Effect of Bifunctional Montmorillonite on the Thermal and Tribological Properties of Polystyrene/Montmorillonite Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
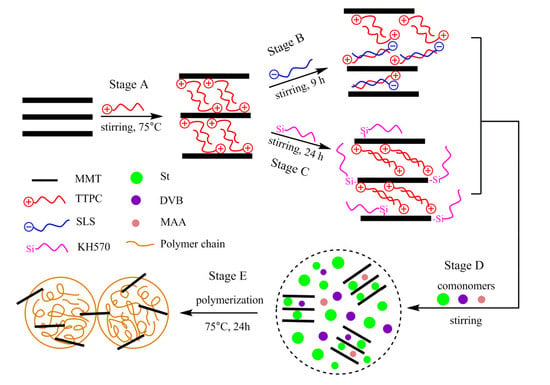
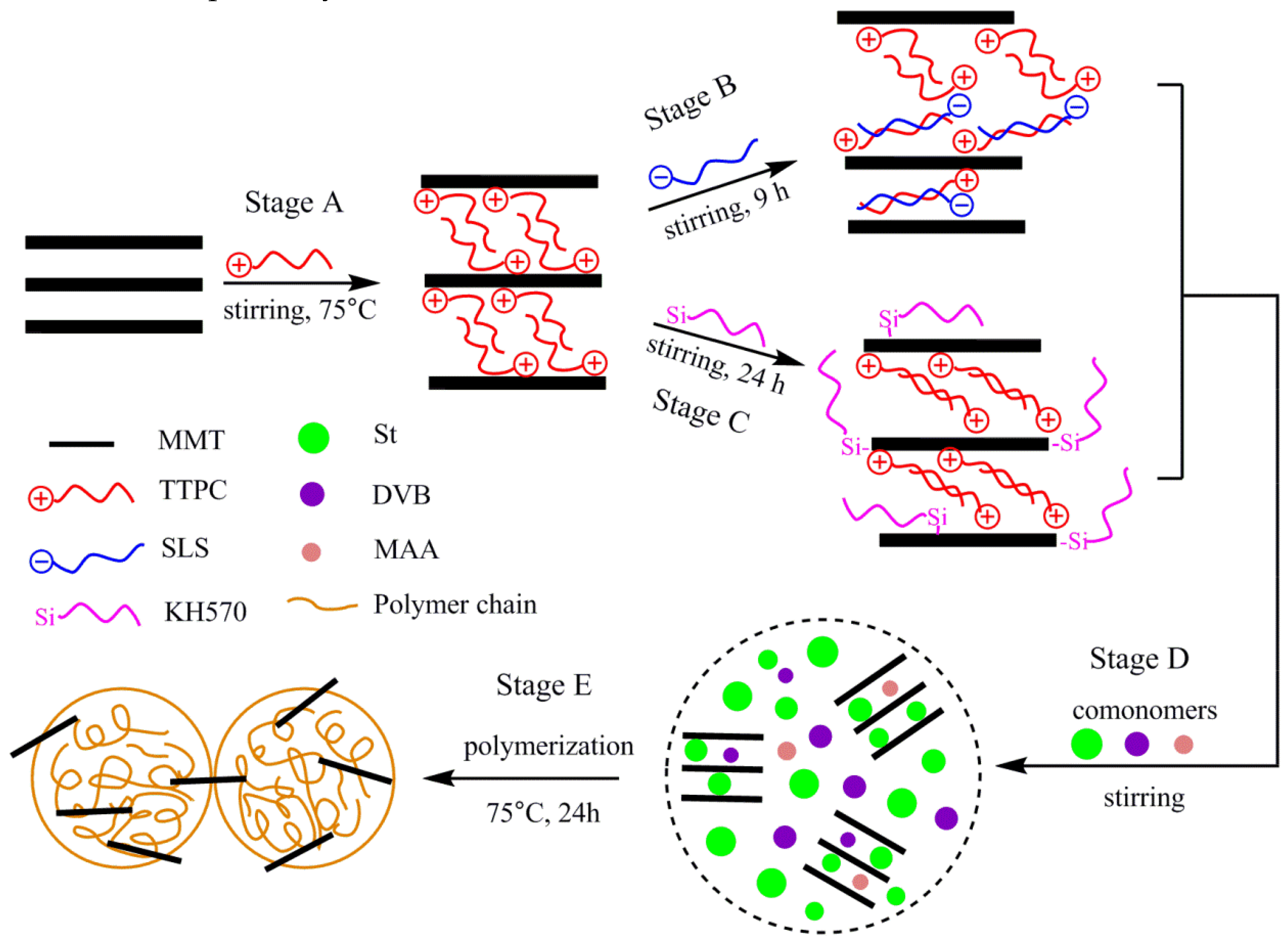
2.2. Preparation of OMMT
2.2.1. Preparation of P-MMT
2.2.2. Preparation of P-SLS-MMT
2.2.3. Preparation of P-KH570-MMT
2.3. Preparation of PS/OMMT Nanocomposite
2.4. Preparation of the PAO-PS/OMMT Lubricant
2.5. Characterization
3. Results
3.1. Surface Treatments of Montmorillonite
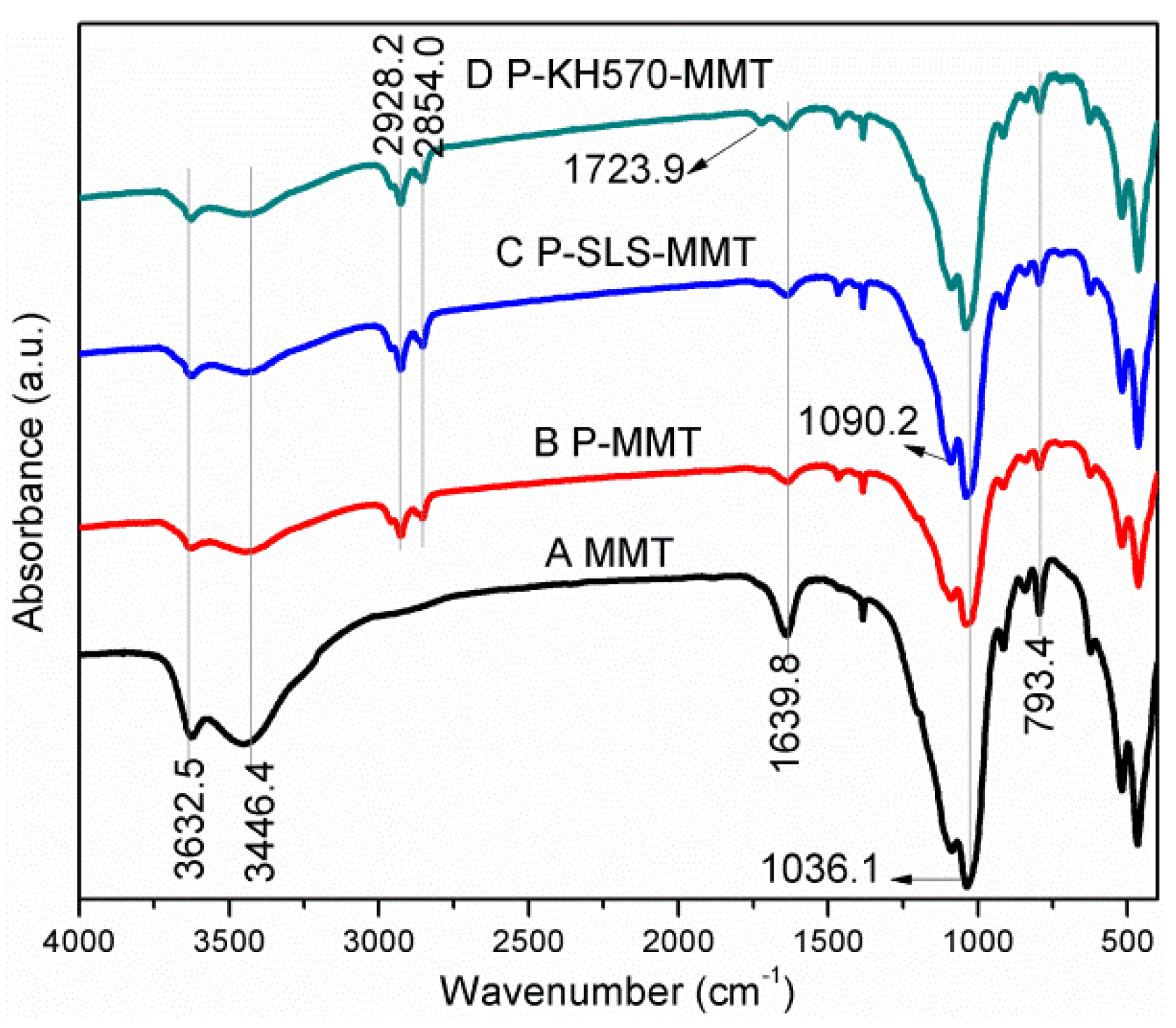
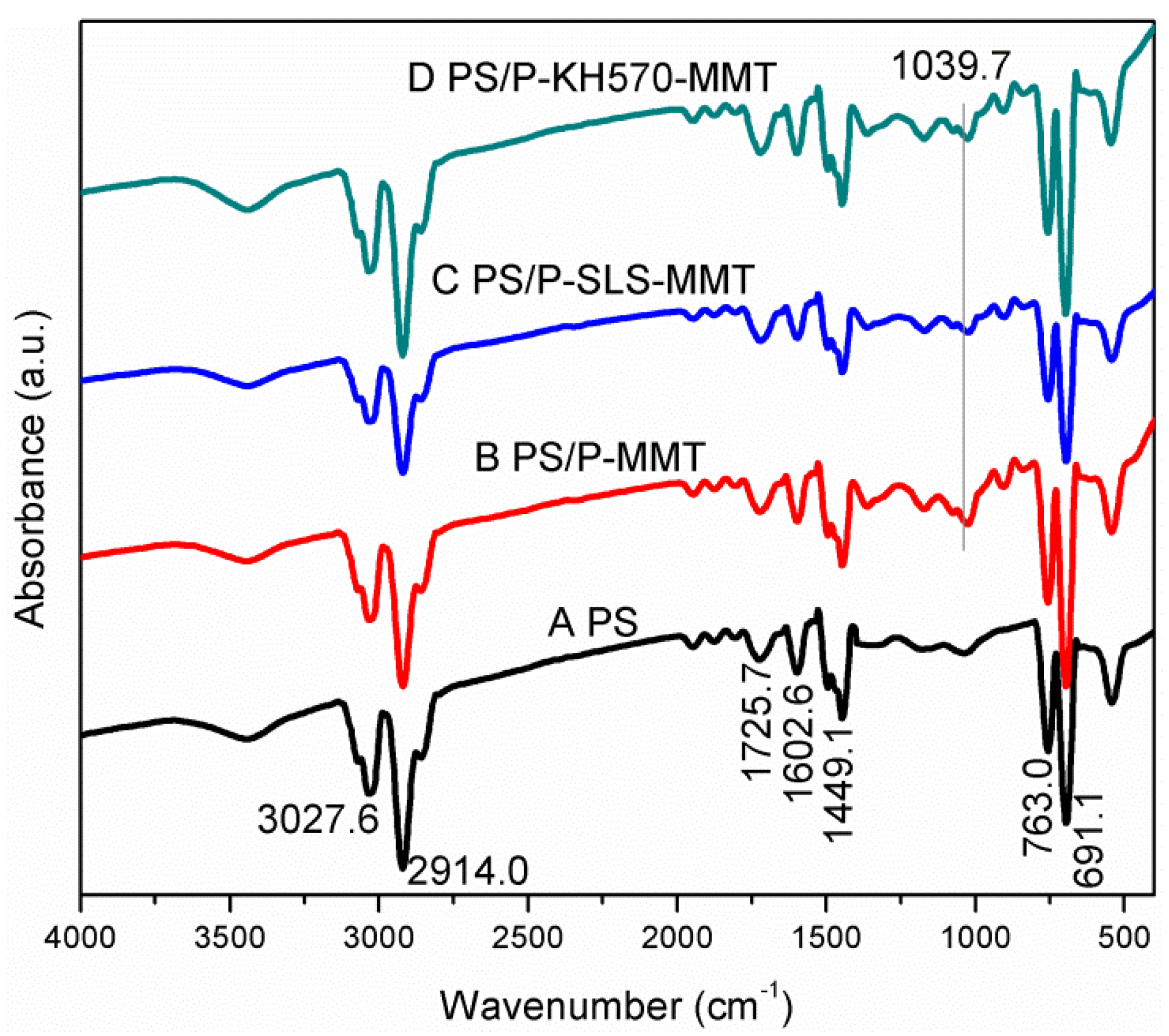
3.1.1. FTIR Analysis
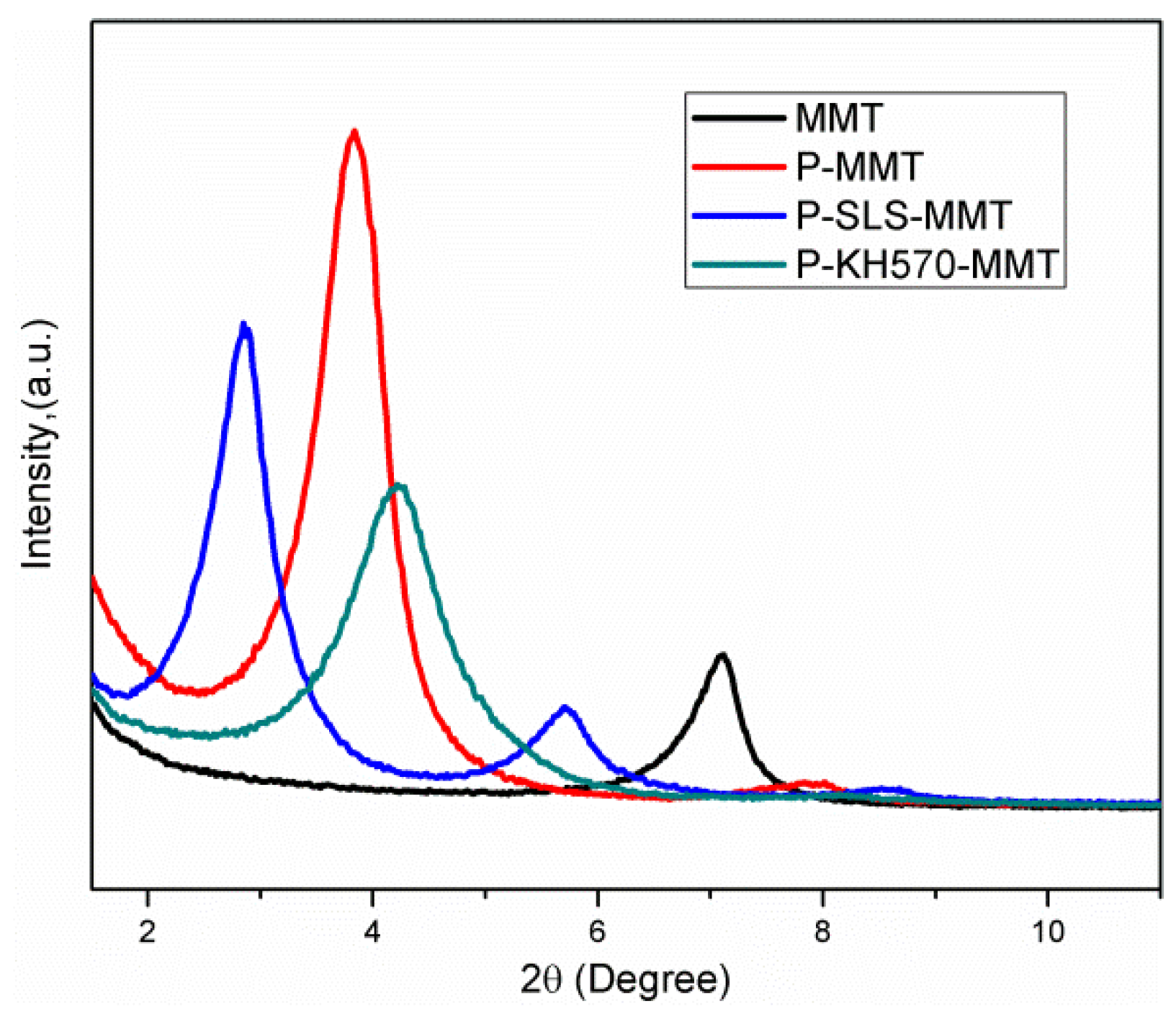
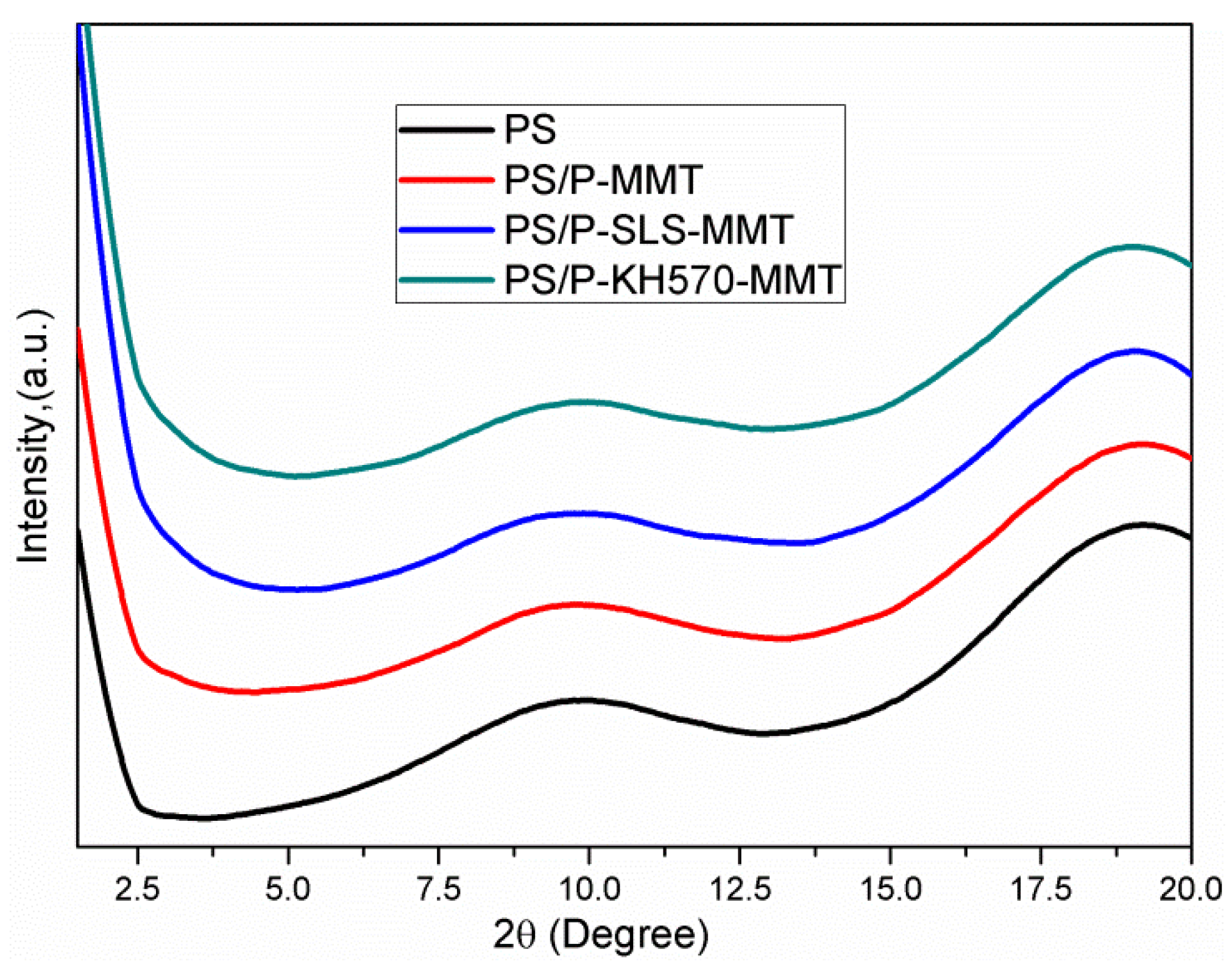
3.1.2. XRD Analysis
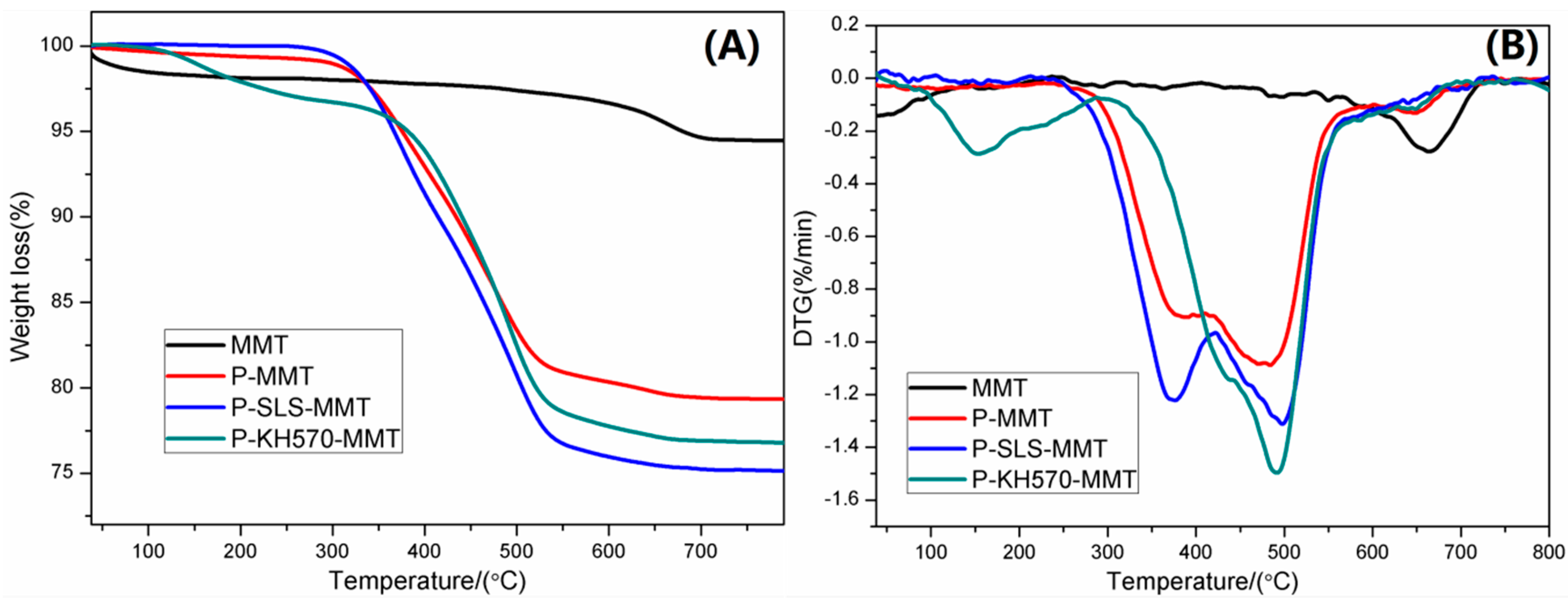
3.1.3. TGA and DTG Analysis
3.2. Characterization of PS/OMMT Nanocomposite
3.2.1. FTIR Analysis
3.2.2. XRD Analysis
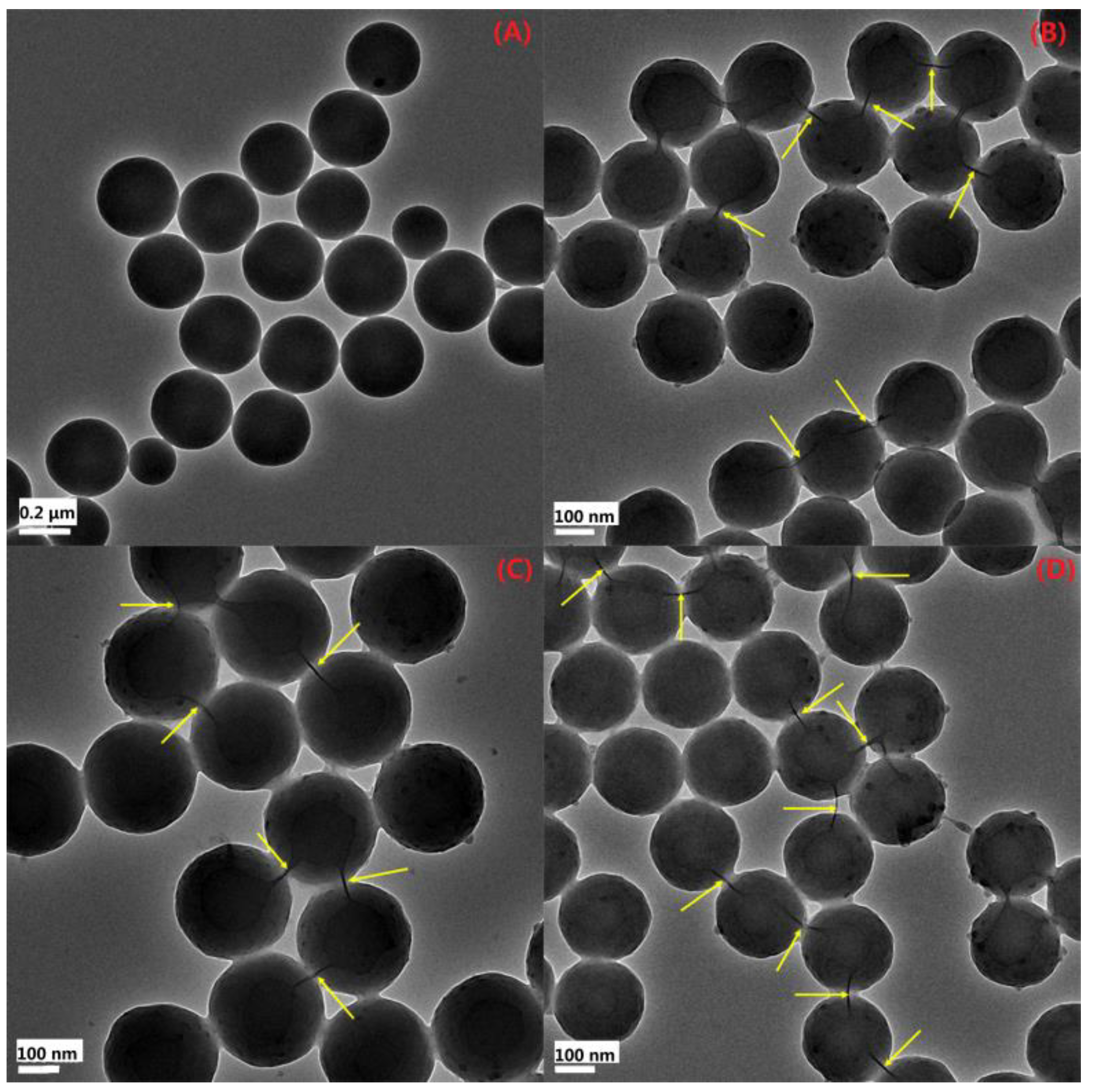
3.2.3. TEM Analysis
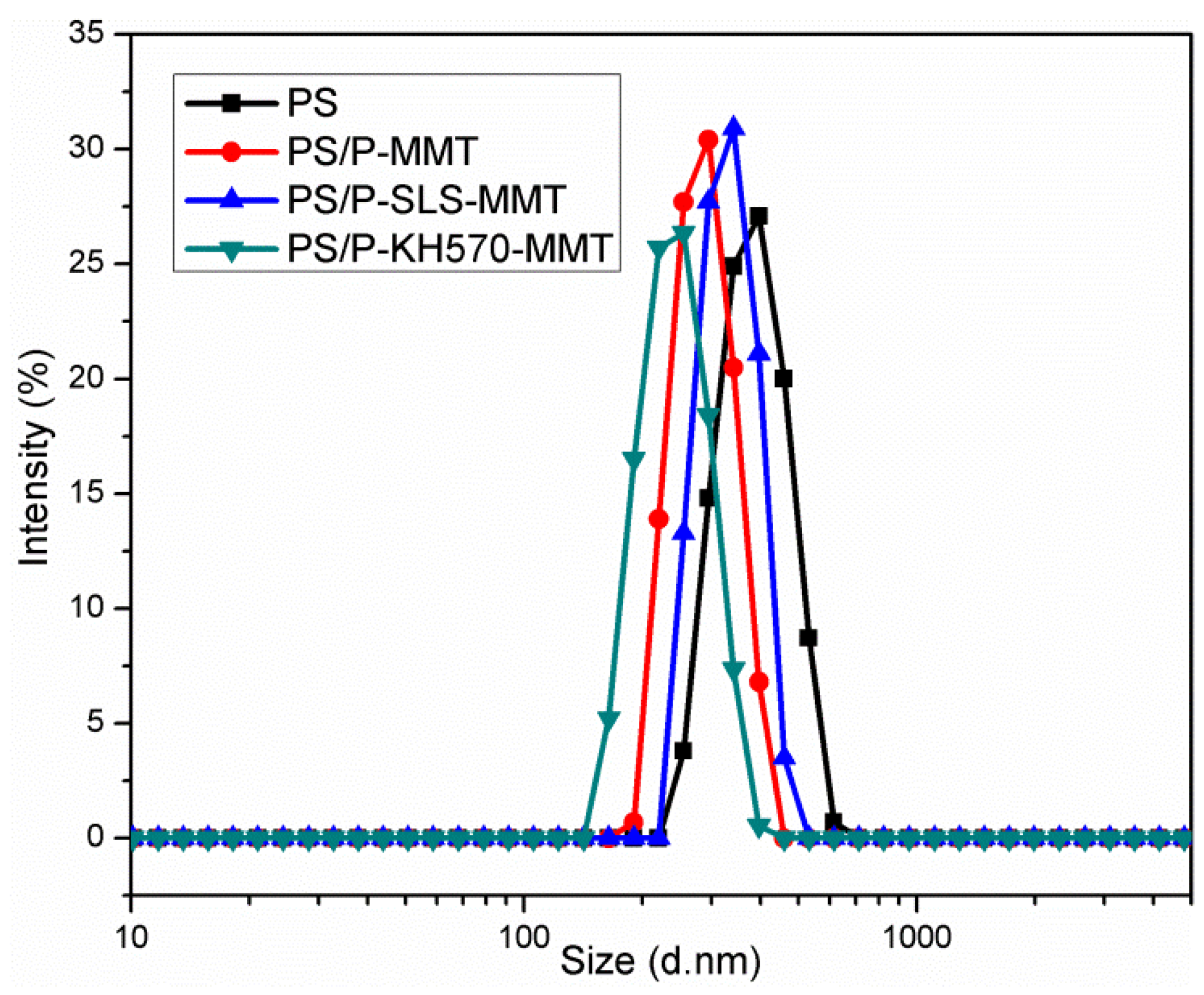
3.2.4. DLS Analysis
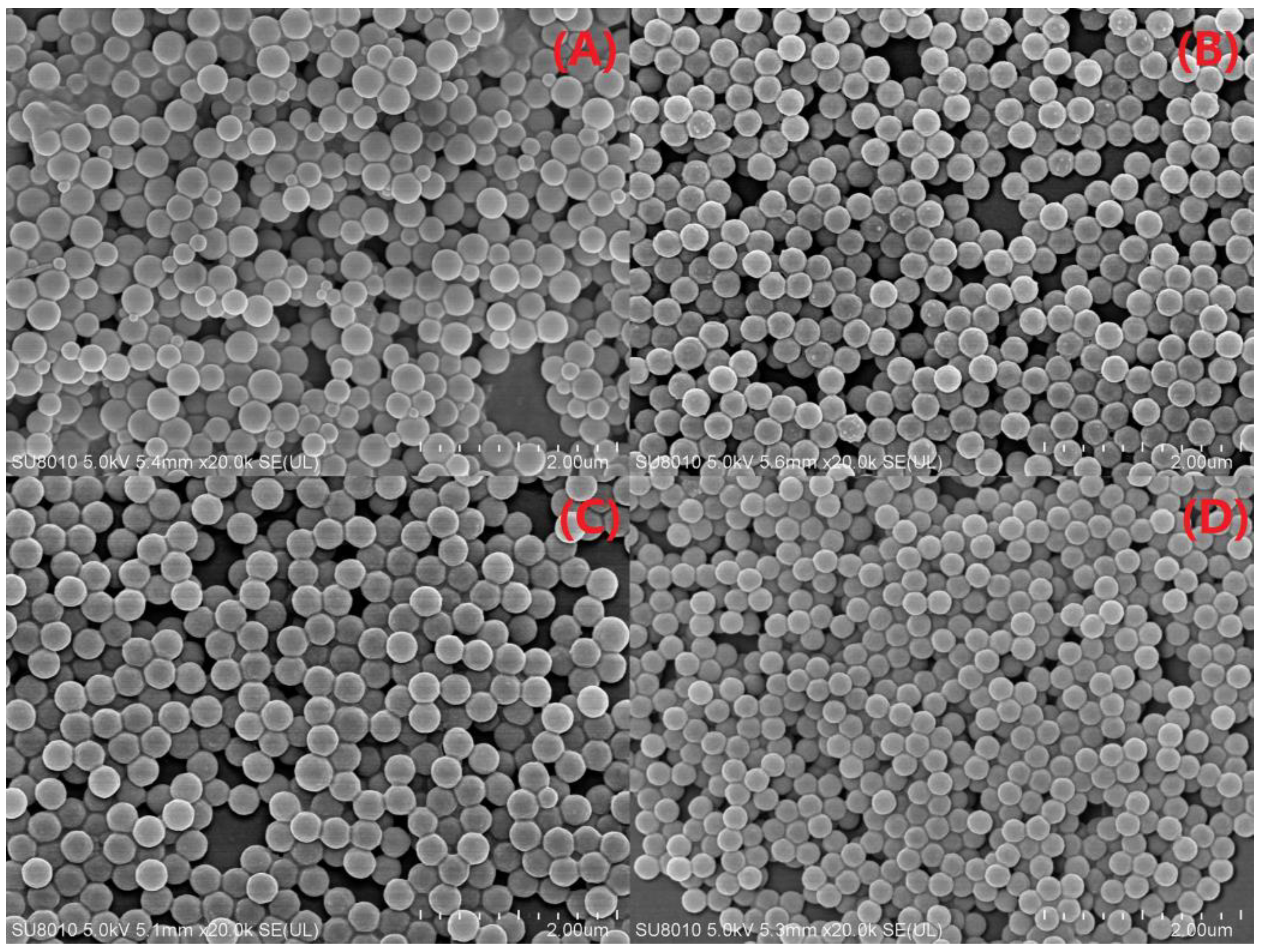
3.2.5. SEM-EDS Analysis
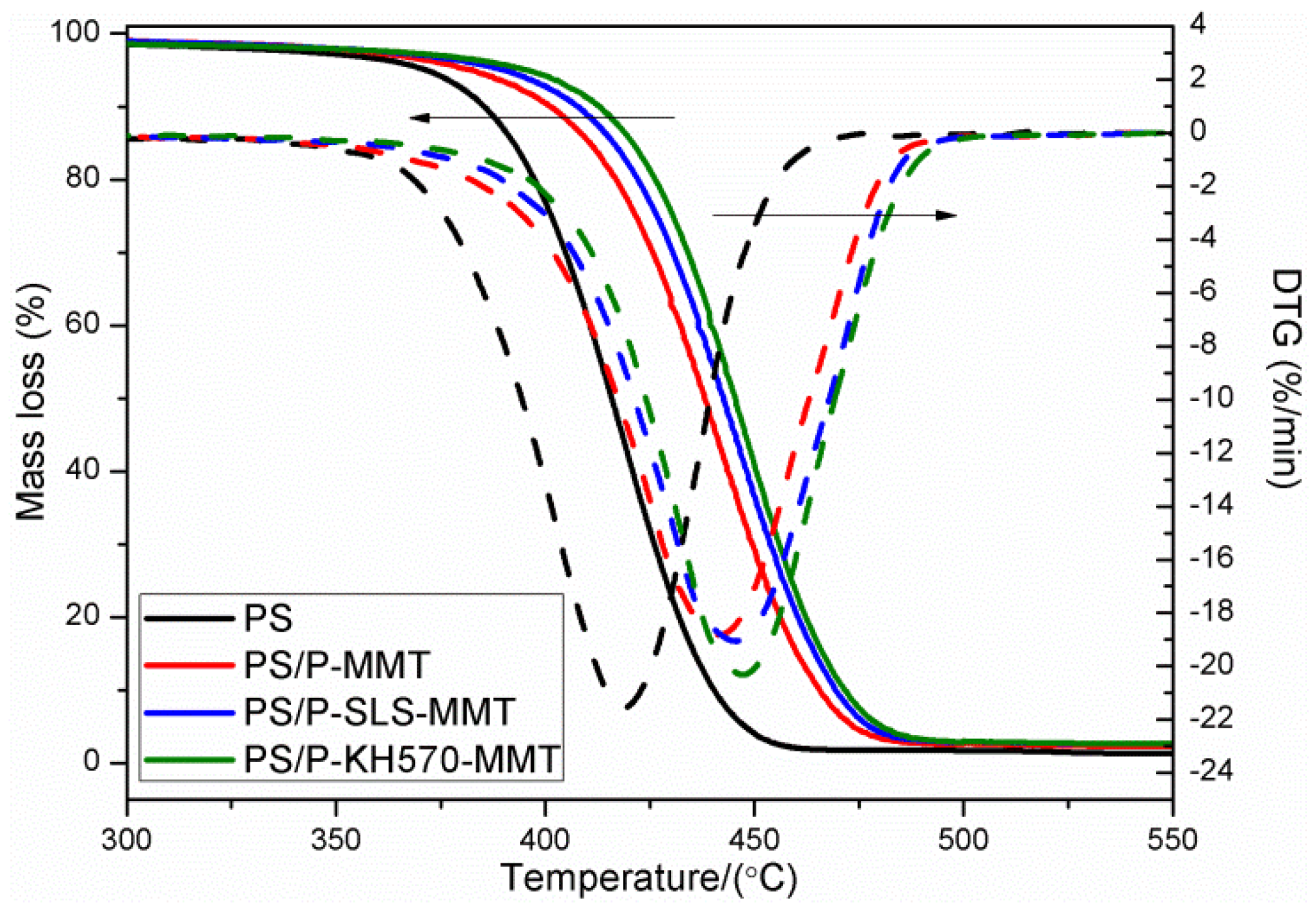
3.2.6. TGA and DTG Analysis
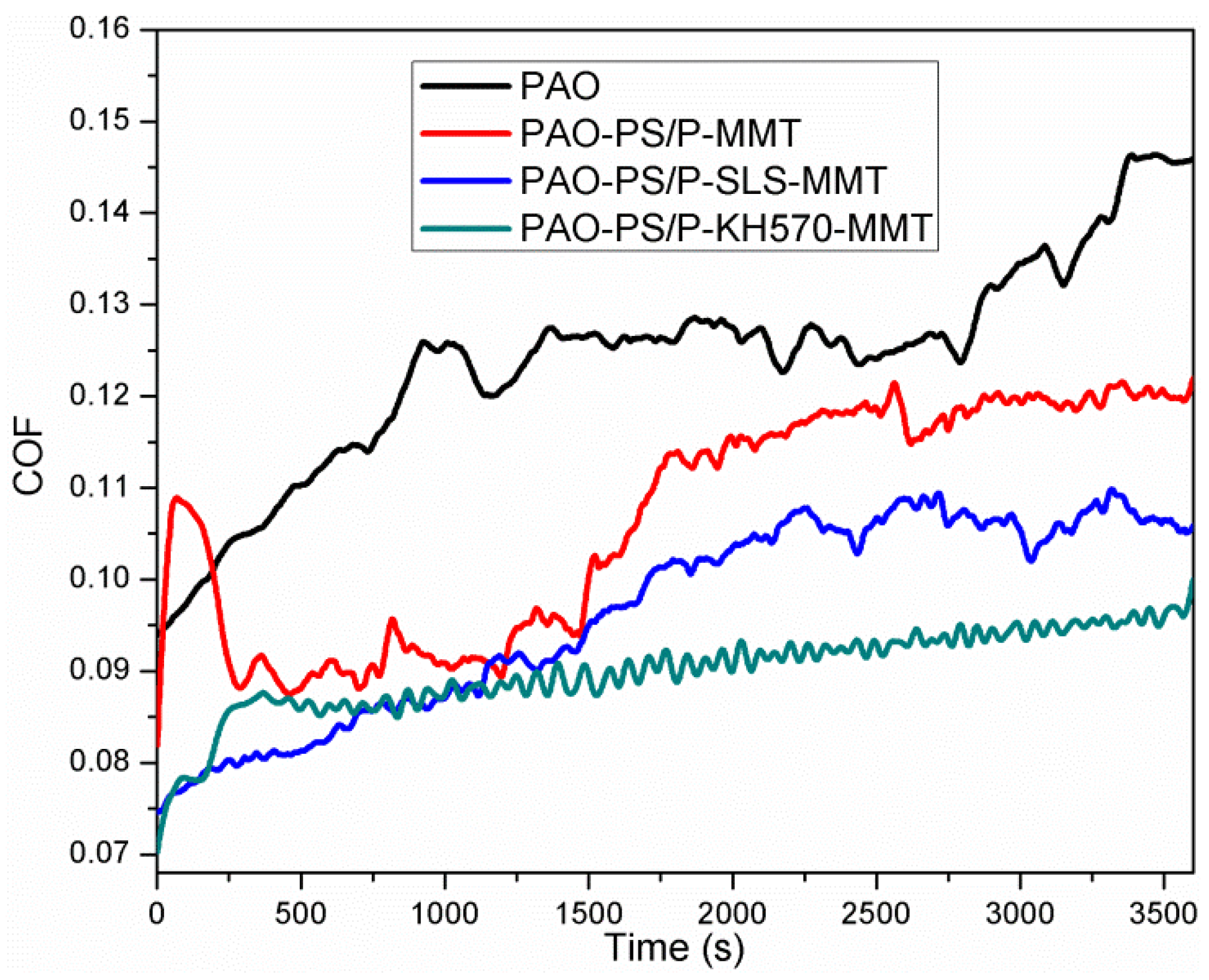
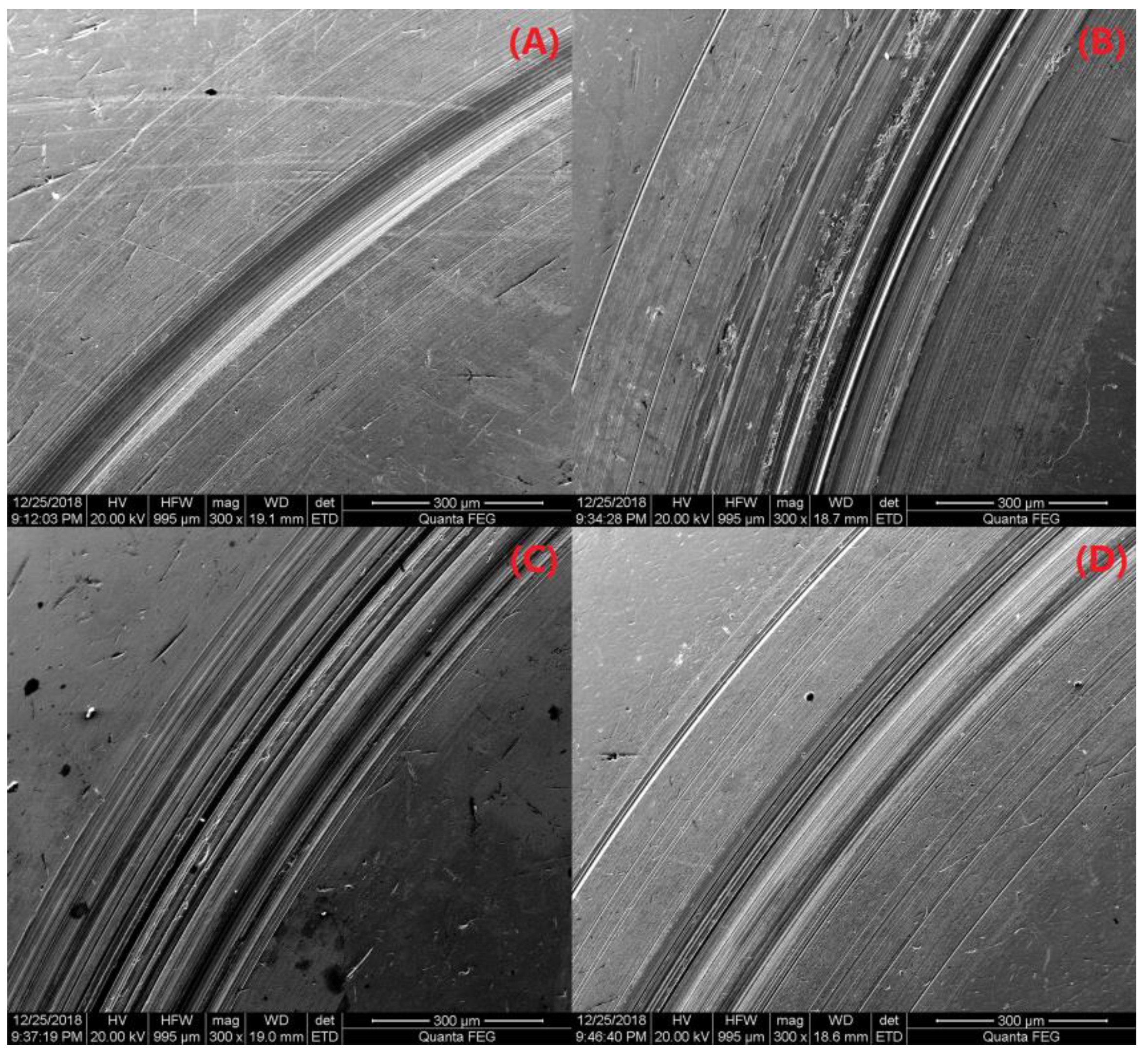
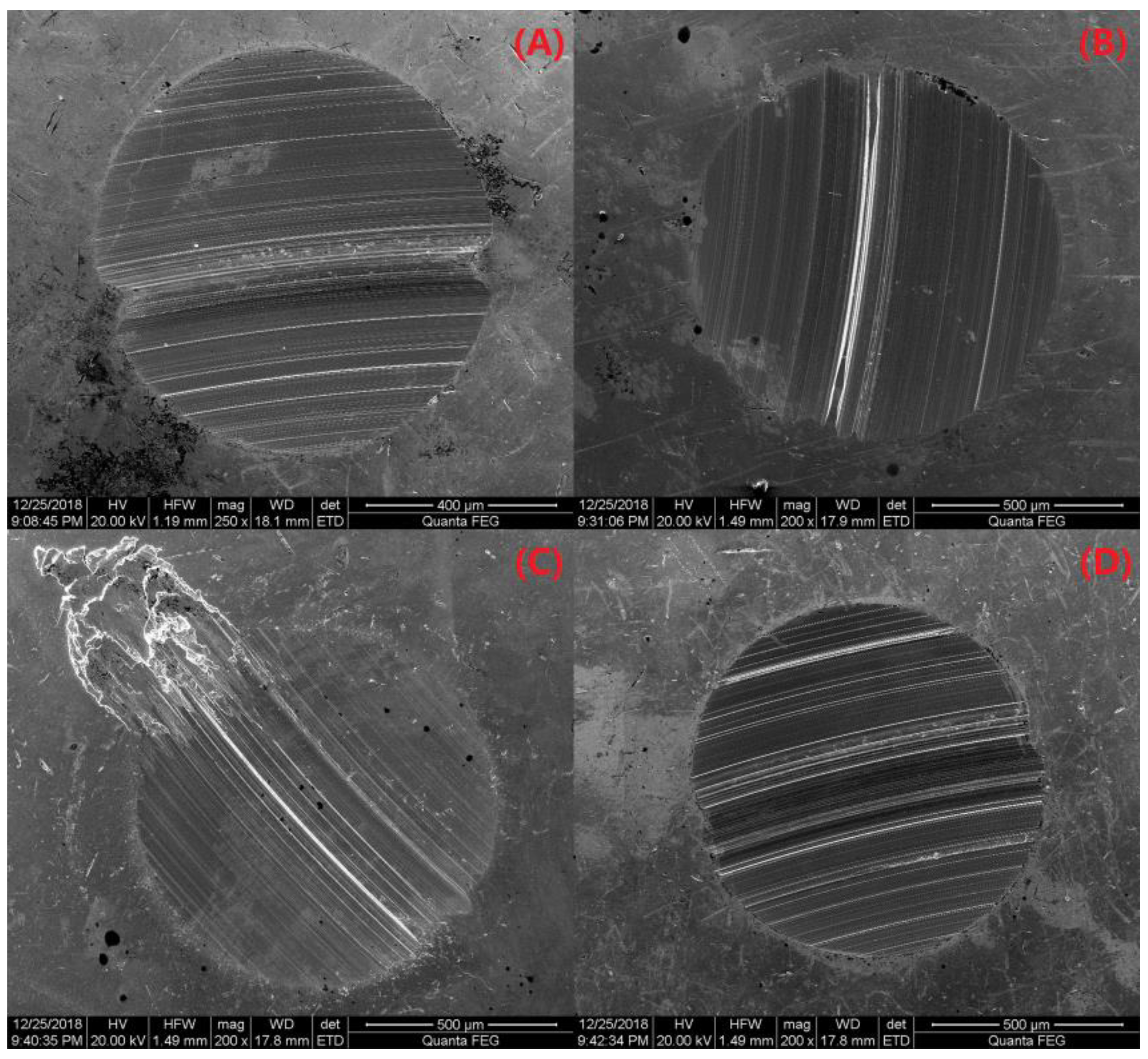
3.2.7. Friction Performance Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kawasumi, M. The discovery of polymer–clay hybrids. J. Polym. Sci. A: Polym. Chem. 2004, 42, 819–824. [Google Scholar] [CrossRef]
- Chafidz, A.; Ali, I.; Mohsin, M.E.A.; Elleithy, R.; Al-Zahrani, S. Nanoindentation and dynamic mechanical properties of PP/clay nanocomposites. J. Polym. Res. 2012, 19, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Tang, S.; Zhou, X.; Gu, S.; Huang, K.; Xu, J.; Wang, X.; Li, Y. Synergistic effect of amino silane functional montmorillonite on intumescent flame-retarded SEBS and its mechanism. J. Appl. Polym. Sci. 2017, 134, 44953. [Google Scholar] [CrossRef]
- Silva, A.A.; Dahmouche, K.; Soares, B.G. Nanostructure and dynamic mechanical properties of silane-functionalized montmorillonite/epoxy nanocomposites. Appl. Clay Sci. 2011, 54, 151–158. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E.; Ersoy, S. The modification of Na-montmorillonite by salts of fatty acids: An easy intercalation process. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 40–49. [Google Scholar] [CrossRef]
- Owusu-Adom, K.; Guymon, C.A. Chemical compatibility and reaction-induced exfoliation in photopolymerizable clay nanocomposites. Macromolecules 2009, 42, 180–187. [Google Scholar] [CrossRef]
- Xidas, P.I.; Triantafyllidis, K.S. Effect of the type of alkylammonium ion clay modifier on the structure and thermal/mechanical properties of glassy and rubbery epoxy–clay nanocomposites. Eur. Polym. J. 2010, 46, 404–417. [Google Scholar] [CrossRef]
- Giannakas, A.; Spanos, C.G.; Kourkoumelis, N.; Vaimakis, T.; Ladavos, A. Preparation, characterization and water barrier properties of PS/organo-montmorillonite nanocomposites. Eur. Polym. J. 2008, 44, 3915–3921. [Google Scholar] [CrossRef]
- Ezquerro, C.S.; Ric, G.I.; Miñana, C.C.; Bermejo, J.S. Characterization of montmorillonites modified with organic divalent phosphonium cations. Appl. Clay Sci. 2015, 111, 1–9. [Google Scholar] [CrossRef]
- Mittal, V. Modification of montmorillonites with thermally stable phosphonium cations and comparison with alkylammonium montmorillonites. Appl. Clay Sci. 2012, 56, 103–109. [Google Scholar] [CrossRef]
- Abdallah, W.; Yilmazer, U. Novel thermally stable organo-montmorillonites from phosphonium and imidazolium surfactants. Thermochim. Acta 2011, 525, 129–140. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Chen, K.; Song, Y.; Wei, Z.; Xue, M. The modification of lanthanum-exchanged montmorillonite with anionic surfactants to enhance the thermal stability of polyvinyl chloride. J. Appl. Polym. Sci. 2015, 132, 41535. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, Z.; Wu, L.; Zhuang, G.; Zhang, S.; Yuan, J.; Liao, L. Investigation on the co-modification process of montmorillonite by anionic and cationic surfactants. Appl. Clay Sci. 2016, 132–133, 694–701. [Google Scholar] [CrossRef]
- Bertuoli, P.T.; Piazza, D.; Scienza, L.C.; Zattera, A.J. Preparation and characterization of montmorillonite modified with 3-aminopropyltriethoxysilane. Appl. Clay Sci. 2014, 87, 46–51. [Google Scholar] [CrossRef]
- Asgari, M.; Sundararaj, U. Silane functionalization of sodium montmorillonite nanoclay: The effect of dispersing media on intercalation and chemical grafting. Appl. Clay Sci. 2018, 153, 228–238. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Pinjari, D.V.; Gogate, P.R.; Sonawane, S.H.; Pandit, A.B. Synthesis of exfoliated poly(styrene-co-methyl methacrylate)/montmorillonite nanocomposite using ultrasound assisted in situ emulsion copolymerization. Chem. Eng. J. 2012, 181–182, 770–778. [Google Scholar] [CrossRef]
- Mirzataheri, M.; Khamisabadi, S.; Salimi, A. Characterization of styrene-co-butyl acrylate/Cloisite Na+ nanocomposite film synthesized via soap free emulsion polymerization. Prog. Org. Coat. 2016, 99, 274–281. [Google Scholar] [CrossRef]
- Bourgeat-Lami, E.; Lansalot, M. Organic/inorganic composite latexes: The marriage of emulsion polymerization and inorganic chemistry. Adv. Polym. Sci. 2010, 233, 53–123. [Google Scholar]
- Yamamoto, T.; Kawaguchi, K.; Takahashi, Y. Particle size control in the soap-free emulsion polymerization of styrene by an oil-soluble initiator with a weakly acidic water-soluble initiator. Colloids Surf. A Physicochem. Eng. Asp. 2016, 502, 1–5. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, X.; Liu, J.; Yan, Y.; Wang, L.; Zeng, Y. An investigation of the effect of sodium dodecyl sulfate on quasi-emulsifier-free emulsion polymerization for highly monodisperse polystyrene nanospheres. Eur. Polym. J. 2011, 47, 24–30. [Google Scholar]
- Hojiyev, R.; Ulcay, Y.; Celik, M.S.; Carty, W.M. Effect of CEC coverage of hexadecyltributylphosphonium modified montmorillonite on polymer compatibility. Appl. Clay Sci. 2017, 141, 204–211. [Google Scholar] [CrossRef]
- Sun, J.; Zhuang, G.; Wu, S.; Zhang, Z. Structure and performance of anionic-cationic-organo-montmorillonite in different organic solvents. RSC Adv. 2016, 6, 54747–54753. [Google Scholar] [CrossRef]
- Alvi, M.U.; Zulfiqar, S.; Yavuz, C.T.; Kweon, H.; Sarwar, M.I. Influence of aminosilane coupling agent on aromatic polyamide/intercalated clay nanocomposites. Ind. Eng. Chem. Res. 2013, 52, 6908–6915. [Google Scholar] [CrossRef]
- Cao, X.; Pan, G.; Huang, P.; Guo, D.; Xie, G. Silica-coated core-shell structured polystyrene nanospheres and their size-dependent mechanical properties. Langmuir 2017, 33, 8225–8232. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H.; Hong, X.; Wang, M.; Cai, C.; Zhang, Q. Polystyrene/MMT nanocomposites prepared by soap-free emulsion polymerization with high solids content. Colloid Polym. Sci. 2012, 290, 1955–1963. [Google Scholar] [CrossRef]
- Angaji, M.T.; Rafiee, R.; Hemmati, M.; Abdollahi, M.; Razavi Aghjeh, M.K. Parametric studies on the grafting of poly (methyl methacrylate) onto organophilic montmorillonite using silylated clay platelets. J. Macromol. Sci. Part B 2014, 53, 957–974. [Google Scholar] [CrossRef]
- Park, S.; Kim, B.; Seo, D.; Rhee, K.; Lyu, Y. Effects of a silane treatment on the mechanical interfacial properties of montmorillonite/epoxy nanocomposites. Mater. Sci. Eng. A 2009, 526, 74–78. [Google Scholar] [CrossRef]
- Benbayer, C.; Saidi-Besbes, S.; Taffin De Givenchy, E.; Amigoni, S.; Guittard, F.; Derdour, A. Synergistic effect of organoclay fillers based on fluorinated surfmers for preparation of polystyrene nanocomposites. J. Appl. Polym. Sci. 2015, 132, 1–12. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Liao, L.; Xia, Z. Synergistic effect of cationic and anionic surfactants for the modification of Ca-montmorillonite. Mater. Res. Bull. 2013, 48, 1811–1816. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Z.; Wang, Y.; Liao, L.; Zhang, J. Influence of montmorillonites exchange capacity on the basal spacing of cation–anion organo-montmorillonites. Mater. Res. Bull. 2014, 59, 59–64. [Google Scholar]
- Silva, T.F.; Soares, B.G.; Ferreira, S.C.; Livi, S. Silylated montmorillonite as nanofillers for plasticized PVC nanocomposites: Effect of the plasticizer. Appl. Clay Sci. 2014, 99, 93–99. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Z.; Pan, W.P.; Hunter, D.; Singh, A.; Vaia, R. Thermal degradation chemistry of alkyl quaternary ammonium montmorillonite. Chem. Mater. 2001, 13, 2979–2990. [Google Scholar] [CrossRef]
- Romanzini, D.; Piroli, V.; Frache, A.; Zattera, A.J.; Amico, S.C. Sodium montmorillonite modified with methacryloxy and vinylsilanes: Influence of silylation on the morphology of clay/unsaturated polyester nanocomposites. Appl. Clay Sci. 2015, 114, 550–557. [Google Scholar] [CrossRef]
- Yu, C.; Ke, Y.; Deng, Q.; Lu, S.; Ji, J.; Hu, X.; Zhao, Y. Synthesis and characterization of polystyrene-montmorillonite nanocomposite particles using an anionic-surfactant-modified clay and their friction performance. Appl. Sci. 2018, 8, 964. [Google Scholar] [CrossRef]
- Romanzini, D.; Frache, A.; Zattera, A.J.; Amico, S.C. Effect of clay silylation on curing and mechanical and thermal properties of unsaturated polyester/montmorillonite nanocomposites. J. Phys. Chem. Solids 2015, 87, 9–15. [Google Scholar] [CrossRef]
- Zhang, J.; Gupta, R.K.; Wilkie, C.A. Controlled silylation of montmorillonite and its polyethylene nanocomposites. Polymer 2006, 47, 4537–4543. [Google Scholar] [CrossRef]
- Bourgeat-Lami, E.; Guimara, T.R.; Pereira, A.M.C.; Alves, G.M.; Moreira, J.C.; Putaux, J.; Santos, A.M. High solids content, soap-free, film-forming latexes stabilized by laponite clay platelets. Macromol. Rapid Comm. 2010, 31, 1874–1880. [Google Scholar] [CrossRef]
- Ianchis, R.; Donescu, D.; Petcu, C.; Ghiurea, M.; Anghel, D.F.; Stanga, G.; Marcu, A. Surfactant-free emulsion polymerization of styrene in the presence of silylated montmorillonite. Appl. Clay Sci. 2009, 45, 164–170. [Google Scholar] [CrossRef]
- Ianchis, R.; Corobea, M.C.; Donescu, D.; Rosca, I.D.; Cinteza, L.O.; Nistor, L.C.; Vasile, E.; Marin, A.; Preda, S. Advanced functionalization of organoclay nanoparticles by silylation and their polystyrene nanocomposites obtained by miniemulsion polymerization. J. Nanopart. Res. 2012, 14, 1233. [Google Scholar] [CrossRef]
- Bee, S.L.; Abdullah, M.A.A.; Mamat, M.; Bee, S.T.; Sin, L.T.; Hui, D.; Rahmat, A.R. Characterization of silylated modified clay nanoparticles and its functionality in PMMA. Compos. Part B: Eng. 2017, 110, 83–95. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Chen, Z.; Ma, P. Effect of doubly organo-modified vermiculite on the properties of vermiculite/polystyrene nanocomposites. Appl. Clay Sci. 2013, 75–76, 74–81. [Google Scholar] [CrossRef]
- Chen, S.; Lu, X.; Zhang, Z.; Wang, T.; Pan, F. Preparation and Characterization of Poly(methyl methacrylate)/Reactive Montmorillonite Nanocomposites. Polym. Composite 2016, 37, 2396–2403. [Google Scholar] [CrossRef]
- Sui, T.; Song, B.; Zhang, F.; Yang, Q. Effects of functional groups on the tribological properties of hairy silica nanoparticles as an additive to polyalphaolefin. RSC Adv. 2016, 6, 393–402. [Google Scholar] [CrossRef]
- Sui, T.; Song, B.; Wen, Y.; Zhang, F. Bifunctional hairy silica nanoparticles as high-performance additives for lubricant. Sci. Rep. 2016, 6, 22696. [Google Scholar] [CrossRef] [PubMed]
| Sample | Temperature Range | ||
|---|---|---|---|
| <200 ± 1.5 °C | 200–600 ± 1.9 °C | 30–800 ± 2.2 °C | |
| MMT | 1.9 | 1.5 | 5.6 |
| P-MMT | 0.6 | 19.0 | 20.7 |
| P-SLS-MMT | 0.2 | 23.9 | 24.9 |
| P-KH570-MMT | 2.3 | 19.9 | 23.3 |
| Sample | Z-Average (nm) | PDI |
|---|---|---|
| PS | 378.6 ± 8.9 | 0.232 |
| PS/P-MMT | 290.4 ± 3.8 | 0.159 |
| PS/P-SLS-MMT | 338.8 ± 3.6 | 0.160 |
| PS/P-KH570-MMT | 241.3 ± 2.1 | 0.074 |
| Sample | C (wt %) | O (wt %) | Al (wt %) | Si (wt %) |
|---|---|---|---|---|
| PS | 68.44 ± 0.46 | 30.33 ± 0.36 | 0.70 ± 0.09 | 0.53 ± 0.07 |
| PS/P-MMT | 63.62 ± 0.24 | 31.45 ± 0.40 | 3.52 ± 0.17 | 1.41 ± 0.12 |
| PS/P-SLS-MMT | 64.15 ± 0.30 | 31.36 ± 0.38 | 3.05 ± 0.19 | 1.44 ± 0.13 |
| PS/P-KH570-MMT | 67.92 ± 0.33 | 28.61 ± 0.29 | 2.33 ± 0.11 | 1.14 ± 0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Ke, Y.; Hu, X.; Zhao, Y.; Deng, Q.; Lu, S. Effect of Bifunctional Montmorillonite on the Thermal and Tribological Properties of Polystyrene/Montmorillonite Nanocomposites. Polymers 2019, 11, 834. https://doi.org/10.3390/polym11050834
Yu C, Ke Y, Hu X, Zhao Y, Deng Q, Lu S. Effect of Bifunctional Montmorillonite on the Thermal and Tribological Properties of Polystyrene/Montmorillonite Nanocomposites. Polymers. 2019; 11(5):834. https://doi.org/10.3390/polym11050834
Chicago/Turabian StyleYu, Chengcheng, Yangchuan Ke, Xu Hu, Yi Zhao, Qingchun Deng, and Shichao Lu. 2019. "Effect of Bifunctional Montmorillonite on the Thermal and Tribological Properties of Polystyrene/Montmorillonite Nanocomposites" Polymers 11, no. 5: 834. https://doi.org/10.3390/polym11050834
APA StyleYu, C., Ke, Y., Hu, X., Zhao, Y., Deng, Q., & Lu, S. (2019). Effect of Bifunctional Montmorillonite on the Thermal and Tribological Properties of Polystyrene/Montmorillonite Nanocomposites. Polymers, 11(5), 834. https://doi.org/10.3390/polym11050834