Preparation, Structure and Properties of Acid Aqueous Solution Plasticized Thermoplastic Chitosan
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
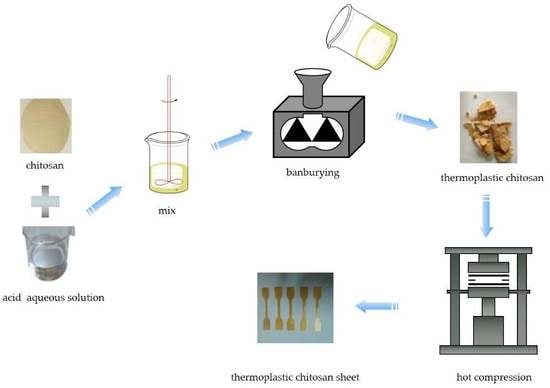
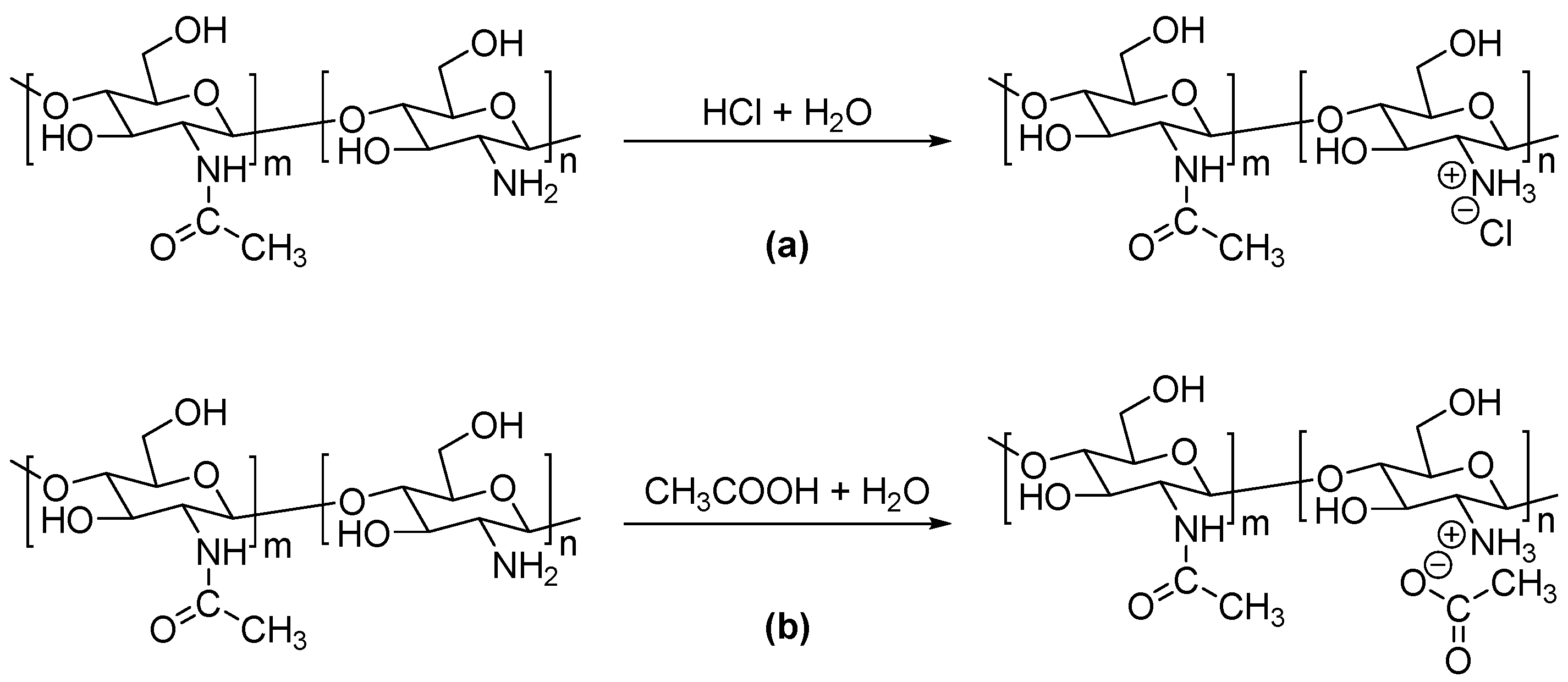
2.2.1. Preparation of Modified Chitosan Materials
2.2.2. Preparation of a Thermoplastic Chitosan Film
2.3. Characterizations
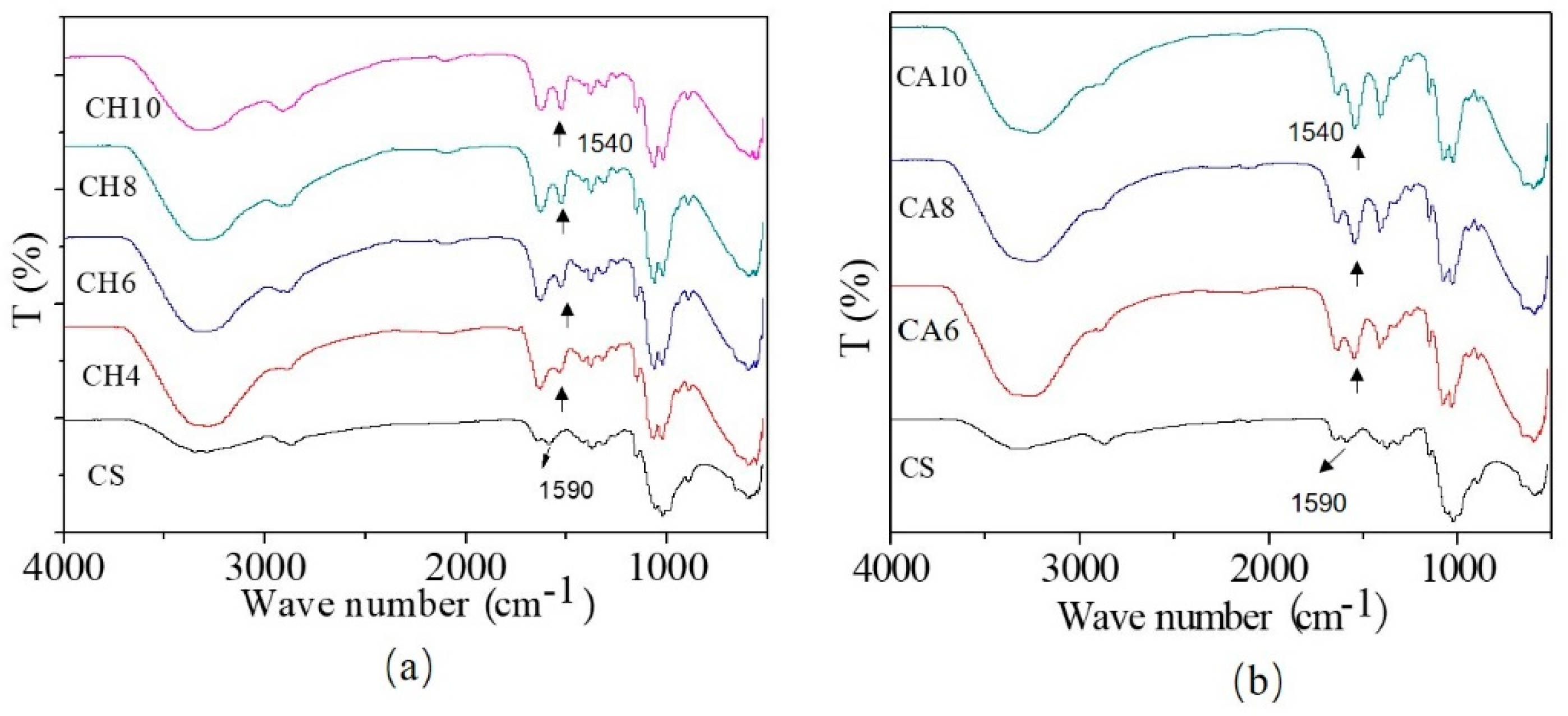
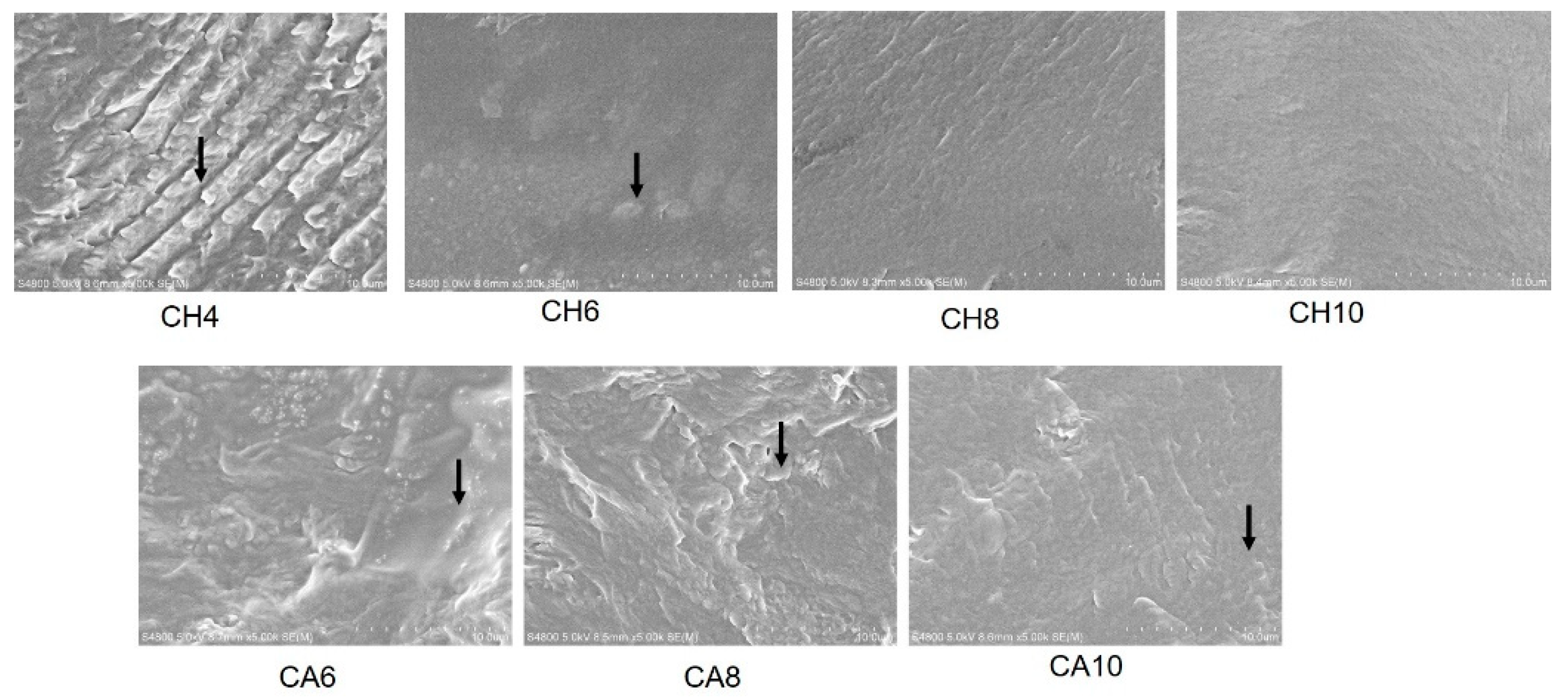
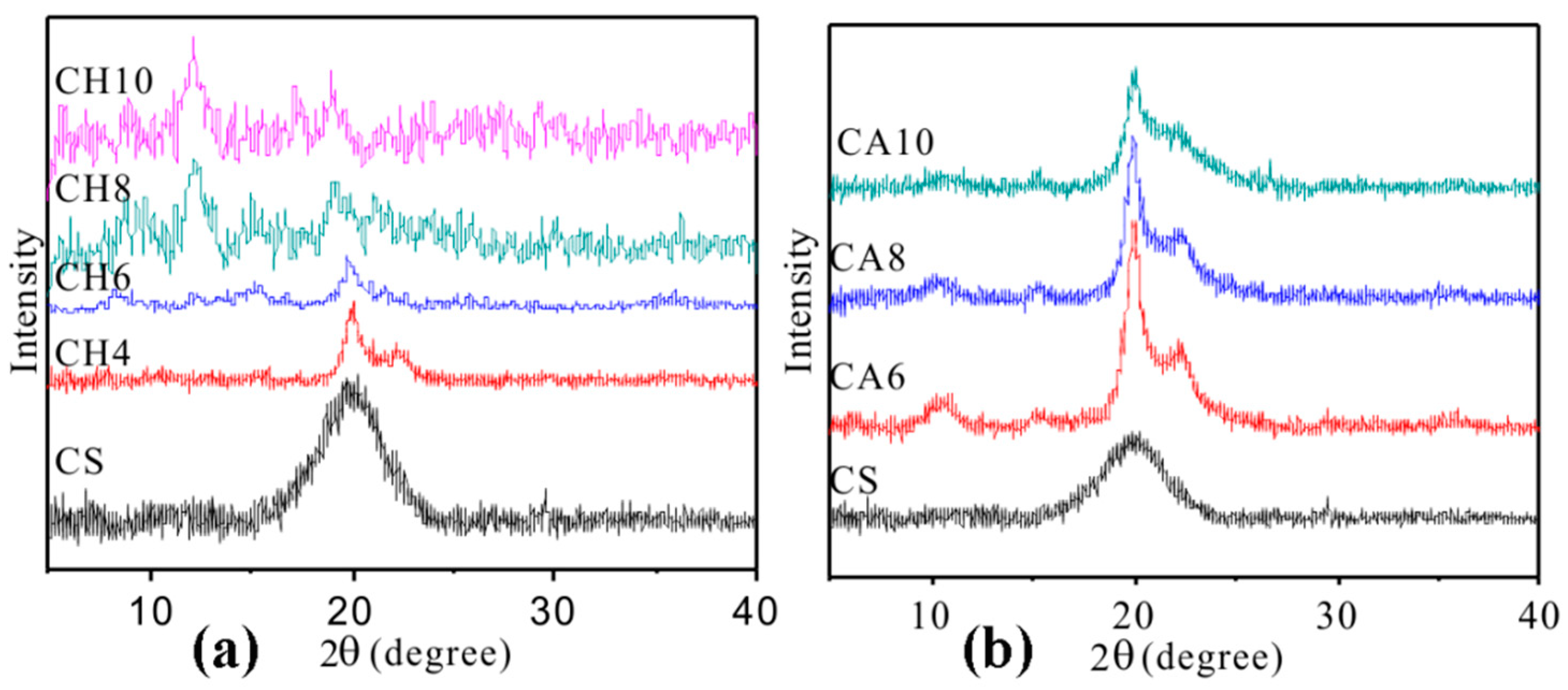
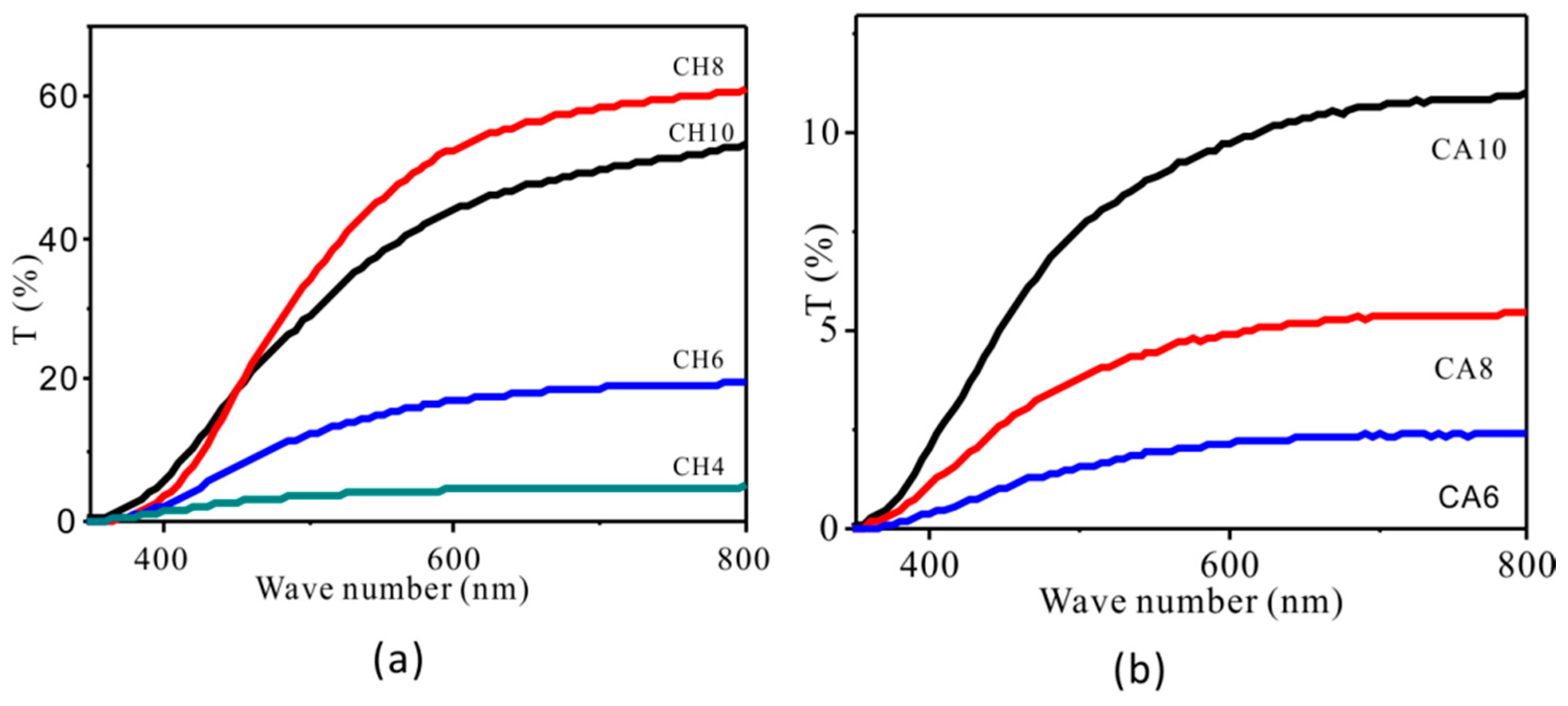
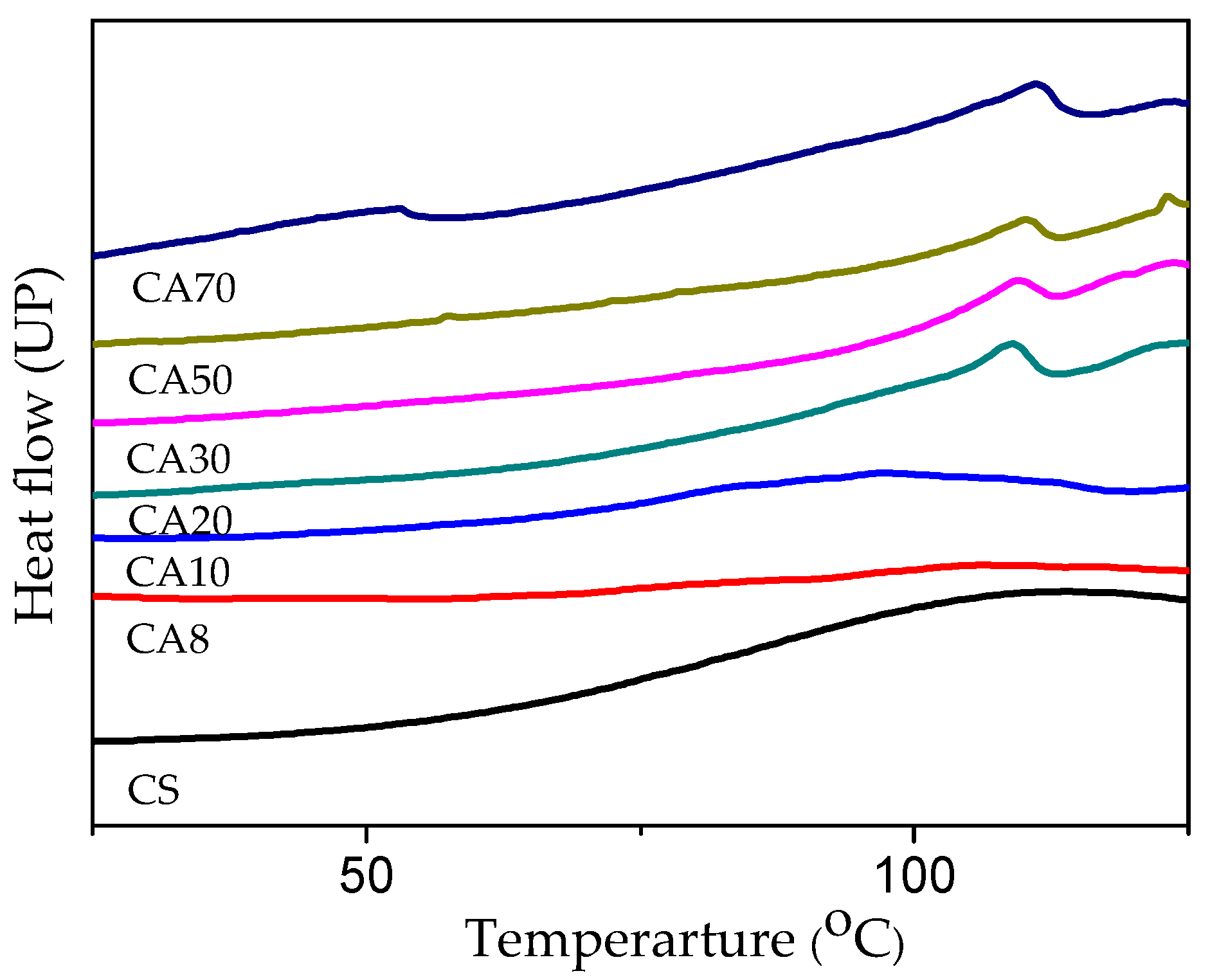
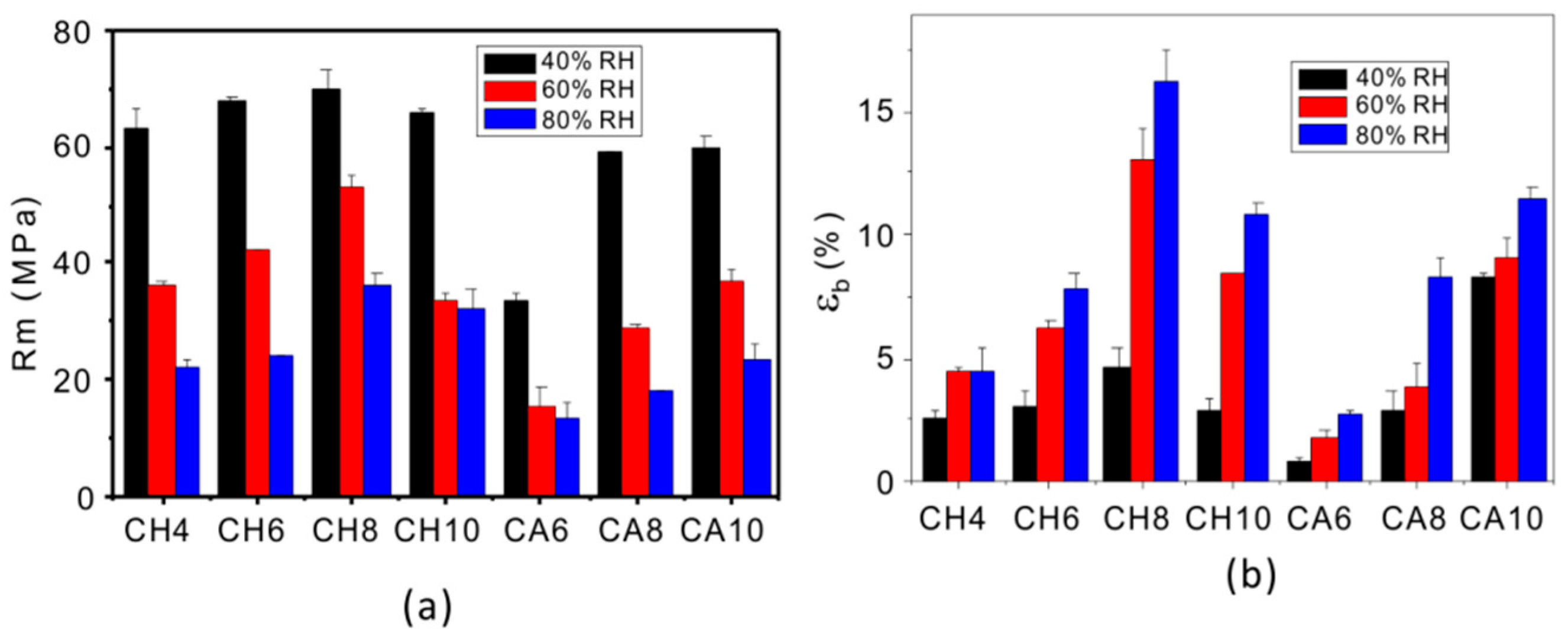
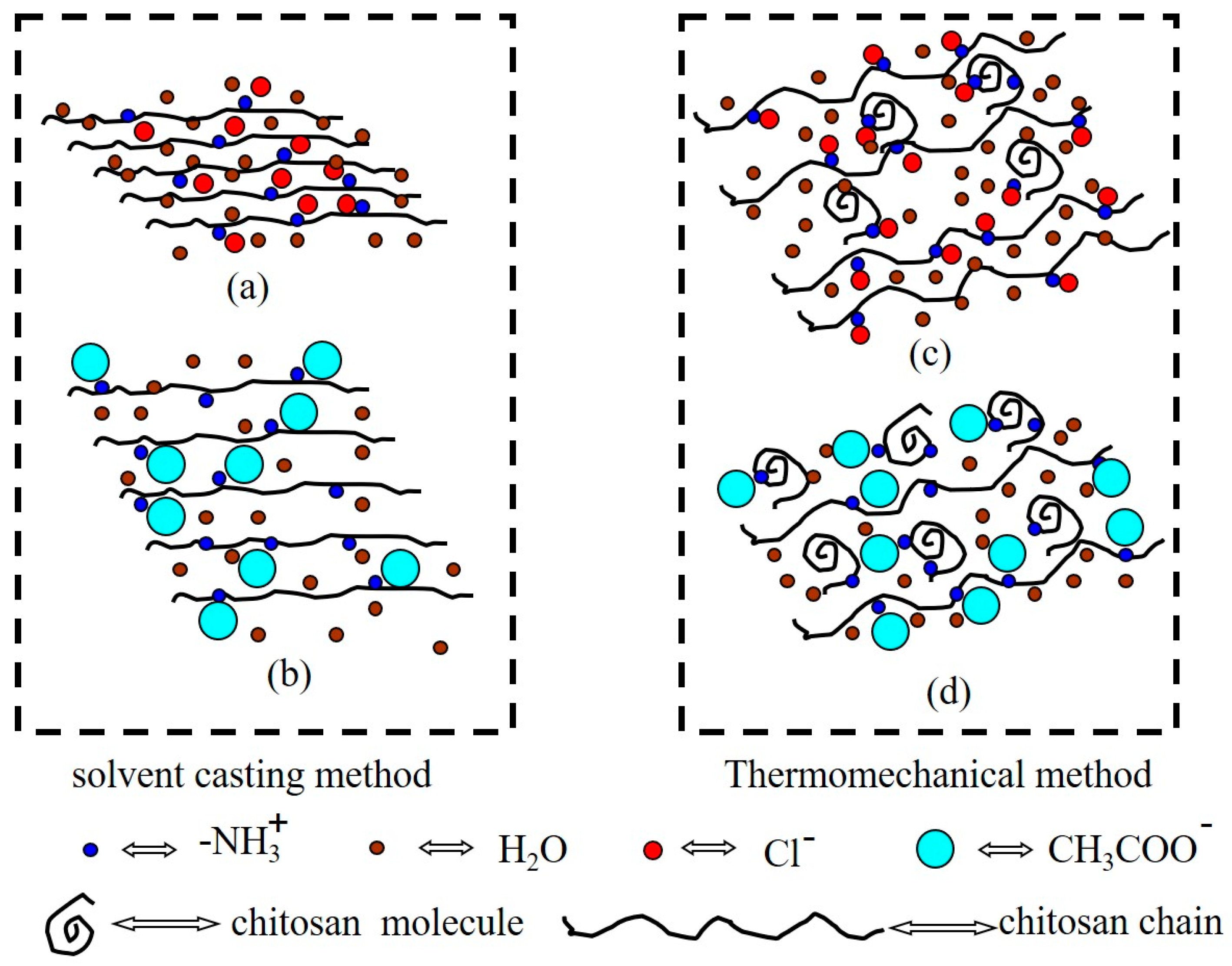
3. Results
Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, X.C.; Zhao, Y.L.; Hou, L.X. The effect of glycerol on properties of chitosan/poly (vinyl alcohol) films with AlCl3•6H2O aqueous solution as the solvent for chitosan. Carbohydr. Polym. 2016, 135, 191–198. [Google Scholar] [CrossRef]
- Huda, E.A.; Seham, Y.H.; Galila, A.Y.; Mohamed, A.M.; Mohamed, M.E.S. Synthesis and Biological Evaluation of New Imine- and Amino-Chitosan Derivatives. Polymers 2015, 7, 2690–2700. [Google Scholar]
- Xiao, Y.; Gong, T.; Jiang, Y.; Wang, Y.P.; Wen, Z.Z.; Zhou, S.B.; Bao, C.Y.; Xu, X.M. Fabrication and Characterization of a Glucose-sensitive Antibacterial Chitosan-Polyethylene Oxide Hydrogel. Polymer 2016, 82, 1–10. [Google Scholar] [CrossRef]
- Chiu, C.W.; Wu, M.T.; Lee, J.C.M.; Cheng, T.Y. Isothermal adsorption properties for the adsorption and removal of reactive blue 221 dye from aqueous solutions by cross-linked -chitosan glycan as acid-resistant adsorbent. Polymers 2016, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Tamer, M.T.; Mohamed, A.H.; Ahmed, M.O.; Katarína, V.; Mohamed, S.; Mohy, E.; Maurice, N.C.; Ladislav, S. Antibacterial and antioxidative activity of O-amine functionalized chitosan. Carbohydr. Polym. 2017, 135, 191–198. [Google Scholar] [CrossRef]
- Marie, M.; Marie, C.H.; Abdellah, A.; Pierre, S. Plasticized chitosan/polyolefin films produced by extrusion. Carbohydr. Polym. 2015, 117, 177–184. [Google Scholar]
- Maurice, N.C.; Katarina, V.; Mohamed, A.H.; Ahmed, M.O.; Mohamed, S.M.; Karol, Š.; Rastislav, J.; L’ubomír, O.; Csaba, B.; Ahmad, B.A.; et al. MitoQ loaded chitosan-hyaluronan composite membranes for wound healing. Materials 2018, 11, 569. [Google Scholar]
- Van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef]
- Sougata, J.; Nirmal, M.; Amit, K.N.; Kalyan, K.S.; Sanat, K.B. Development of chitosan-based nanoparticles through inter-polymeric complexation for oral drug delivery. Carbohydr. Polym. 2013, 98, 870–876. [Google Scholar]
- Iosody, S.C.; Pablom, M.R.; Petruta, M.M.; Marciabela, F.C.; Salvador, H.N.; Jesús, M.G. Eco-friendly nanocomposites of chitosan with natural extracts, antimicrobial agents, and nanometals. Handb. Compos. Renew. Mater. 2017, 8, 35–60. [Google Scholar]
- Monika, Y.; Priynshi, G.; Kunwar, P.; Manish, K.; Nidhi, P.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8–28. [Google Scholar]
- Muhammad, M.; Rania, E.M.; Garry, K.; Maher, Z.E.; Murat, K.; Jalel, L.; Khalid, M.K. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2018, 121, 889–904. [Google Scholar]
- Avérous, L. Biodegradable multiphase systems based on plasticized starch: A review. J. Macromol. Sci. Part C 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Wang, H.Y.; Huang, M.F. Preparation, characterization and performances of biodegradable thermoplastic starch. Polym. Adv. Technol. 2007, 18, 910–915. [Google Scholar] [CrossRef]
- Gao, C.C.; Pollet, E.; Avérous, L. Innovative plasticized alginate obtained by thermo-mechanical mixing: Effect of different biobased polyols systems. Carbohydr. Polym. 2017, 157, 669–676. [Google Scholar] [CrossRef]
- Olivier, M.; Jean, F.B.; Isabelle, D. Chitosan: An upgraded polysaccharide waste for organocatalysis. Eur. J. Org. Chem. 2015, 32, 2559–2578. [Google Scholar]
- Khanh, M.D.; Rangrong, Y. Development of thermoplastic starch blown film by incorporating plasticized chitosan. Carbohydr. Polym. 2015, 115, 575–581. [Google Scholar]
- Epure, V.; Griffon, M.; Pollet, E.; Avérous, L. Structure and properties of glycerol-plasticized chitosan obtained by mechanical kneading. Carbohydr. Polym. 2011, 83, 947–952. [Google Scholar] [CrossRef]
- Cano, L.; Pollet, E.; Avérous, L.; Agnieszka, T. Thermoplastic chitosan-based nano-biocomposites obtained by mechanical kneading. Compos. Part A 2017, 93, 33–40. [Google Scholar] [CrossRef]
- Mendes, J.F.; Paschoalin, R.T.; Carmona, V.B.; Sena, N.; Alfredo, R.; Marques, A.C.P.; Marconcini, J.M.; Mattoso, L.H.C.; Medeiros, E.S.; Oliveira, J.E. Biodegradable polymer blends based on cornstarch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 13, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Heuzey, M.C.; Carreau, P.J. Hierarchical structure and physicochemical properties of plasticized chitosan. Biomacromolecules 2014, 15, 1216–1224. [Google Scholar] [CrossRef]
- Andrea, C.G.S.; Maria, C.R.C.; Krzysztof, B.; Maria, P.G.; Hiléia, K.S.S. Natural deep eutectic solvents as green plasticizers for chitosan thermoplastic production with controlled/desired mechanical and barrier properties. Food Hydrocoll. 2018, 82, 478–489. [Google Scholar]
- Li, J.L.; Liu, D.G.; Hu, C.M.; Sun, F.X.; Gustave, W.; Tian, H.F.; Yang, S.J. Flexible fibers wet-spun from formic acid modified chitosan. Carbohydr. Polym. 2016, 136, 1137–1143. [Google Scholar] [CrossRef]
- Marguerite, R. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar]
- Kittur, F.S.; Vishu, K.A.B.; Tharanathan, R.N. Low molecular weight chitosans–preparation by depolymerization with Aspergillus niger pectinase, and characterization. Carbohydr. Res. 2003, 338, 1283–1290. [Google Scholar] [CrossRef]
- Okuyama, K.; Noguchi, K.; Miyazawa, T.; Yui, T.; Ogawa, K. Molecular and crystal structure of hydrated chitosan. Macromolecules 1997, 30, 5849–5855. [Google Scholar] [CrossRef]
- Carmína, G.; Betty, L.L.; Ligia, S.; Robert, G.; Hans, W.S.; Marianne, G. Interplay between structure and dynamics in chitosan films. Investigated with solid-state NMR, dynamic mechanical analysis, and X-ray diffraction. Biomacromolecules 2011, 12, 1380–1386. [Google Scholar]
| Sample Name | Chitosan (g) | 36 wt % HCl (g) | HAc (g) | Water (g) | Mass Fraction of Acid (%) |
|---|---|---|---|---|---|
| CH4 | 30 | 5 | -- | 40 | 4 |
| CH6 | 30 | 7.5 | -- | 37.5 | 6 |
| CH8 | 30 | 10 | -- | 35 | 8 |
| CH10 | 30 | 12.5 | -- | 32.5 | 10 |
| CA6 | 30 | -- | 2.7 | 42.3 | 6 |
| CA8 | 30 | -- | 3.6 | 41.4 | 8 |
| CA10 | 30 | -- | 4.5 | 40.5 | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, B.-L.; Wang, L.-J.; Deng, Y.-H.; Zhou, S.-Y.; Feng, J.-W. Preparation, Structure and Properties of Acid Aqueous Solution Plasticized Thermoplastic Chitosan. Polymers 2019, 11, 818. https://doi.org/10.3390/polym11050818
Zhang Y, Liu B-L, Wang L-J, Deng Y-H, Zhou S-Y, Feng J-W. Preparation, Structure and Properties of Acid Aqueous Solution Plasticized Thermoplastic Chitosan. Polymers. 2019; 11(5):818. https://doi.org/10.3390/polym11050818
Chicago/Turabian StyleZhang, Yu, Biao-Lan Liu, Liang-Jie Wang, Ying-Hua Deng, Shi-Yi Zhou, and Ji-Wen Feng. 2019. "Preparation, Structure and Properties of Acid Aqueous Solution Plasticized Thermoplastic Chitosan" Polymers 11, no. 5: 818. https://doi.org/10.3390/polym11050818
APA StyleZhang, Y., Liu, B.-L., Wang, L.-J., Deng, Y.-H., Zhou, S.-Y., & Feng, J.-W. (2019). Preparation, Structure and Properties of Acid Aqueous Solution Plasticized Thermoplastic Chitosan. Polymers, 11(5), 818. https://doi.org/10.3390/polym11050818