Lignin Transformation of One-Year-Old Plants During Anaerobic Digestion (AD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sampling
2.2. Chemical Analysis
2.2.1. Determination of Lignin Percentage
2.2.2. Determination of Functional Groups
2.2.3. Fourier Transform-Infrared Spectroscopy of Lignin
2.3. Statistical Analysis
Anaerobic Digestion
3. Results and Discussion
3.1. Percentage of Lignin
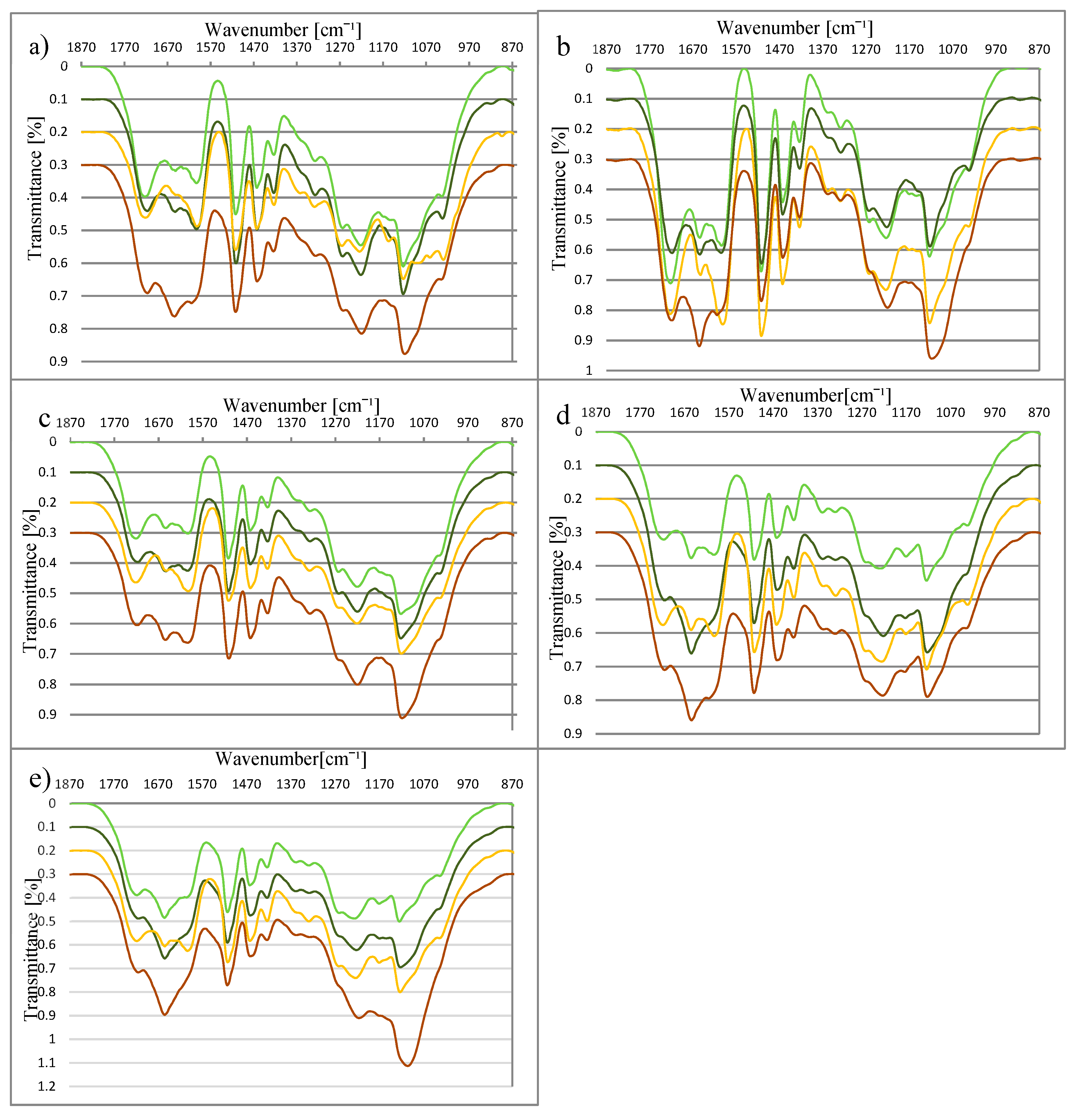
3.2. Structure of Lignin
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Derkacheva, O.; Sukhov, D. Investigation of lignins by FTIR spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
- Banoub, J.; Delmas, G.H.; Mackenzie, G.; Cachet, N.; Benjelloun Mlayah, B.; Delmas, M. A critique on the structural analysis of lignins and application of novel tandem mass spectrometric strategies to determine lignin sequencing. J. Mass Spectr. 2014, 50, 5–48. [Google Scholar] [CrossRef] [PubMed]
- Martone, P.T.; Estevez, J.M.; Lu, F.; Ruel, K.; Denny, M.W.; Somerville, C.; Ralph, J. Discovery of lignin in seaweed reveals convergent evolution of cell-wall architecture. Curr. Biol. 2009, 19, 169–175. [Google Scholar] [CrossRef]
- Doherty, W.; Mousavioun, P.; Fellows, C. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crop. Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Singh, S.; Parthasarathi, R.; Simmons, B.A.; Henry, R.J. Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew. Sustain. Energy Rev. 2015, 49, 871–906. [Google Scholar] [CrossRef]
- Freudenberg, K. Biosynthesis and constitution of lignin. Nature 1959, 183, 1152. [Google Scholar] [CrossRef]
- Davison, B.H.; Drescher, S.R.; Tuskan, G.A.; Davis, M.F.; Nghiem, N.P. Variation of S/G ratio and lignin content in a Populus family influences the release of xylose by dilute acid hydrolysis. Appl. Biochem. Biotechnol. 2006, 129–132, 427–435. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood formation in trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tsukahara, K.; Yagishita, T.; Sawayama, S. Performance of a fixed-bed reactor packed with carbon felt during anaerobic digestion of cellulose. Bioresour. Technol. 2004, 94, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Brosse, N.; Dufour, F.A.; Meng, X.; Sun, Q.; Ragauskas, A. Miscanthus: A fast-growing crop for biofuels and chemicals production. Biofuels Bioprod. Bioref. 2012, 6, 580–598. [Google Scholar] [CrossRef]
- Dukiewicz, H.; Waliszewska, B.; Zborowska, M. Higher and lower heating values of selected lignocellulose materials. Ann. Wars. Univ. Life Sci-SGGW For. Wood Technol. 2014, 87, 60–63. [Google Scholar]
- Kozłowski, S.; Zielewicz, W.; Lutyński, A. Określanie wartości energetycznej Sorghum saccharatum (L.) Moench, Zea mays L. i Malva verticillata L. Łąkarstwo W Polsce 2007, 10, 131–140. [Google Scholar]
- She, D.; Xu, F.; Geng, Z.C.; Sun, R.C.; Jones, G.L.; Baird, M.S. Physicochemical characterization of extracted lignin from sweet sorghum stem. Ind. Crop. Prod. 2010, 32, 21–28. [Google Scholar] [CrossRef]
- Stefaniak, T.R.; Dahlberg, J.A.; Bean, B.W.; Dighe, N.; Wolfrum, E.J.; Rooney, W.L. Variation in biomass composition components among forage, biomass, sorghum-sudangrass, and sweet sorghum types. Crop Sci. 2012, 52, 1949–1954. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels. Bioprod. Biorefining 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Lee, W.-C.; Kuan, W.-C. Miscanthus as cellulosic biomass for bioethanol production. Biotechnol. J. 2015, 10, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Anami, S.E.; Zhang, L.-M.; Xia, Y.; Zhang, Y.-M.; Liu, Z.-Q.; Jing, H.-C. Sweet sorghum ideotypes: Genetic improvement of the biofuel syndrome. Food Energy Secur. 2015, 4, 159–177. [Google Scholar] [CrossRef]
- Mayer, F.; Gerin, P.A.; Noo, A.; Lemaigre, S.; Stilmant, D.; Schmit, T.; Leclech, N.; Ruelle, L.; Gennen, J.; von Francken-Welz, H.; et al. Assessment of energy crops alternative to maize for biogas production in the Greater Region. Bioresour. Technol. 2014, 166, 358–367. [Google Scholar] [CrossRef]
- Michalska, K.; Ledakowicz, S. Degradacja struktur lignocelulozowych oraz produktów ich hydrolizy. Inżynieria I Aparatura Chemiczna [Degradation of lignocellulosic structures and products of their hydrolysis. Eng. Chem. Appar. 2012, 51, 157. [Google Scholar]
- Castro, F.B.; Hotten, P.M.; Ørskov, E.R.; Rebeller, M. Inhibition of Rumen microbes by compounds formed in the steam treatment of wheat straw. Bioresour. Technol. 1994, 50, 25–30. [Google Scholar] [CrossRef]
- Pu, Y.; Hu, F.; Huang, F.; Davison, B.H.; Ragauskas, A.J. Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol. Biofuels 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Theuretzbacher, F.; Lizasoain, J.; Lefever, C.; Saylor, M.K.; Enguidanos, R.; Weran, N.; Gronauer, A.; Bauer, A. Steam explosion pretreatment of wheat straw to improve methane yields: Investigation of the degradation kinetics of structural compounds during anaerobic digestion. Bioresour. Technol. 2015, 179, 299–305. [Google Scholar] [CrossRef]
- Mulat, D.G.; Dibdiakova, J.; Horn, S.J. Microbial biogas production from hydrolysis lignin: Insight into lignin structural changes. Biotechnol. Biofuels 2018, 11, 61–77. [Google Scholar] [CrossRef]
- Whittaker, C.; Hunt, J.; Misselbrook, T.; Shiel, I. How well does Miscanthus ensile for use in an anaerobic digestion plant? Biomass Bioenergy 2016, 88, 24–34. [Google Scholar] [CrossRef]
- Godin, B.; Agneessens, R.; Schmit, T.; Lamaudiere, S.; Goffart, J.P.; Gerin, P.A. Evolution of Sorghum and Corn Composition with the Harvest Period, with Focus on the Hemicelluloses Monosaccharidic Composition; Journée Annuelle de l’EDT Geproc and Envitam: Gembloux, Belgium, 2013. [Google Scholar]
- Galbe, M.; Zacchi, G. Pretreatment: The key to efficient utilization of lignocellulosic materials. Biomass Bioenergy 2012, 46, 70–78. [Google Scholar] [CrossRef]
- Mao, J.D.; Holtman, K.M.; Franqui-Villanueva, D. Chemical structures of corn stover and its residue after dilute acid prehydrolysis and enzymatic hydrolysis: Insight into factors limiting enzymatic hydrolysis. J. Agric. Food Chem. 2010, 58, 11680–11687. [Google Scholar] [CrossRef]
- Jung, S.; Foston, M.; Sullards, M.C.; Ragauskas, A.J. Surface characterization of dilute acid pretreated Populus deltoides by ToF-SIMS. Energy Fuels 2010, 24, 1347–1357. [Google Scholar] [CrossRef]
- Waliszewska, H.; Zborowska, M.; Waliszewska, B.; Borysiak, S.; Antczak, A.; Czekała, W. Transformation of miscanthus and sorghum cellulose during methane fermentation. Cellulose 2018, 25, 1207–1216. [Google Scholar] [CrossRef]
- Stachowiak-Wencek, A.; Zborowska, M.; Waliszewska, H. Waliszewska B., Zmiany struktury ligniny osadków kukurydzy pod wpływem fermentacji metanowej. Przemysł Chem. 2018, 97, 2162–2165. [Google Scholar]
- TAPPI method T 222 om-83. Acid-insoluble lignin in wood and pulp. In Test Methods, 1998–1999; TAPPI Press: Atlanta, GA, USA, 1999.
- Kačík, F.; Kačíková, D.; Bubenikova, T.; Veľková, V. Determination of methoxy groups in lignocellulosic materials. Drewno 2004, 47, 113–119. [Google Scholar]
- Lin, S.Y.; Dence, C.W. Methods in Lignin Chemistry, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar] [CrossRef]
- Fan, M.; Dai, D.; Huang, B. Fourier Transform Infrared Spectroscopy for Natural Fibres, Fourier Transform Salih Mohammed Salih, IntechOpen. Available online: https://www.intechopen.com/books/fourier-transform-materials-analysis/fourier-transform-infrared-spectroscopy-for-natural-fibres (accessed on 23 May 2012). [CrossRef]
- Ungureanu, E.; Ungureanu, O.; Căpraru, A.-M.; Popa, V.I. Chemical modification and characterization of straw lignin. Cellul. Chem. Technol. 2009, 43, 263–269. [Google Scholar]
- DIN 38 414-S8. Bestimmung des Faulverhaltens “Schlamm und Sedimente”; BeuthVerlag GmbH: Berlin, Germany, 1985.
- Lewicki, A.; Pilarski, K.; Janczak, D.; Czekała, W.; Rodríguez Carmona, P.C.; Cieślik, M.; Witaszek, K. The biogas production from herbs and waste from herbal industry. J. Res. Appl. Agric. Eng. 2013, 58, 114–117. [Google Scholar]
- Shugang, Z.; Jing, W.; Hongxia, W.; Zhihua, Z.; Xibo, L. Changes in lignin content and activity of related enzymes in the endocarp during the walnut shell development period. Hortic. Plant J. 2016, 2, 141–146. [Google Scholar]
- Sannigrahi, P.; Ragauskas, A.J. Characterization of fermentation residues from the production of bio-ethanol from lignocellulosic feedstocks. J. Biobased Mater. Bioenergy 2011, 5, 514–519. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood—Chemistry, Ultrastructure, Reactions, 2nd ed.; Walter de Gruyter: Berlin, Germany, 1989. [Google Scholar]
- Popescu, C.M.; Popescu, M.C.; Singurel, G.; Vasile, C.; Argyropoulos, D.S.; Willfor, S. Spectral characterization of eucalyptus wood. Appl. Spectrosc. 2007, 61, 1168–1177. [Google Scholar] [CrossRef]
- Hatfield, R.; Ralph, J.; Grabber, J.H. A potential role for sinapyl p-coumarate as a radical transfer mechanism in grass lignin formation. Planta 2008, 228, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Faix, O.; Mozuch, M.D.; Kent Kirk, T. Degradation of gymnosperm (guaiacyl) vs. angiosperm (syringyl/guaiacyl) lignins by phanerochaete chrysosporium. Holzforschung 1985, 39, 203–208. [Google Scholar] [CrossRef]
- Lewis, N.; Yamamoto, E. Lignin: Occurrence, biogenesis and biodegradation. Annu. Rev. Plant Biol. 1990, 41, 455–496. [Google Scholar] [CrossRef] [PubMed]
- Skyba, O.; Douglas, C.J.; Mansfield, S.D. Syringyl-rich lignin renders poplars more resistant to degradation by wood decay fungi. Appl. Environ. Microbiol. 2013, 79, 2560–2571. [Google Scholar] [CrossRef]
- Obst, J.R.; Highley, T.L.; Miller, R.B. Influence of Lignin Type on the Decay of Woody Angiosperms by Trametes Versicolor. In Mycotoxins, Wood Decay, Plant Stress: Biocorrosion, and General Biodeterioration; Llewellyn, G.C., Dashek, W.V., O’Rear, C.E., Eds.; Biodeterioration Research; Springer: Boston, MA, USA, 1994; Volume 4. [Google Scholar]
- Chua, M.; Chen, C.; Chang, H. 13C NMR Spectroscopic study of spruce lignin degraded Phanerochaete Chrysosporium. Holzforsch.—Int. J. Biol. Chem. Phys. Technol. Wood 2009, 36, 165–172. [Google Scholar]
- Ruiz-Dueñas, F.J.; Martínez, A.T. Microbial degradation of lignin: How a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb. Biotechnol. 2009, 2, 164–177. [Google Scholar] [CrossRef]
- Brauns, F.E.; Brauns, D.A. The Chemistry of Lignin: Covering the Literature for the Years 1949–1958; Academic Press: New York, NY, USA; London, UK, 1960. [Google Scholar]
- Zhao, Q. Lignification: Flexibility, Biosynthesis and Regulation. Trends Plant Sci. 2016, 21, 713–721. [Google Scholar] [CrossRef]
- Marques, G.; Rencoret, J.; Gutiérrez, A.; del Río, J.C. Evaluation of the chemical composition of different non-woody plant fibers used for pulp and paper manufacturing. Open Agric. J. 2010, 3, 93–101. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Smith, E.A. Characterization of woody and herbaceous biomasses lignin composition with 1064 nm dispersive multichannel Raman spectroscopy. Appl. Spectrosc. 2012, 66, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Palmer, N.A.; Saballos, A.; Greene, A.M.; Xin, Z.; Sarath, G.; Vermerris, W.; Pedersen, J.F. “Identification and Characterization of Four Missense Mutations in Brown midrib12 (Bmr12), the Caffeic O-Methyltranferase (COMT) of Sorghum”. Bioenerg. Res. 2012, 5, 855–865. [Google Scholar] [CrossRef]
- Mazumder, B. Fortunate Sons: New Estimates of Intergenerational Mobility in the United States Using Social Security Earnings Data. Rev. Econ. Stat. 2005, 87, 235–255. [Google Scholar] [CrossRef]
- Todorciuc, T.; Căpraru, A.-M.; Kratochvílová, I.; Popa, V.I. Characterization of non-wood lignin and its hydoxymethylated derivatives by spectroscopy and self-assembling investigations. Cellul. Chem. Technol. 2009, 43, 399–408. [Google Scholar]
- Xiao, Z.; Li, Y.; Wu, X.; Qi, G.; Li, N.; Zhang, K.; Wang, D.; Sun, X.S. Utilization of sorghum lignin to improve adhesion strength of soy protein adhesives on wood veneer. Ind. Crop. Prod. 2013, 50, 501–509. [Google Scholar] [CrossRef]
| Varieties | Harvest Growing Phase | Lignin (% d.m.) | Increase of Lignin Content During AD (%) | |
|---|---|---|---|---|
| NL | RL | |||
| Miscanthus ×giganteus | DV | 18.4a ± 0.9 | 27.6b ± 0.1 | 55.0 |
| AV | 24.4a ± 0.1 | 31.9b ± 0.8 | 30.9 | |
| M. sacchariflorus | DV | 18.4a ± 0.1 | 29.3b ± 3.1 | 59.4 |
| AV | 20.0a ± 0.1 | 26.6b ± 1.2 | 33.2 | |
| M. sinensis | DV | 17.9a ± 0.2 | 28.1b ± 0.2 | 57.3 |
| AV | 19.1a ± 0.2 | 25.4b ± 0.7 | 33.2 | |
| Mean of Miscanthus | DV | 18.2 ± 0.2 | 28.3 ± 0.7 | 57.2 |
| AV | 21.1 ± 2.3 | 28.0 ± 2.8 | 32.3 | |
| S. bicolor | DV | 15.4a ± 0.2 | 34.2b ± 0.2 | 122.3 |
| AV | 16.9a ± 0.1 | 32.6b ± 0.2 | 93.3 | |
| S. saccharatum | DV | 14.5a ± 0.1 | 41.1b ± 0.1 | 183.4 |
| AV | 16.4a ± 0.4 | 34.3b ± 0.2 | 109.4 | |
| Mean of Sorghum | DV | 15.0 ± 0.4 | 37.7 ± 3.4 | 152.0 |
| AV | 16.6 ± 0.2 | 33.4 ± 0.8 | 101.2 | |
| Position [cm−1] | Band Origin |
|---|---|
| 2936–2850 | C–H stretching in methyl and methylene groups |
| 1715–1705 | C=O stretching nonconjugated to the aromatic ring |
| 1660–1650 | C=O stretching in conjugation to the aromatic ring |
| 1620–1600 | Aromatic ring vibration |
| 1515–1510 | Aromatic ring vibration |
| 1460–1455 | C–H deformations |
| 1420 | Aromatic ring vibration |
| 1360–1350 | C–H deformations |
| 1330–1325 | Aromatic (syringyl) ring breaching |
| 1260–1220 | Aromatic (guaiacyl) ring breaching |
| 1160–1120 | C–O–C stretching |
| 1035 | C–H, C–O deformations |
| Varieties | Harvest Season | OCH3 (%) | Changes of OCH3 Content during AD (%) | OH (%) | Change of OH Content during AD (%) | ||
|---|---|---|---|---|---|---|---|
| NL | RL | NL | RL | ||||
| Miscanthus ×giganteus | DV | 12.8 | 12.6 | −1.56 | 3.6 | 1.5 | 58.33 |
| AV | 13.9 | 10.7 | −23.02 | 0.4 | 0.2 | 50.00 | |
| M. sacchariflorus | DV | 10.6 | 4.5 | −57.55 | 1.7 | 0.4 | 76.47 |
| AV | 12.6 | 12.3 | −2.38 | 1.6 | 0.8 | 50.00 | |
| M. sinensis | DV | 11.1 | 4.7 | −57.66 | 4.9 | 2.4 | 51.02 |
| AV | 10.5 | 9.7 | −7.62 | 1.7 | 0.8 | 52.94 | |
| Mean of miscanthus | DV | 11.5 | 7.2 | −37.39 | 3.4 | 1.4 | 58.82 |
| AV | 10.5 | 10.9 | −11.00 | 1.2 | 0.6 | 50.00 | |
| S. bicolor | DV | 10.8 | 8.4 | −22.22 | 5.0 | 3.4 | 32.00 |
| AV | 12.2 | 8.8 | −27.87 | 0.8 | 0.4 | 50.00 | |
| S. saccharatum | DV | 10.3 | 7.6 | −26.21 | 1.7 | 1.4 | 17.65 |
| AV | 9.3 | 2.5 | −73.12 | 1.0 | 0.8 | 20.00 | |
| Mean of sorghum | DV | 10.5 | 8.0 | −23.81 | 3.3 | 1.4 | 57.58 |
| AV | 10.7 | 5.6 | −47.66 | 0.9 | 0.6 | 33.33 | |
| Varieties | Harvest Season | S/G A1325/A1267 | Changes of S/G during AD (%) | Al/Ar A2930/A1510 | Changes of Al/Ar during AD (%) | ||
|---|---|---|---|---|---|---|---|
| NL | RL | NL | RL | ||||
| Miscanthus ×giganteus | DV | 0.59 | 0.61 | 3.39 | 0.54 | 0.39 | −27.78 |
| AV | 0.66 | 0.62 | −6.06 | 0.47 | 0.77 | 63.83 | |
| M. sacchariflorus | DV | 0.53 | 0.56 | 5.66 | 0.99 | 0.92 | −7.07 |
| AV | 0.65 | 0.53 | −18.46 | 0.85 | 1.11 | 30.59 | |
| M. sinensis | DV | 0.38 | 0.34 | −10.53 | 0.83 | 0.71 | −14.46 |
| AV | 0.49 | 0.28 | −42.86 | 0.61 | 1.02 | 67.21 | |
| Mean of miscanthus | DV | 0.5 | 0.55 | 10.00 | 0.79 | 0.67 | −15.19 |
| AV | 0.6 | 0.47 | −21.67 | 0.64 | 0.96 | 50.00 | |
| S. bicolor | DV | 0.59 | 0.55 | −6.78 | 0.74 | 0.89 | 20.27 |
| AV | 0.64 | 0.62 | −3.13 | 0.73 | 0.71 | 1.37 | |
| S. saccharatum | DV | 0.57 | 0.54 | −5.26 | 0.61 | 0.87 | 42.62 |
| AV | 0.61 | 0.44 | −27.87 | 0.54 | 0.81 | 50.00 | |
| Mean of sorghum | DV | 0.58 | 0.55 | −5.17 | 0.88 | 0.88 | 37.50 |
| AV | 0.63 | 0.53 | −15.87 | 0.76 | 0.76 | 18.75 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waliszewska, H.; Zborowska, M.; Stachowiak-Wencek, A.; Waliszewska, B.; Czekała, W. Lignin Transformation of One-Year-Old Plants During Anaerobic Digestion (AD). Polymers 2019, 11, 835. https://doi.org/10.3390/polym11050835
Waliszewska H, Zborowska M, Stachowiak-Wencek A, Waliszewska B, Czekała W. Lignin Transformation of One-Year-Old Plants During Anaerobic Digestion (AD). Polymers. 2019; 11(5):835. https://doi.org/10.3390/polym11050835
Chicago/Turabian StyleWaliszewska, Hanna, Magdalena Zborowska, Agata Stachowiak-Wencek, Bogusława Waliszewska, and Wojciech Czekała. 2019. "Lignin Transformation of One-Year-Old Plants During Anaerobic Digestion (AD)" Polymers 11, no. 5: 835. https://doi.org/10.3390/polym11050835
APA StyleWaliszewska, H., Zborowska, M., Stachowiak-Wencek, A., Waliszewska, B., & Czekała, W. (2019). Lignin Transformation of One-Year-Old Plants During Anaerobic Digestion (AD). Polymers, 11(5), 835. https://doi.org/10.3390/polym11050835