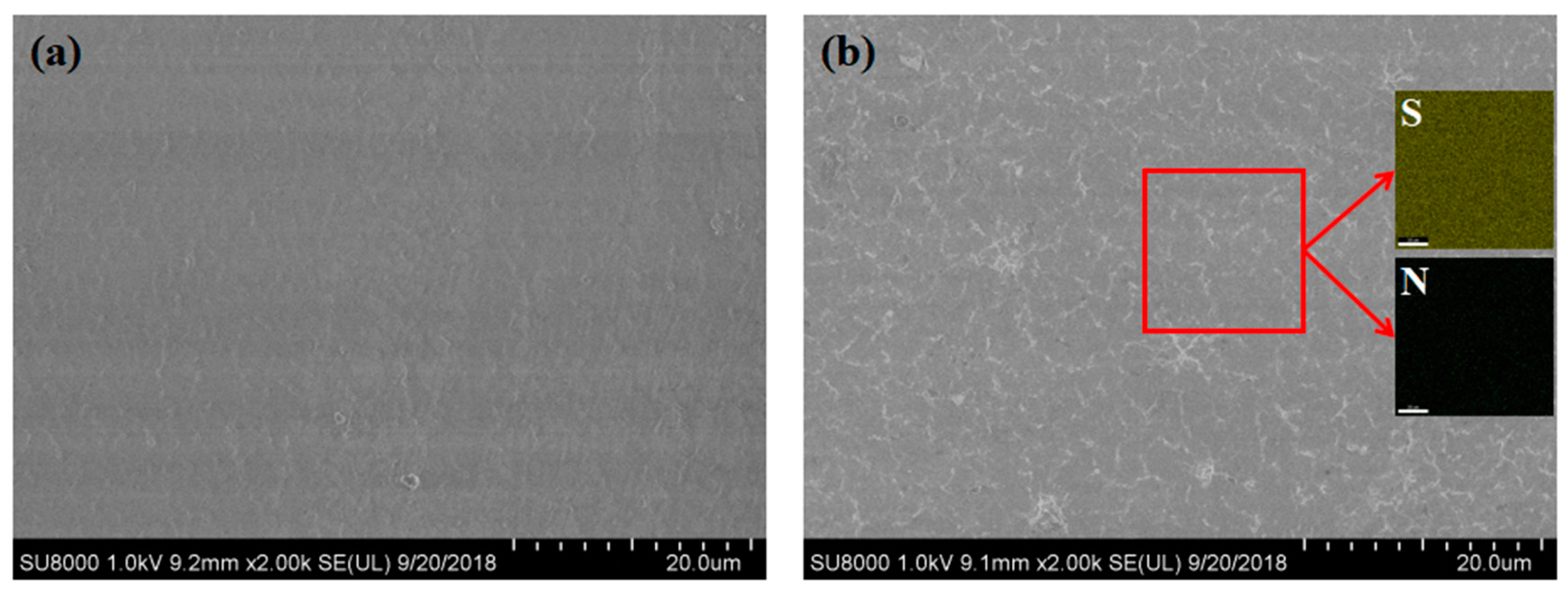
3.2.5. Surface Morphology
3.2.6. Elemental Analysis
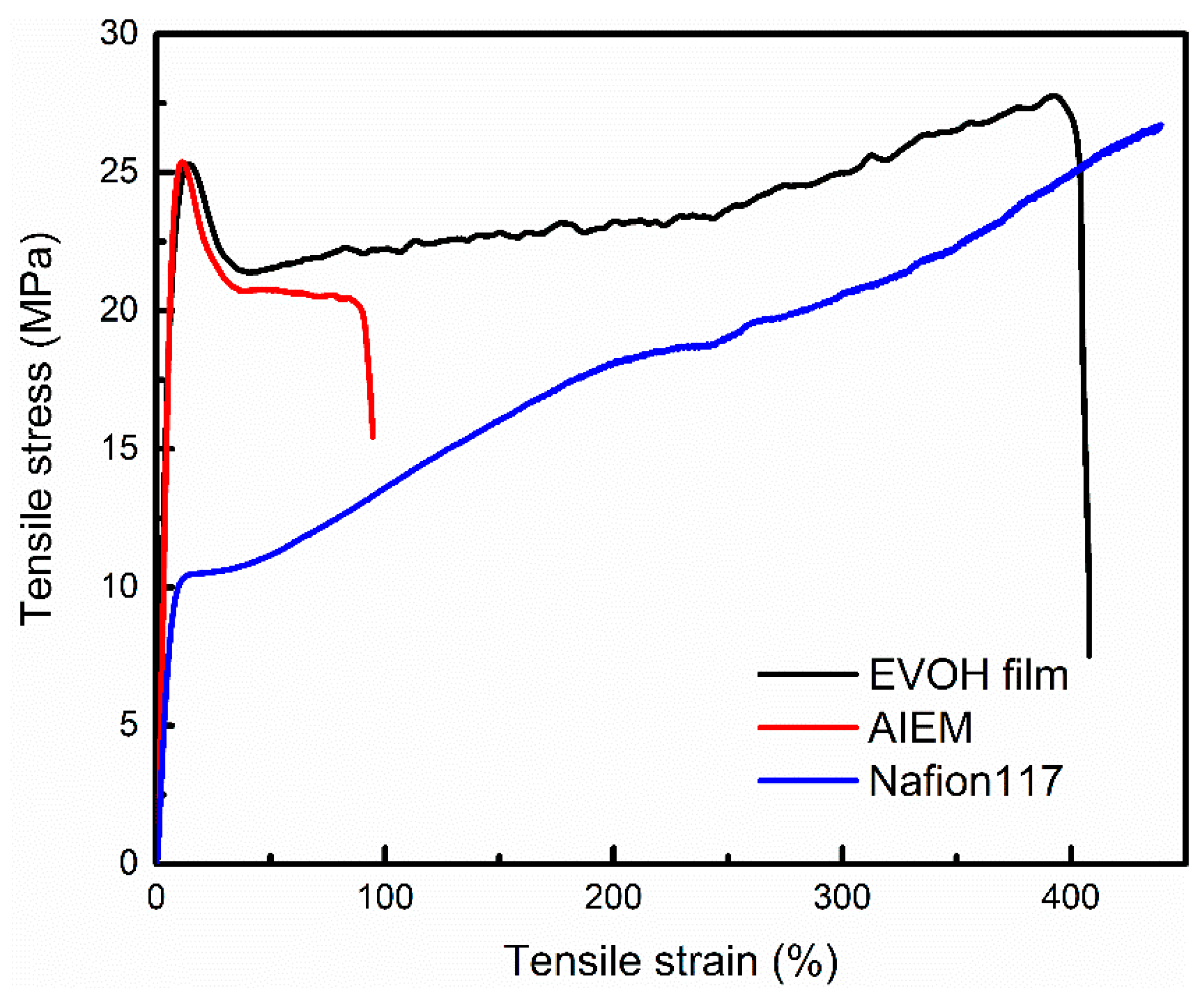
3.2.7. Mechanical Property
3.2.8. Water Uptake, Ion Exchange Capacity and Proton Conductivity
3.2.9. Permeability of V (IV) Ions through AIEM
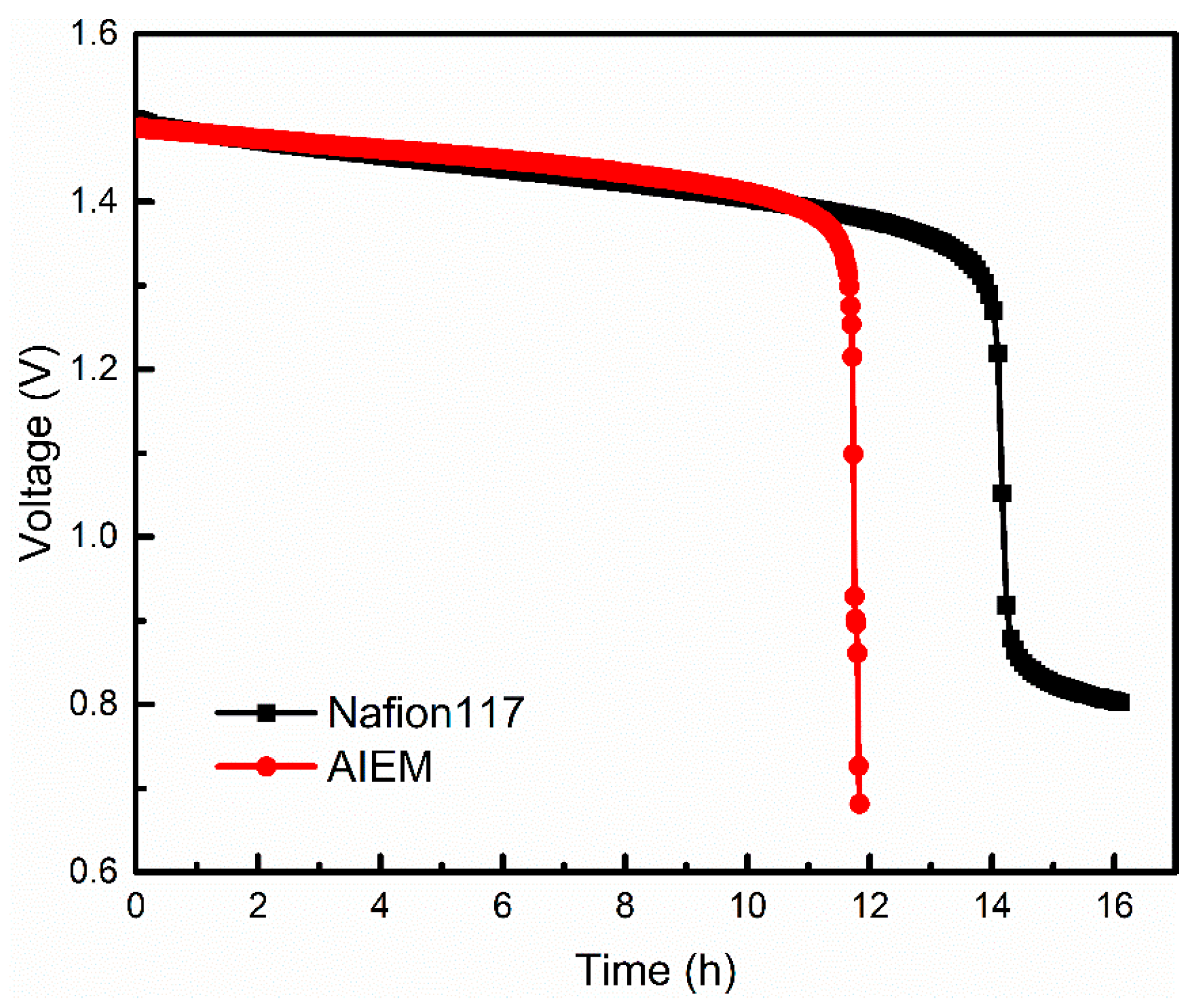
3.2.10. Open Circuit Voltage of the VRFB
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeng, S.; Zeng, L.; Wang, R.; Guo, W.; Tang, H. Effect of Elevated Temperature Annealing on Nafion/SiO2 Composite Membranes for the All-Vanadium Redox Flow Battery. Polymers 2018, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Cheng, Y.; Sun, L.; Ding, M.; Wu, C.; Yuan, D.; Jia, C. A green SPEEK/lignin composite membrane with high ion selectivity for vanadium redox flow battery. J. Membr. Sci. 2019, 572, 110–118. [Google Scholar] [CrossRef]
- Zeng, L.; Ye, J.; Zhang, J.; Liu, J.; Jia, C. A promising SPEEK/MCM composite membrane for highly efficient vanadium redox flow battery. Surf. Coat. Technol. 2019, 358, 167–172. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- Sun, C.; Zlotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Di Noto, V. [Nafion/(WO3)x] hybrid membranes for vanadium redox flow batteries. Solid State Ion. 2018, 319, 110–116. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, H.; Chen, J.; Qian, P.; Zhai, Y. Modification of Nafion membrane using interfacial polymerization for vanadium redox flow battery applications. J. Membr. Sci. 2008, 311, 98–103. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Z.; Qiu, X.; Chen, L. Nafion/SiO2 hybrid membrane for vanadium redox flow battery. J. Power Sources 2007, 166, 531–536. [Google Scholar] [CrossRef]
- Oldenburg, F.J.; Nilsson, E.; Schmidt, T.J.; Gubler, L. Tackling Capacity Fading in Vanadium Redox Flow Batteries with Amphoteric PBI/Nafion Bilayer Membranes. ChemSusChem 2019. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.B.; Zhao, L.; Sui, X.L.; Meng, L.H.; Wang, Z.B. Phosphotungstic acid immobilized nanofibers-Nafion composite membrane with low vanadium permeability and high selectivity for vanadium redox flow battery. J. Colloid Interface Sci. 2019, 542, 177–186. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.; Hempelmann, R.; Jang, J.H.; Kim, H.J.; Han, J.; Henkensmeier, D. Nafion membranes with a sulfonated organic additive for the use in vanadium redox flow batteries. J. Appl. Polym. Sci. 2019, 136, 47547. [Google Scholar] [CrossRef]
- Zeng, J.; Jiang, C.; Wang, Y.; Chen, J.; Zhu, S.; Zhao, B.; Wang, R. Studies on polypyrrole modified nafion membrane for vanadium redox flow battery. Electrochem. Commun. 2008, 10, 372–375. [Google Scholar] [CrossRef]
- Teng, X.; Dai, J.; Su, J.; Yin, G. Modification of Nafion membrane using fluorocarbon surfactant for all vanadium redox flow battery. J. Membr. Sci. 2015, 476, 20–29. [Google Scholar] [CrossRef]
- Kim, B.G.; Han, T.H.; Cho, C.G. Sulfonated graphene oxide/Nafion composite membrane for vanadium redox flow battery. J. Nanosci. Nanotechnol. 2014, 14, 9073–9077. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Yuan, Z.; Li, X.; Zhang, H.; Vankelecom, I.F. Advanced charged sponge—Like membrane with ultrahigh stability and selectivity for vanadium flow batteries. Adv. Funct. Mater. 2016, 26, 210–218. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J.; Li, J.; Zhai, M. PVDF based ion exchange membrane prepared by radiation grafting of ethyl styrenesulfonate and sequent hydrolysis. Radiat. Phys. Chem. 2017, 130, 252–258. [Google Scholar] [CrossRef]
- Qiu, J.; Ni, J.; Zhai, M.; Peng, J.; Zhou, H.; Li, J.; Wei, G. Radiation grafting of styrene and maleic anhydride onto PTFE membranes and sequent sulfonation for applications of vanadium redox battery. Radiat. Phys. Chem. 2007, 76, 1703–1707. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, L.; Zhai, M.; Ni, J.; Zhou, H.; Peng, J.; Wei, G. Pre-irradiation grafting of styrene and maleic anhydride onto PVDF membrane and subsequent sulfonation for application in vanadium redox batteries. J. Power Sources 2008, 177, 617–623. [Google Scholar] [CrossRef]
- Nasef, M.M.; Saidi, H.; Dahlan, K.Z.M. Single-step radiation induced grafting for preparation of proton exchange membranes for fuel cell. J. Membr. Sci. 2009, 339, 115–119. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Peng, J.; Qiu, J.; Xu, L.; Li, J.; Zhai, M. Designing a new process to prepare amphoteric ion exchange membrane with well-distributed grafted chains for vanadium redox flow battery. J. Membr. Sci. 2012, 419, 1–8. [Google Scholar] [CrossRef]
- Krepker, M.; Zhang, C.; Nitzan, N.; Prinz-Setter, O.; Massad-Ivanir, N.; Olah, A.; Segal, E. Antimicrobial LDPE/EVOH layered films containing carvacrol fabricated by multiplication extrusion. Polymers 2018, 10, 864. [Google Scholar] [CrossRef]
- Maes, C.; Luyten, W.; Herremans, G.; Peeters, R.; Carleer, R.; Buntinx, M. Recent updates on the barrier properties of ethylene vinyl alcohol copolymer (EVOH): A review. Polym. Rev. 2018, 58, 209–246. [Google Scholar] [CrossRef]
- Lopez-de-Dicastillo, C.; Alonso, J.M.; Catala, R.; Gavara, R.; Hernández-Muñoz, P. Improving the antioxidant protection of packaged food by incorporating natural flavonoids into ethylene-vinyl alcohol copolymer (EVOH) films. J. Agric. Food Chem. 2010, 58, 10958–10964. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; López-Carballo, G.; Hernández-Muñoz, P.; Gavara, R. Characterization of ethylene-vinyl alcohol copolymer containing lauril arginate (LAE) as material for active antimicrobial food packaging. Food Packag. Shelf Life 2014, 1, 10–18. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Huang, Y.D.; Wang, L. Study of EVOH based single ion polymer electrolyte: Composition and microstructure effects on the proton conductivity. Solid State Ion. 2006, 177, 65–71. [Google Scholar] [CrossRef]
- Chiba, Y.; Tominaga, Y. Poly (ethylene-co-vinyl alcohol)/sulfonated mesoporous organosilicate composites as proton-conductive membranes. J. Power Sources 2012, 203, 42–47. [Google Scholar] [CrossRef]
- Du, J.; Dong, Z.; Yang, X.; Zhao, L. Facile fabrication of sodium styrene sulfonate-grafted ethylene-vinyl alcohol copolymer as adsorbent for ammonium removal from aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 27235–27244. [Google Scholar] [CrossRef]
- Linlin, M.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Poly(2,5-benzimidazole)–silica nanocomposite membranes for high temperature proton exchange membrane fuel cell. J. Membr. Sci. 2012, 411, 91–98. [Google Scholar] [CrossRef]

| DMAEMA/SSS in the Feed | Weight Percent (%) | GY (%) | DMAEMA/SSS in the AIEM | ||
|---|---|---|---|---|---|
| C | N | S | |||
| 0.3:1 | 59.13 | 0.38 | 2.63 | 15.2 | 0.33 |
| 0.3:1 | 57.67 | 0.47 | 3.37 | 31.6 | 0.32 |
| 0.3:1 | 56.97 | 0.55 | 3.59 | 38.7 | 0.35 |
| 0.3:1 | 56.02 | 0.61 | 3.78 | 40.9 | 0.37 |
| Sample | GY (%) | WU (%) | IEC (mmol g−1) | Conductivity (mS/cm) | Thickness (μm) |
|---|---|---|---|---|---|
| AIEM | 15.2 | 34.7 | 0.43 | 3.4 | 118 |
| 31.6 | 61.3 | 0.73 | 29.9 | 121 | |
| 38.7 | 81.2 | 0.94 | 35.2 | 123 | |
| 40.9 | 96.0 | 1.05 | 40.0 | 120 | |
| Nafion117 | - | 30.0 | 0.98 | 50.1 | 175 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, K.; Dong, Z.; Wang, Y.; Qi, W.; Zhai, M.; Zhao, L. Facile Preparation of EVOH-Based Amphoteric Ion Exchange Membrane Using Radiation Grafting Technique: A Preliminary Investigation on Its Application for Vanadium Redox Flow Battery. Polymers 2019, 11, 843. https://doi.org/10.3390/polym11050843
Xie K, Dong Z, Wang Y, Qi W, Zhai M, Zhao L. Facile Preparation of EVOH-Based Amphoteric Ion Exchange Membrane Using Radiation Grafting Technique: A Preliminary Investigation on Its Application for Vanadium Redox Flow Battery. Polymers. 2019; 11(5):843. https://doi.org/10.3390/polym11050843
Chicago/Turabian StyleXie, Kangjun, Zhen Dong, Yicheng Wang, Wei Qi, Maolin Zhai, and Long Zhao. 2019. "Facile Preparation of EVOH-Based Amphoteric Ion Exchange Membrane Using Radiation Grafting Technique: A Preliminary Investigation on Its Application for Vanadium Redox Flow Battery" Polymers 11, no. 5: 843. https://doi.org/10.3390/polym11050843
APA StyleXie, K., Dong, Z., Wang, Y., Qi, W., Zhai, M., & Zhao, L. (2019). Facile Preparation of EVOH-Based Amphoteric Ion Exchange Membrane Using Radiation Grafting Technique: A Preliminary Investigation on Its Application for Vanadium Redox Flow Battery. Polymers, 11(5), 843. https://doi.org/10.3390/polym11050843




