Three New Supramolecular Coordination Polymers Based on 1H-pyrazolo[3,4-b]pyridin-3-amine and 1,3-benzenedicarboxylate Derivatives
Abstract
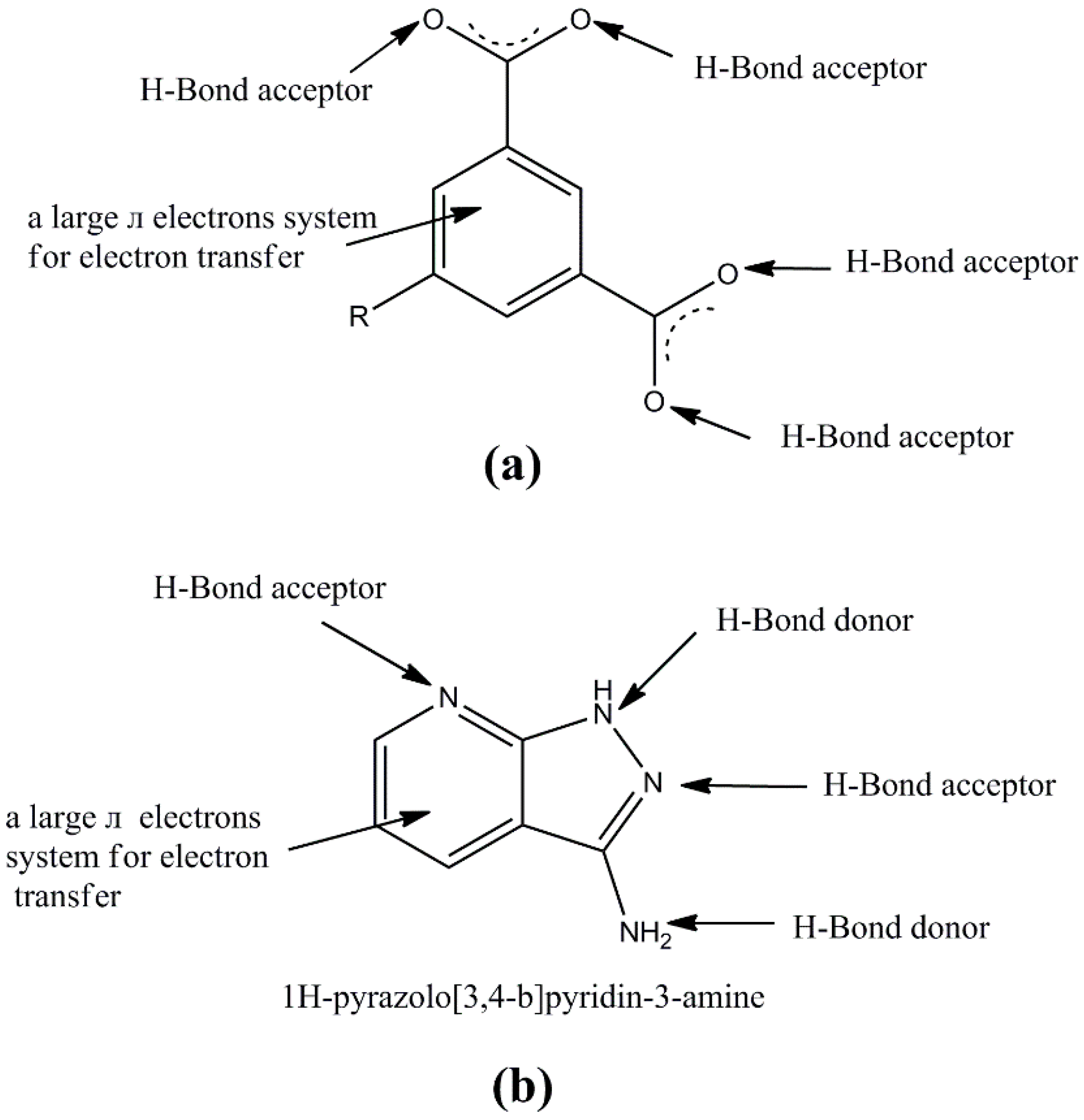
1. Introduction
2. Experimental
2.1. Materials and Instruments
2.2. Preparation
2.2.1. Synthesis of [Zn(1,3-BDC)(HL)]n (Polymer 1)
2.2.2. Synthesis of [Zn3(1,3,5-BTC)2(HL)2(H2O)2]n (Polymer 2)
2.2.3. Synthesis of [Zn9(5-SO3-1,3-BDC)2(L)8(OH)4]n (Polymer 3)
2.3. X-ray Crystallographic Determinations
3. Results and Discussion
3.1. Description of the Structures
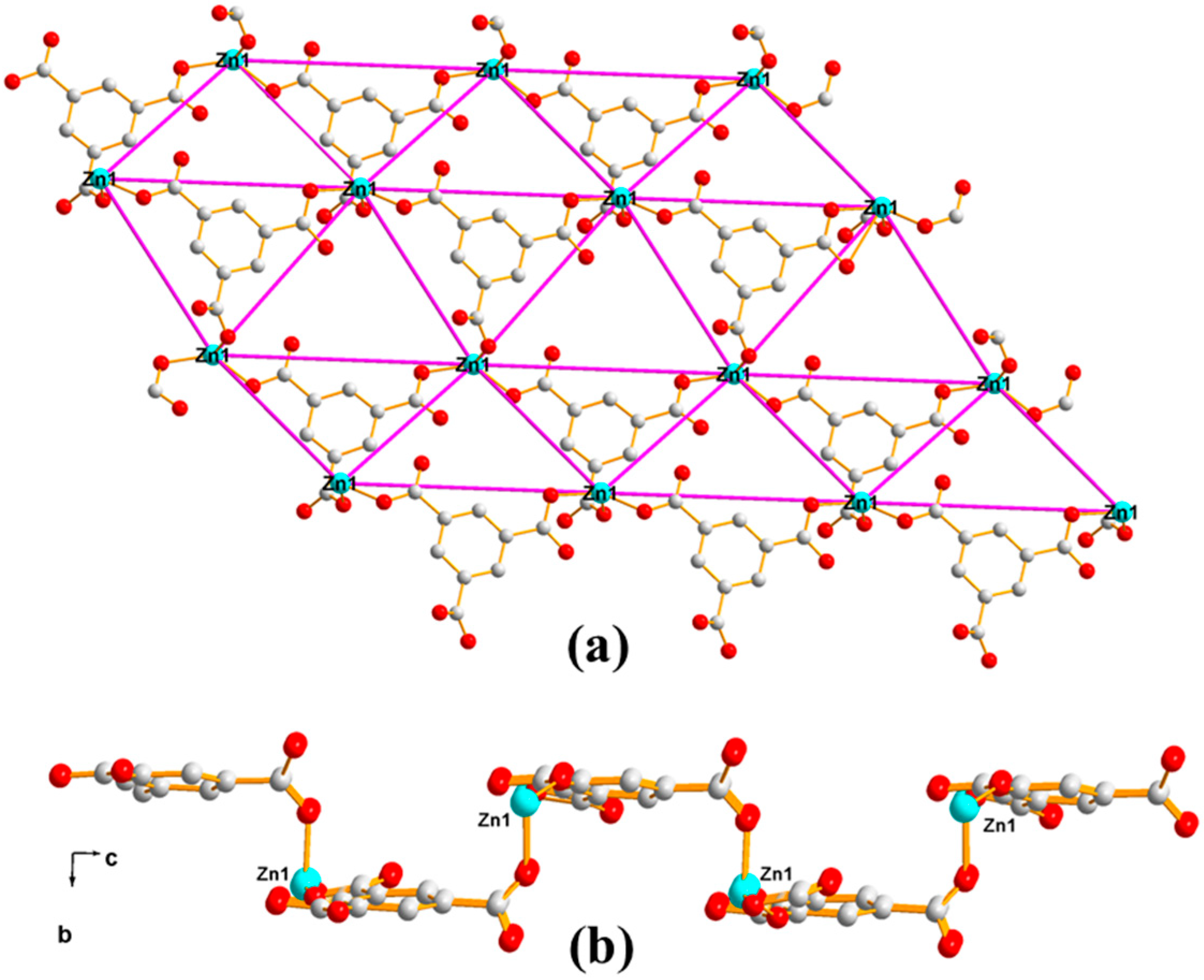
3.1.1. Structural Description of [Zn(1,3-BDC)(HL)]n (Polymer 1)
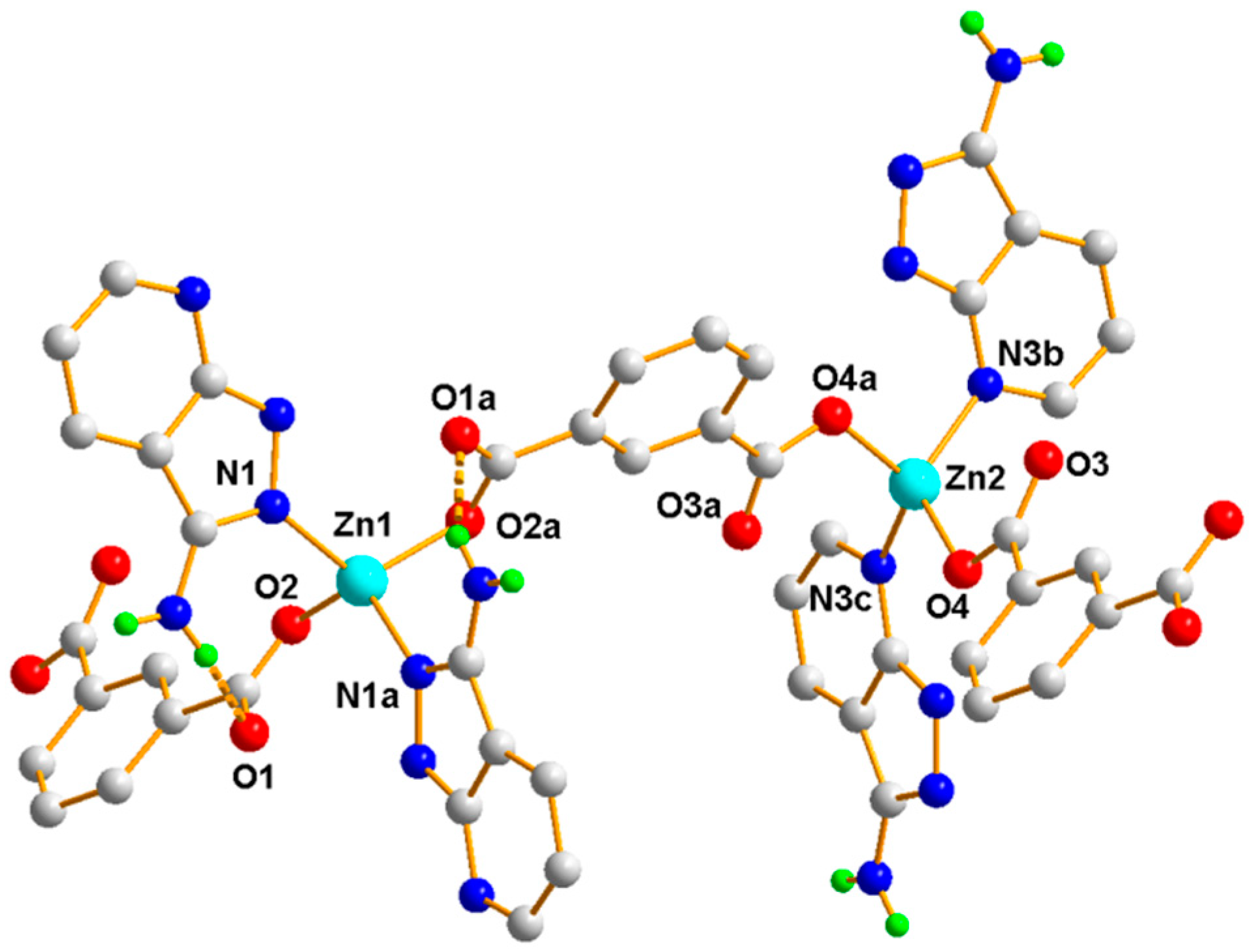
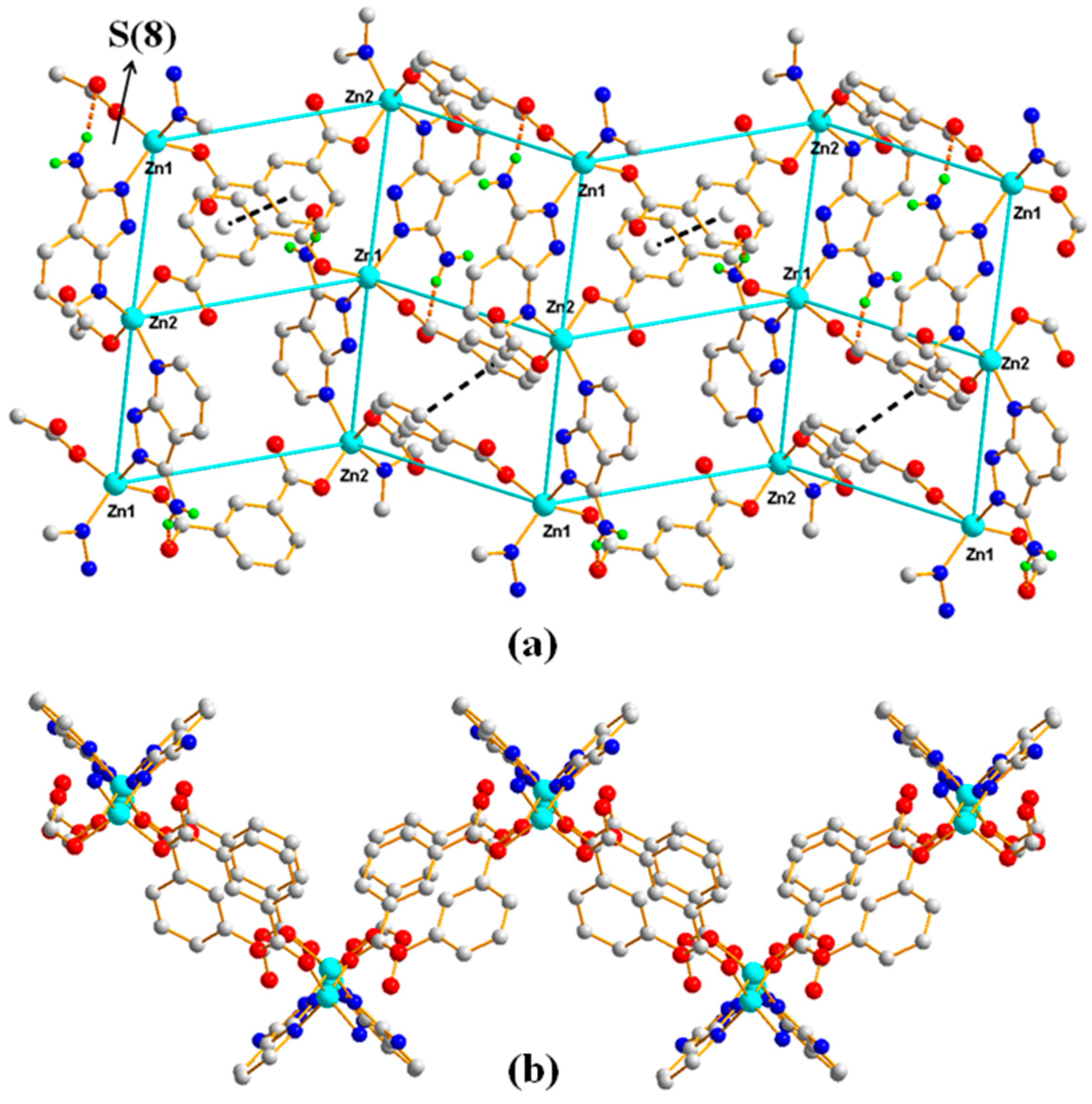
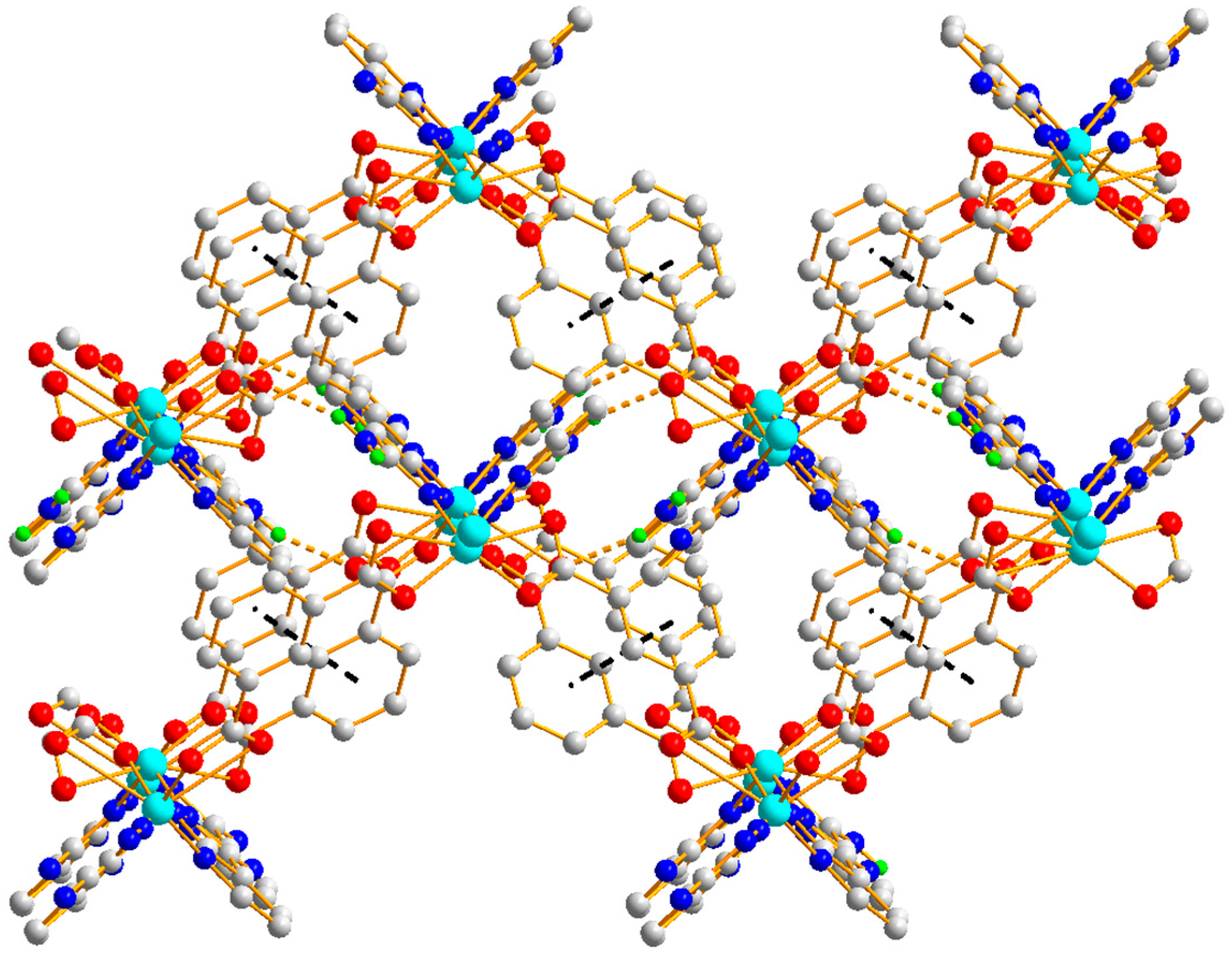
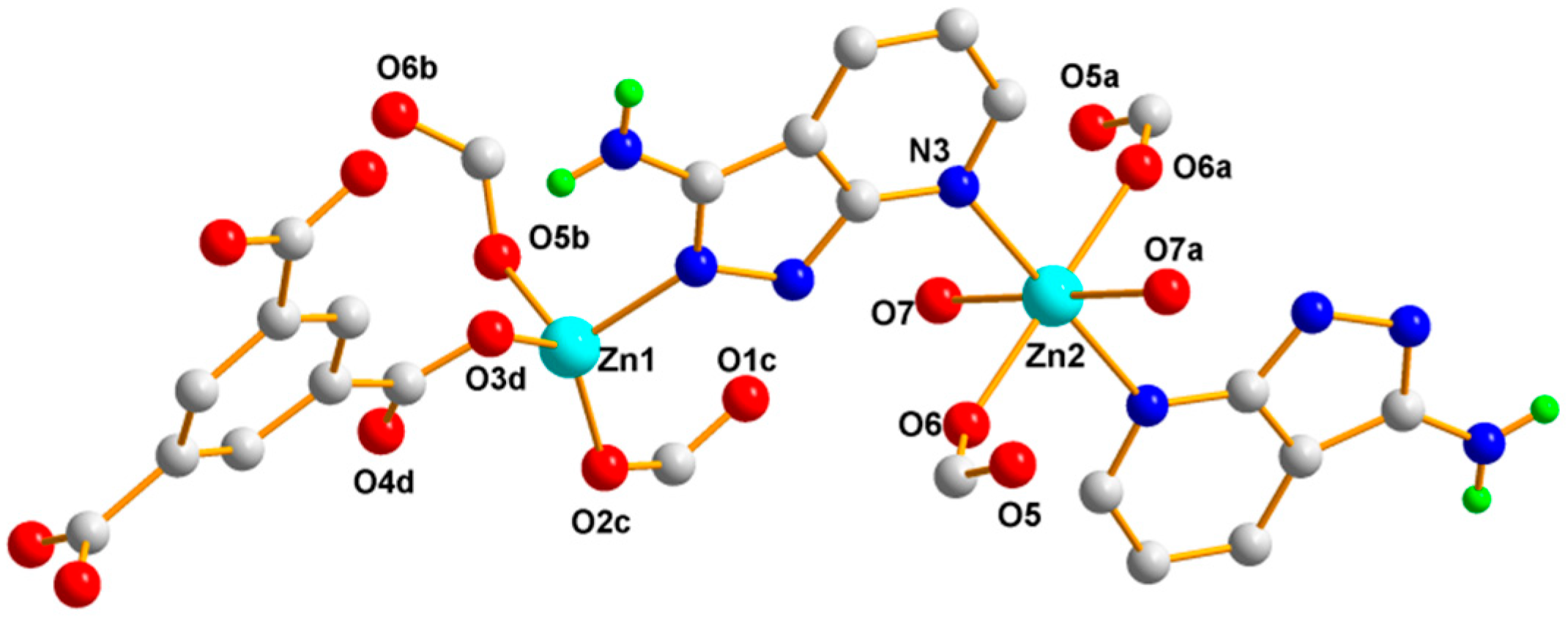
3.1.2. Structural Description of [Zn3(1,3,5-BTC)2(HL)2(H2O)2]n (Polymer 2)
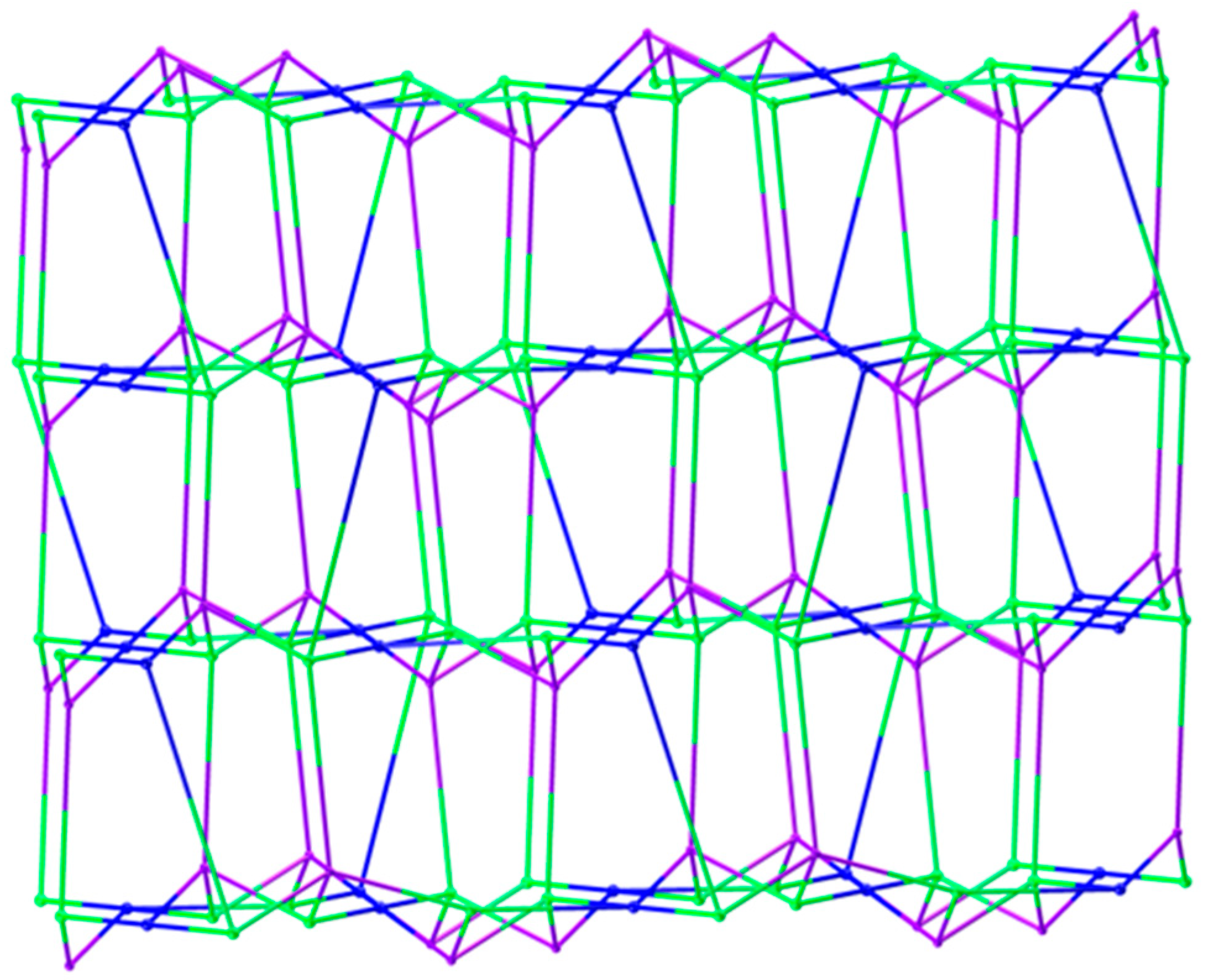
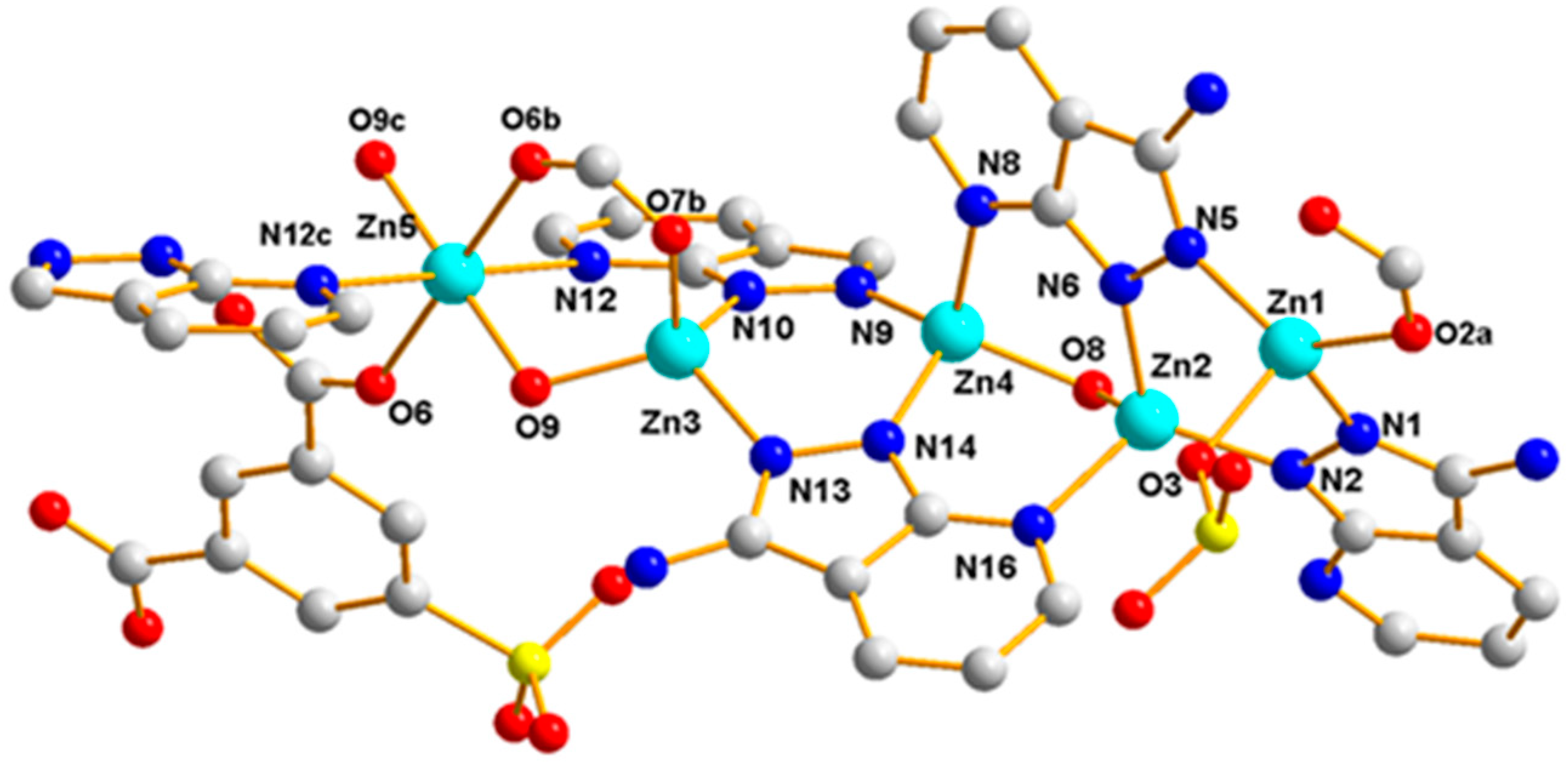
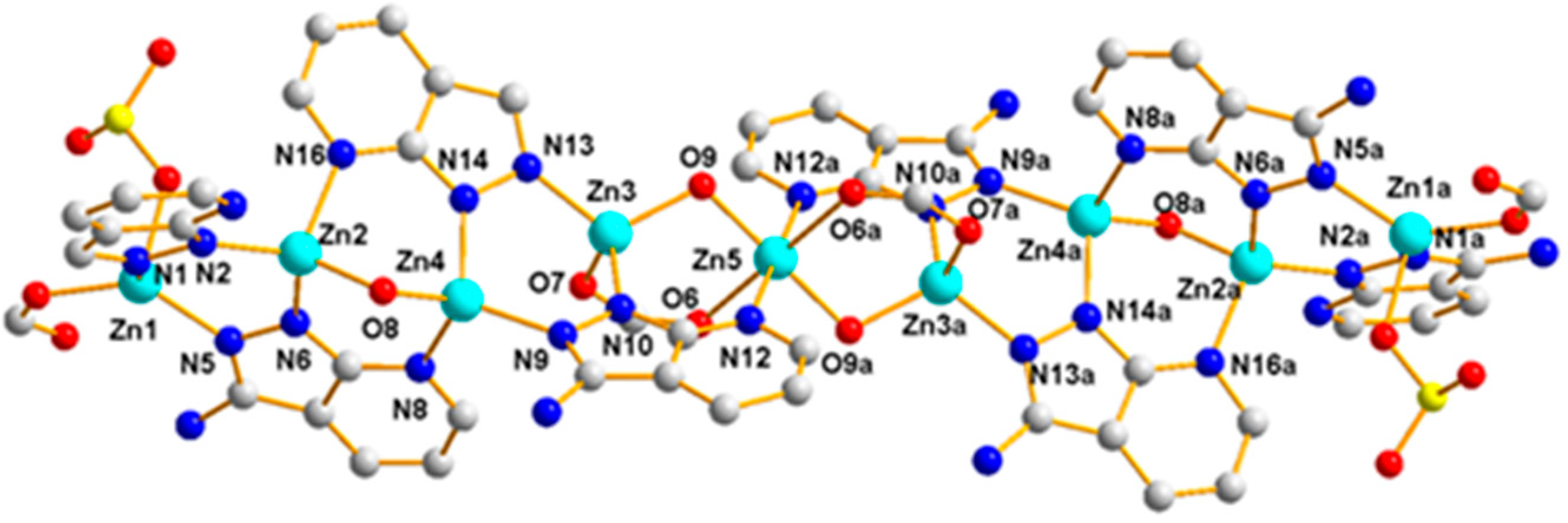
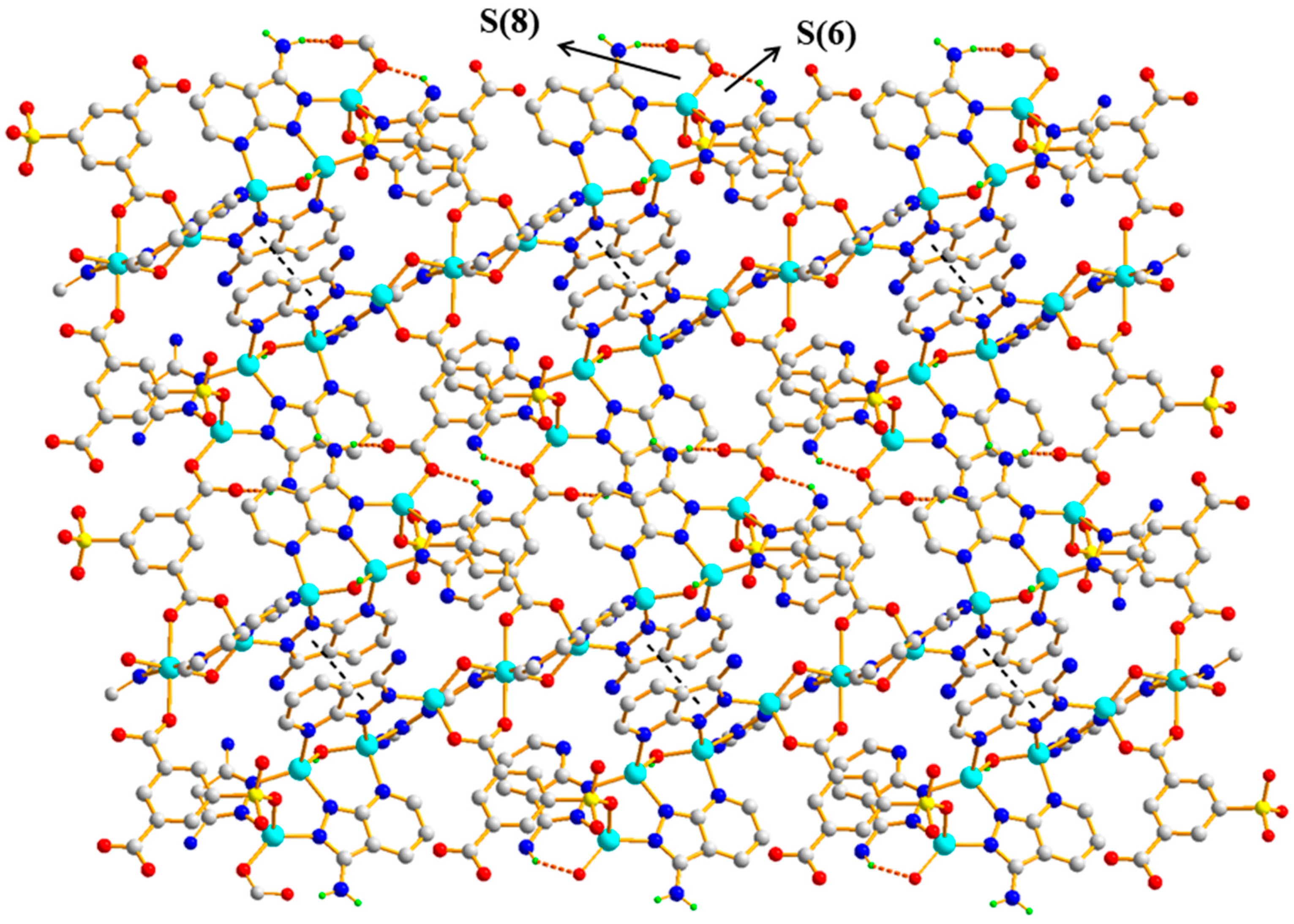
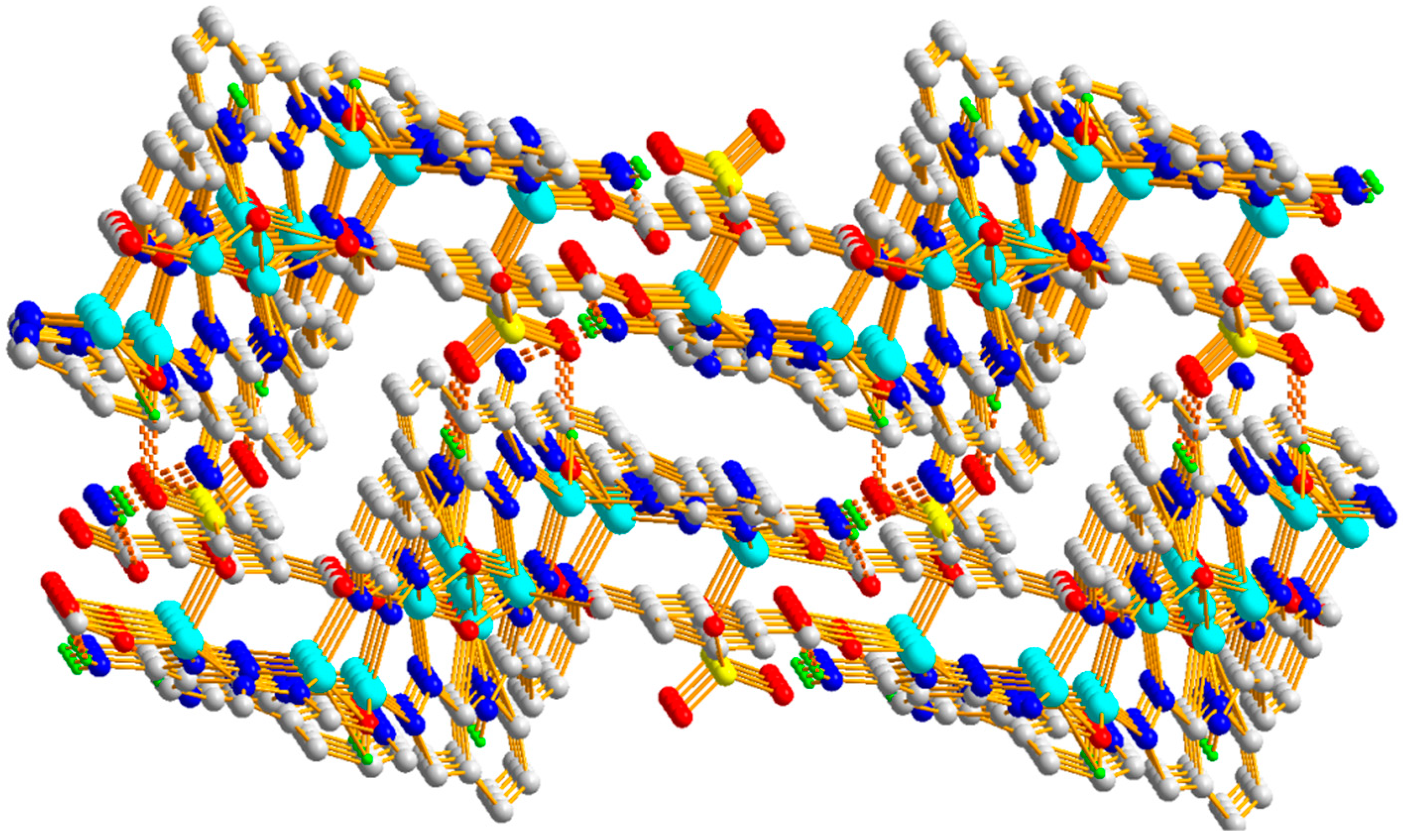
3.1.3. Structural Description of [Zn9(5-SO3-1,3-BDC)2(L)8(OH)4]n (Polymer 3)
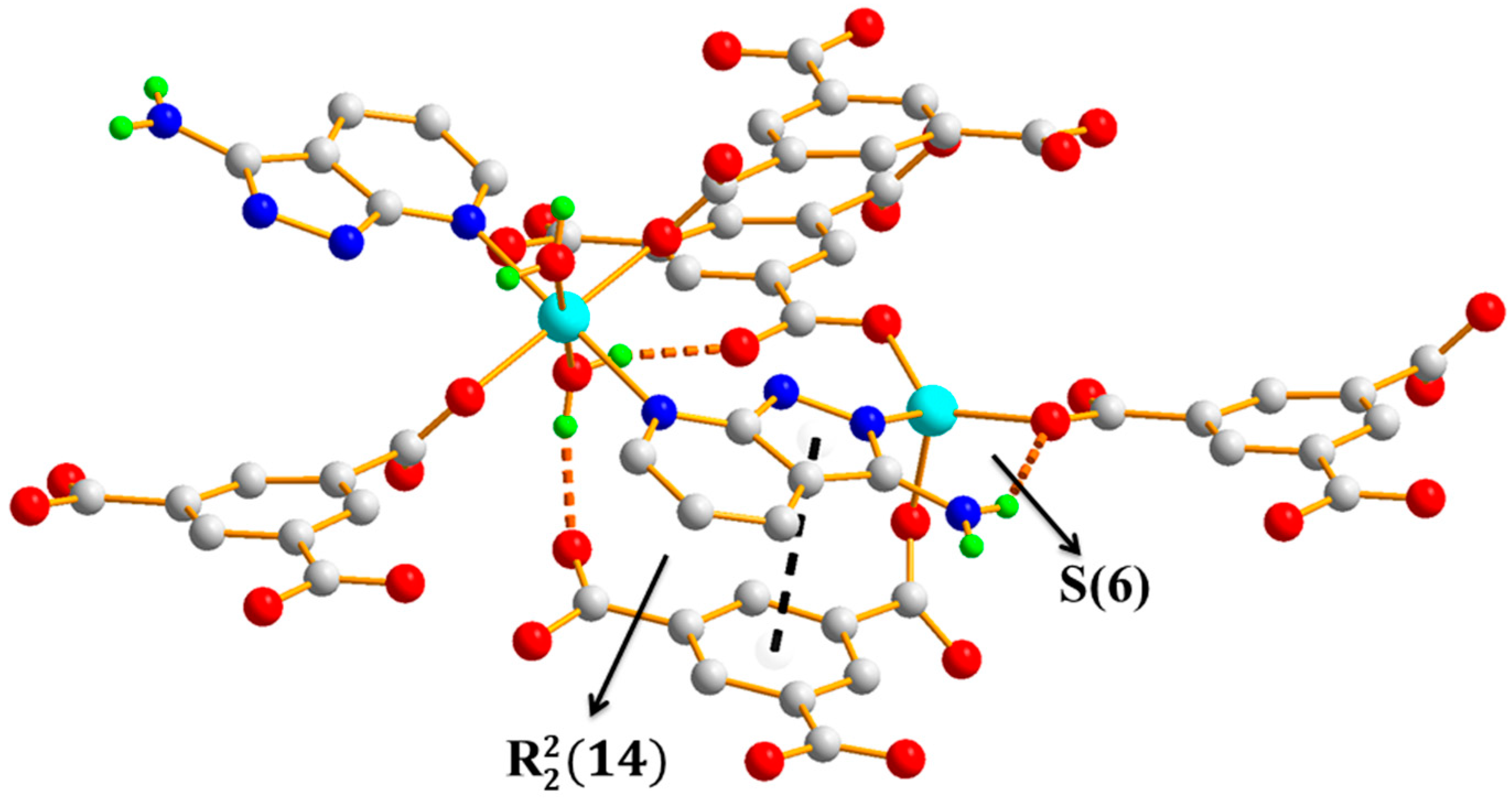
3.1.4. Comparison of the Structures of Polymers 1–3
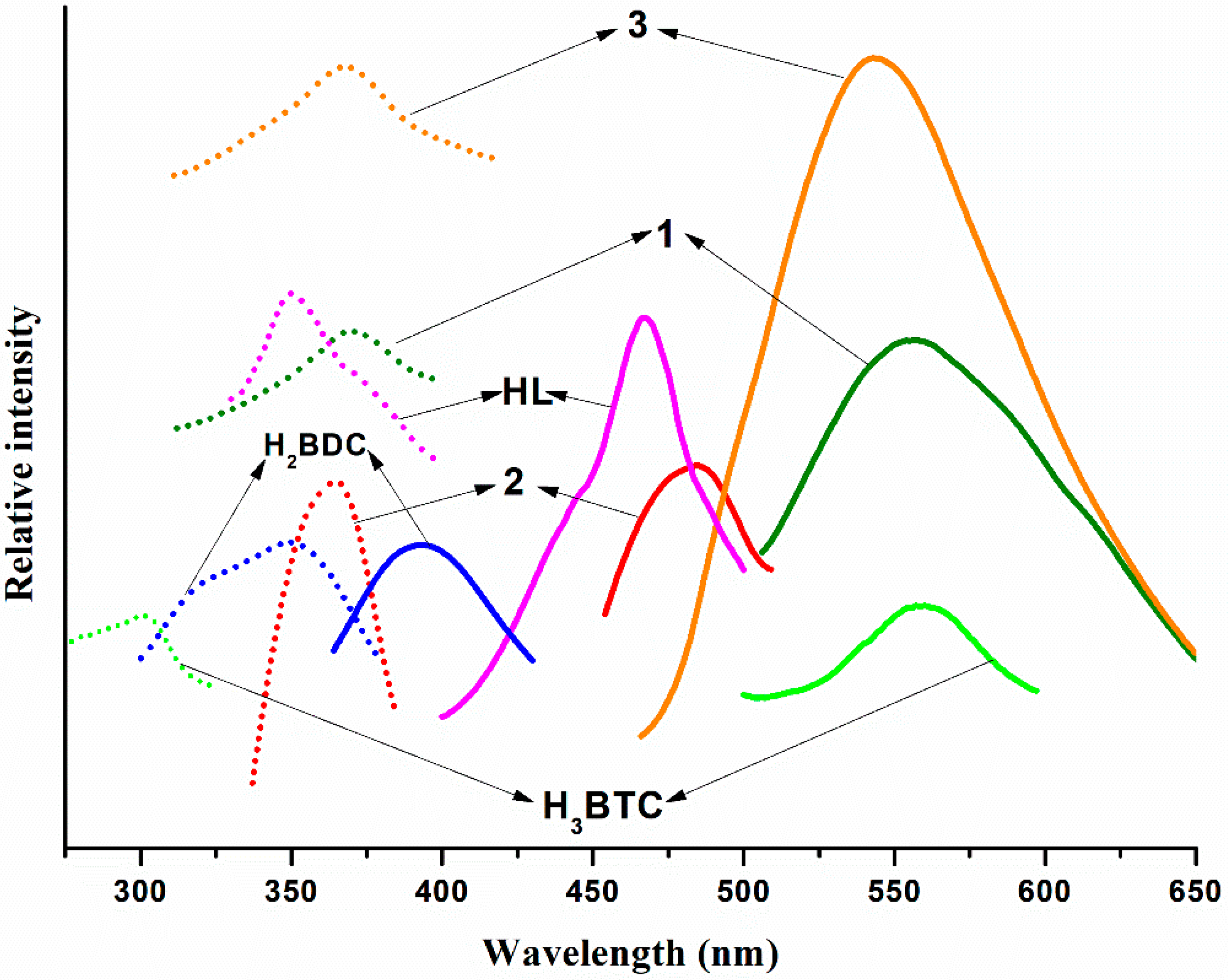
3.2. PXRD and Photoluminescence Properties of the Three Polymers
3.3. Thermogravimetric Analyses (TGA) of the Three Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shang, X.B.; Song, I.; Jung, G.Y.; Choi, W.; Ohtsu, H.; Lee, J.H.; Koo, J.Y.; Liu, B.; Ahn, J.; Kawano, M.; et al. Chiral self-sorted multifunctional supramolecular biocoordination polymers and their applications in sensors. Nat. Commun. 2018, 9, 3933. [Google Scholar] [CrossRef]
- Guillerm, V.; Kim, D.; Eubank, J.F.; Luebke, R.; Liu, X.F.; Adil, K.; Lah, M.S.; Eddaoudi, M. A supermolecular building approach for the design and construction of metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 6141–6172. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: From Molecule to Crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Knichal, J.V.; Gee, W.J.; Burrows, A.D.; Raithby, P.R.; Teat, S.J.; Wilson, C.C. A facile single crystal to single crystal transition with significant structural contraction on desolvation. Chem. Commun. 2014, 50, 14436–14439. [Google Scholar] [CrossRef] [PubMed]
- Madrid, E.; Buckingham, M.A.; Stone, J.M.; Rogers, A.T.; Gee, W.J.; Burrows, A.D.; Raithby, P.R.; Celorrio, V.; Fermind, D.J.; Marken, F. Ion flow in a zeolitic imidazolate framework results in ionic diode phenomena. Chem. Commun. 2016, 52, 2792–2794. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Uemura, K. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem. Soc. Rev. 2005, 34, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Aida, T.; Meijer, E.W.; Stupp, S.I. Functional Supramolecular Polymers. Science 2012, 335, 813–817. [Google Scholar] [CrossRef]
- Sengupta, S.; Goswami, A.; Ganguly, S.; Bala, S.; Bhunia, M.K.; Mondal, R. Influence of chloro/chloro interaction and p–p stacking in 3D supramolecular framework construction. CrystEngComm 2011, 13, 6136–6149. [Google Scholar] [CrossRef]
- Servati-Gargari, M.; Mahmoudi, G.; Batton, S.; Stilinovi, V.; Butler, D.; Beauvais, L.; Kassel, W.S.; Dougherty, W.G.; VanDerveer, D.G. Control of Interpenetration in 2D MOF-s by Modification of Hydrogen Bonding Capability of the Organic Bridging Subunits. Cryst. Growth Des. 2015, 15, 1336–1343. [Google Scholar] [CrossRef]
- Masaoka, S.; Tanaka, D.; Nakanishi, Y.; Kitagawa, S. Reaction-Temperature-Dependent Supramolecular Isomerism of Coordination Networks Based on the Organometallic Building Block [CuI2(μ2-BQ)(μ2-OAc)2]. Angew. Chem. Int. Ed. 2004, 43, 2530–2534. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, F.; Liu, D.; Yang, P.P.; Zhu, L.L. Syntheses, structures and properties of four Cd(II) coordination polymers induced by the pH regulator. J. Mol. Struct. 2018, 1155, 72–77. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.M.; Zhang, K.; Teo, B.K. Functional Crystals: Search Criteria and Design Principles. J. Solid State Chem. 2000, 152, 191–198. [Google Scholar] [CrossRef]
- Rachuri, Y.; Parmar, B.; Bisht, K.K.; Suresh, E. Solvothermal Self-assembly of Cd2+ Coordination Polymers with Supramolecular Networks Involving N-donor Ligands and Aromatic Dicarboxylates: Synthesis, Crystal Structure and Photoluminescence Studies. Dalton Trans. 2017, 46, 3623–3630. [Google Scholar] [CrossRef] [PubMed]
- Burattini, S.; Greenland, B.W.; Merino, D.H.; Weng, W.; Seppala, J.; Colquhoun, H.M.; Hayes, W.; Mackay, M.E.; Hamley, I.W.; Rowan, S.J. A Healable Supramolecular Polymer Blend Based on Aromatic π–π Stacking and Hydrogen-Bonding Interactions. J. Am. Chem. Soc. 2010, 132, 12051–12058. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zhou, X.W.; Zhou, S.R.; Tian, Y.P.; Wu, J.Y. A series of coordination polymers constructed from R-isophthalic acid (R = –SO3H, –NO2, and –OH) and N-donor ligands: Syntheses, structures and fluorescence properties. J. Solid State Chem. 2017, 245, 190–199. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, F.; Hu, Q.-Q.; Yang, P.-P.; Liu, D. A New Mn(II) Coordination Polymer Based on 1H-pyrazolo[3,4-b]pyridin-3-amine: Crystal Structure, Magnetic and Electrochemical Properties. Chin. J. Struct. Chem. 2017, 36, 1864–1870. [Google Scholar]
- Xu, Y.; Ding, F.; Liu, D.; Yang, P.-P. Two New d10 Supramolecular Polymers Constructed by 1H-Pyrazolo[3,4-b]pyridin-3-amine and 1,4-Benzenedicarboxylic Acids. Chin. J. Struct. Chem. 2019. [Google Scholar] [CrossRef]
- Gu, Y.F.; Liu, X.T.; Zhang, Y.; Zhang, S.M.; Chang, Z.; Bu, X.H. Supramolecular recognition of benzene homologues in a 2D coordination polymer through variable inter-layer π–π interaction. CrystEngComm 2018, 20, 3313–3317. [Google Scholar] [CrossRef]
- Mendiratta, S.; Lee, C.H.; Lee, S.Y.; Kao, Y.C.; Chang, B.C.; Lo, Y.H.; Lu, K.L. Structural Characteristics and Non-Linear Optical Behaviour of a 2-Hydroxynicotinate-Containing Zinc-Based Metal-Organic Framework. Molecules 2015, 20, 8941–8951. [Google Scholar] [CrossRef]
- Zang, S.; Su, Y.; Li, Y.; Ni, Z.; Meng, Q. Assemblies of a new flexible multicarboxylate ligand and d10 metal centers toward the construction of homochiral helical coordination polymers: Structures, luminescence, and NLO-active properties. Inorg. Chem. 2006, 45, 174–180. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Program for the Siemens Area Detector Absorption Correction; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXS-97: Program for X-ray Crystal Structure Determination; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Lin, X.M.; Niu, J.L.; Chen, D.N.; Lu, Y.N.; Zhang, G.; Cai, Y.P. Four metal–organic frameworks based on a semirigid tripodal ligand and different secondary building units: Structures and electrochemical performance. CrystEngComm 2016, 18, 6841–6848. [Google Scholar] [CrossRef]
- Yao, Q.X.; Jin, X.H.; Ju, Z.F.; Zhang, H.X.; Zhang, J. Supramolecular Borromean sheet consisting of threefold parallel interwoven 44-sql layers assembled by a flexible bipyridinium ligand. CrystEngComm 2009, 11, 1502–1504. [Google Scholar] [CrossRef]
- Li, X.Y.; Su, H.F.; Zhou, R.Q.; Feng, S.; Tan, Y.Z.; Wang, X.P.; Jia, J.; Kurmoo, M.; Sun, D.; Zheng, L.S. General Assembly of Twisted Trigonal-Prismatic Nonanuclear Silver(I) Clusters. Chem. Eur. J. 2016, 22, 3019–3028. [Google Scholar] [CrossRef]
- Zhang, B.; Xiao, T.; Liu, C.; Li, Q.; Zhu, Y.; Tang, M.; Du, C.; Song, M. Systematic Study of the Luminescent Europium-Based Nonanuclear Clusters with Modified 2-Hydroxybenzophenone Ligands. Inorg. Chem. 2013, 52, 13332–13340. [Google Scholar] [CrossRef]
- Boudalis, A.K.; Donnadieu, B.; Nastopoulos, V.; Clemente-Juan, J.M.; Mari, A.; Sanakis, Y.; Tuchagues, J.P.; Perlepes, S.P. A Nonanuclear Iron(ii) Single-Molecule Magnet. Angew. Chem. Int. Ed. 2004, 43, 2266–2270. [Google Scholar] [CrossRef]
- Baril-Robert, F.; Petit, S.; Pilet, G.; Chastanet, G.; Reber, C.; Luneau, D. Site-Selective Lanthanide Doping in a Nonanuclear Yttrium(III) Cluster Revealed by Crystal Structures and Luminescence Spectra. Inorg. Chem. 2010, 49, 10970–10976. [Google Scholar] [CrossRef]
- Omagari, S.; Nakanishi, T.; Seki, T.; Kitagawa, Y.; Takahata, Y.; Fushimi, K.; Ito, H.; Hasegawa, Y. Effective Photosensitized Energy Transfer of Nonanuclear Terbium Clusters Using Methyl Salicylate Derivatives. J. Phys. Chem. A 2015, 119, 1943–1947. [Google Scholar] [CrossRef]
- Huang, X.L.; Liu, L.; Gao, M.L.; Han, Z.B. A luminescent metal-organic framework for highly selective sensing of nitrobenzene and aniline. RSC Adv. 2016, 6, 87945–87949. [Google Scholar] [CrossRef]
- Wu, Y.; Morton, S.; Kong, X.; Nichol, G.S.; Zheng, Z. Hydrolytic synthesis and structural characterization of lanthanide-acetylacetonato/hydroxo cluster complexes—A systematic study. Dalton Trans. 2011, 40, 1041–1046. [Google Scholar] [CrossRef]
- Li, Y.W.; He, K.H.; Bu, X.H. Bottom-up assembly of a porous MOF based on nanosized nonanuclear zinc precursors for highly selective gas adsorption. J. Mater. Chem. A 2013, 1, 4186–4189. [Google Scholar] [CrossRef]
- Cheng, M.L.; Li, H.X.; Liu, L.L.; Wang, H.H.; Zhang, Y.; Lang, J.P. Unique formation of mono-, tetra- and nona-nuclear zinc complexes from protonolysis reactions of [Zn(dmpzm)Et2]. Dalton Trans. 2009, 11, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Pichon, C.; Mialane, P.; Dolbecq, A.; Marrot, J.; Riviere, E.; Bassil, B.S.; Kortz, U.; Keita, B.; Nadjo, L.; Sécheresse, F. Octa- and nonanuclear nickel(II) polyoxometalate clusters: Synthesis and electrochemical and magnetic characterizations. Inorg. Chem. 2008, 47, 11120–11128. [Google Scholar] [CrossRef] [PubMed]
- Roy, E.; Patra, S.; Madhuri, R.; Sharma, P.K. Development of an imprinted polymeric sensor with dual sensing property for trace level estimation of zinc and arginine. Mater. Sci. Eng. C 2015, 49, 25–33. [Google Scholar] [CrossRef] [PubMed]

| Polymer | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C14H10N4O4Zn | C30H22N8O14Zn3 | C64H50N32O18S2Zn9 |
| Formula weight | 363.63 | 914.67 | 2207.81 |
| Temp (K) | 293(2) | 293(2) | 293(2) |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | P2/c | P21/c | P |
| a (Å) | 12.1658(3) | 9.7940(8) | 9.9428(7) |
| b (Å) | 8.5041(3) | 13.4881(11) | 13.4612(8) |
| c (Å) | 18.0701(4) | 14.9135(9) | 15.1197(10) |
| α (°) | 90 | 90 | 73.567(2) |
| β (°) | 126.7210(10) | 126.970(4) | 84.738(3) |
| γ (°) | 90 | 90 | 74.256(2) |
| V (Å3) | 3878.9(2) | 1574.0(2) | 1867.9(2) |
| Z | 4 | 2 | 1 |
| F(000) | 736 | 920 | 1104 |
| Density (Mg/m3) | 1.612 | 1.930 | 1.963 |
| Absorption Coefficients (mm−1) | 1.664 | 2.357 | 1.093 |
| data/restraints/params | 3443/0/209 | 3617/0/250 | 6416/0/565 |
| GOF | 0.999 | 1.035 | 1.064 |
| R1 [I > 2σ(I)] | 0.0294 | 0.0242 | 0.0522 |
| wR2 [I > 2σ(I)] | 0.0946 | 0.0629 | 0.1026 |
| Polymer 1 | |||
| Zn(1)-O(2)#1 | 1.9572(15) | Zn(2)-O(4) | 1.9445(15) |
| Zn(1)-O(2) | 1.9572(15) | Zn(2)-N(3)#3 | 2.0496(17) |
| Zn(1)-N(1) | 2.0421(17) | Zn(2)-N(3)#4 | 2.0496(17) |
| Zn(1)-N(1)#1 | 2.0421(17) | Zn(2)-O(4)#2 | 1.9445(15) |
| N(3)-Zn(2)#3 | 2.0496(17) | ||
| O(4)#2-Zn(2)-O(4) | 103.18(10) | O(2)#1-Zn(1)-O(2) | 107.29(10) |
| O(4)#2-Zn(2)-N(3)#3 | 100.44(6) | O(2)#1-Zn(1)-N(1) | 117.82(7) |
| O(4)-Zn(2)-N(3)#3 | 124.75(7) | O(2)-Zn(1)-N(1) | 102.43(7) |
| O(4)#2-Zn(2)-N(3)#4 | 124.75(7) | O(2)#1-Zn(1)-N(1)#1 | 102.43(7) |
| O(4)-Zn(2)-N(3)#4 | 100.44(6) | O(2)-Zn(1)-N(1)#1 | 117.82(7) |
| N(3)#3-Zn(2)-N(3)#4 | 105.60(10) | N(1)-Zn(1)-N(1)#1 | 109.77(11) |
| Polymer 2 | |||
| Zn(1)-O(2) | 1.9239(13) | Zn(1)-O(3)#1 | 1.9713(15) |
| Zn(1)-O(5)#2 | 2.0075(15) | Zn(1)-N(1) | 2.0330(16) |
| Zn(2)-O(6) | 2.0596(13) | Zn(2)-O(6) | 2.0596(13) |
| Zn(2)-O(7)#3 | 2.1648(15) | Zn(2)-O(7) | 2.1648(15) |
| Zn(2)-N(3)#4 | 2.1997(16) | Zn(2)-N(3)#5 | 2.1997(16) |
| O(3)-Zn(1)#6 | 1.9713(15) | O(5)-Zn(1)#7 | 2.0075(15) |
| O(2)-Zn(1)-O(3)#1 | 131.93(7) | O(2)-Zn(1)-O(5)#2 | 100.29(6) |
| O(3)#1-Zn(1)-O(5)#2 | 107.37(7) | O(2)-Zn(1)-N(1) | 114.47(6) |
| O(3)#1-Zn(1)-N(1) | 93.61(6) | O(5)#2-Zn(1)-N(1) | 107.79(6) |
| O(6)-Zn(2)-O(6)#3 | 180.000(1) | O(6)-Zn(2)-O(7)#3 | 99.04(6) |
| O(6)#3-Zn(2)-O(7)#3 | 80.96(6) | O(6)-Zn(2)-O(7) | 80.96(6) |
| O(6)#3-Zn(2)-O(7) | 99.04(6) | O(7)#3-Zn(2)-O(7) | 180.000(1) |
| O(6)-Zn(2)-N(3)#4 | 92.84(6) | O(6)#3-Zn(2)-N(3)#4 | 87.16(6) |
| O(7)#3-Zn(2)-N(3)#4 | 86.50(6) | O(7)-Zn(2)-N(3)#4 | 93.50(6) |
| O(6)-Zn(2)-N(3)#5 | 87.16(6) | O(6)#3-Zn(2)-N(3)#5 | 92.84(6) |
| O(7)#3-Zn(2)-N(3)#5 | 93.50(6) | O(7)-Zn(2)-N(3)#5 | 86.50(6) |
| N(3)#4-Zn(2)-N(3)#5 | 180.00(8) | ||
| Polymer 3 | |||
| Zn(1)-O(2)#1 | 1.934(3) | Zn(3)-N(13) | 1.965(4) |
| Zn(1)-N(5) | 1.991(4) | Zn(3)-O(7)#2 | 1.974(4) |
| Zn(1)-O(3) | 1.997(4) | Zn(3)-N(10) | 1.976(5) |
| Zn(1)-N(1) | 2.006(4) | Zn(3)-Zn(5B)#3 | 3.045(17) |
| Zn(2)-O(8) | 1.922(4) | Zn(3)-Zn(5) | 3.1242(6) |
| Zn(2)-N(2) | 1.949(4) | Zn(4)-O(8) | 1.911(4) |
| Zn(2)-N(6) | 2.023(4) | Zn(4)-N(9) | 1.978(4) |
| Zn(2)-N(16) | 2.056(5) | Zn(4)-N(14) | 1.990(5) |
| Zn(2)-Zn(4) | 3.0695(9) | Zn(4)-N(8) | 2.053(4) |
| Zn(3)-O(9) | 1.909(4) | Zn(5)-O(9)#4 | 1.899(4) |
| Zn(5)-O(9) | 1.899(4) | Zn(5B)-O(9)#6 | 1.998(16) |
| Zn(5)-O(6)#3 | 2.183(4) | Zn(5B)-O(9)#3 | 2.121(17) |
| Zn(5)-O(6)#2 | 2.183(4) | Zn(5B)-O(6) | 2.170(18) |
| Zn(5)-Zn(3)#4 | 3.1242(6) | Zn(5B)-O(6)#5 | 2.469(16) |
| Zn(5B)-Zn(5B)#5 | 1.597(14) | Zn(5B)-Zn(3)#3 | 3.045(17) |
| Zn(5B)-N(12)#3 | 1.743(9) | O(7)-Zn(3)#6 | 1.974(4) |
| O(6)-Zn(5B)#5 | 2.469(16) | O(6)-Zn(5)#6 | 2.183(4) |
| O(2)#1-Zn(1)-N(5) | 133.06(17) | O(3)-Zn(1)-N(1) | 96.91(17) |
| O(2)#1-Zn(1)-O(3) | 107.56(16) | O(8)-Zn(2)-N(2) | 122.63(17) |
| N(5)-Zn(1)-O(3) | 103.45(16) | O(8)-Zn(2)-N(6) | 102.80(18) |
| O(2)#1-Zn(1)-N(1) | 100.10(16) | N(2)-Zn(2)-N(6) | 110.20(18) |
| N(5)-Zn(1)-N(1) | 110.24(17) | O(8)-Zn(2)-N(16) | 100.61(17) |
| N(6)-Zn(2)-N(16) | 107.41(19) | N(2)-Zn(2)-N(16) | 111.89(19) |
| O(8)-Zn(2)-Zn(4) | 36.67(11) | O(9)-Zn(3)-N(13) | 116.15(18) |
| N(2)-Zn(2)-Zn(4) | 158.92(14) | O(9)-Zn(3)-O(7)#2 | 104.77(17) |
| N(6)-Zn(2)-Zn(4) | 78.68(12) | N(13)-Zn(3)-O(7)#2 | 105.91(17) |
| N(16)-Zn(2)-Zn(4) | 81.99(12) | O(9)-Zn(3)-N(10) | 112.25(19) |
| O(7)#2-Zn(3)-N(10) | 107.68(19) | N(13)-Zn(3)-N(10) | 109.45(19) |
| O(9)-Zn(3)-Zn(5B)#3 | 43.6(3) | Zn(5B)#3-Zn(3)-Zn(5) | 14.80(13) |
| N(10)-Zn(3)-Zn(5B)#3 | 75.1(2) | O(8)-Zn(4)-N(9) | 125.13(19) |
| O(9)-Zn(3)-Zn(5) | 34.78(12) | O(8)-Zn(4)-N(14) | 101.16(18) |
| N(13)-Zn(3)-Zn(5) | 150.93(13) | N(9)-Zn(4)-N(14) | 111.05(19) |
| O(7)#2-Zn(3)-Zn(5) | 88.18(11) | O(8)-Zn(4)-N(8) | 104.06(18) |
| N(10)-Zn(3)-Zn(5) | 89.41(13) | N(9)-Zn(4)-N(8) | 108.08(18) |
| N(14)-Zn(4)-N(8) | 105.74(19) | O(9)#4-Zn(5)-O(6)#2 | 88.51(16) |
| O(8)-Zn(4)-Zn(2) | 36.92(12) | O(9)-Zn(5)-O(6)#2 | 91.49(16) |
| N(9)-Zn(4)-Zn(2) | 162.04(14) | O(6)#3-Zn(5)-O(6)#2 | 180.0 |
| N(14)-Zn(4)-Zn(2) | 78.57(12) | O(9)#4-Zn(5)-Zn(3)#4 | 34.99(12) |
| N(8)-Zn(4)-Zn(2) | 82.59(12) | O(9)-Zn(5)-Zn(3)#4 | 145.01(12) |
| O(9)#4-Zn(5)-O(9) | 180.0 | O(6)#3-Zn(5)-Zn(3)#4 | 66.42(11) |
| O(9)#4-Zn(5)-O(6)#3 | 91.49(16) | O(6)#2-Zn(5)-Zn(3)#4 | 113.58(11) |
| O(9)-Zn(5)-O(6)#3 | 88.51(16) | O(9)#4-Zn(5)-Zn(3) | 145.01(12) |
| O(9)-Zn(5)-Zn(3) | 34.99(12) | N(12)#3-Zn(5B)-O(9)#6 | 115.2(8) |
| O(6)#3-Zn(5)-Zn(3) | 113.58(11) | Zn(5B)#5-Zn(5B)-O(9)#3 | 63.2(11) |
| O(6)#2-Zn(5)-Zn(3) | 66.42(11) | N(12)#3-Zn(5B)-O(9)#3 | 110.0(8) |
| Zn(3)#4-Zn(5)-Zn(3) | 180.0 | O(9)#6-Zn(5B)-O(9)#3 | 134.5(4) |
| Zn(5B)#5-Zn(5B)-N(12)#3 | 170.6(18) | Zn(5B)#5-Zn(5B)-O(6) | 80.4(12) |
| Zn(5B)#5-Zn(5B)-O(9)#6 | 71.3(11) | O(6)-Zn(5B)-O(6)#5 | 140.4(3) |
| N(12)#3-Zn(5B)-O(6) | 105.9(7) | Zn(5B)#5-Zn(5B)-Zn(3)#3 | 88.2(12) |
| O(9)#6-Zn(5B)-O(6) | 89.3(6) | N(12)#3-Zn(5B)-Zn(3)#3 | 82.7(6) |
| O(9)#3-Zn(5B)-O(6) | 83.5(6) | O(9)#6-Zn(5B)-Zn(3)#3 | 143.9(7) |
| Zn(5B)#5-Zn(5B)-O(6)#5 | 60.0(11) | O(9)#3-Zn(5B)-Zn(3)#3 | 38.4(3) |
| N(12)#3-Zn(5B)-O(6)#5 | 113.4(8) | O(6)-Zn(5B)-Zn(3)#3 | 117.0(7) |
| O(9)#6-Zn(5B)-O(6)#5 | 78.7(5) | O(6)#5-Zn(5B)-Zn(3)#3 | 65.2(4) |
| O(9)#3-Zn(5B)-O(6)#5 | 78.9(5) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Deng, Q.-H.; Ding, F.; An, R.; Liu, D.; Miao, T.-F. Three New Supramolecular Coordination Polymers Based on 1H-pyrazolo[3,4-b]pyridin-3-amine and 1,3-benzenedicarboxylate Derivatives. Polymers 2019, 11, 819. https://doi.org/10.3390/polym11050819
Xu Y, Deng Q-H, Ding F, An R, Liu D, Miao T-F. Three New Supramolecular Coordination Polymers Based on 1H-pyrazolo[3,4-b]pyridin-3-amine and 1,3-benzenedicarboxylate Derivatives. Polymers. 2019; 11(5):819. https://doi.org/10.3390/polym11050819
Chicago/Turabian StyleXu, Yun, Qing-Hua Deng, Fang Ding, Ran An, Dong Liu, and Ti-Fang Miao. 2019. "Three New Supramolecular Coordination Polymers Based on 1H-pyrazolo[3,4-b]pyridin-3-amine and 1,3-benzenedicarboxylate Derivatives" Polymers 11, no. 5: 819. https://doi.org/10.3390/polym11050819
APA StyleXu, Y., Deng, Q.-H., Ding, F., An, R., Liu, D., & Miao, T.-F. (2019). Three New Supramolecular Coordination Polymers Based on 1H-pyrazolo[3,4-b]pyridin-3-amine and 1,3-benzenedicarboxylate Derivatives. Polymers, 11(5), 819. https://doi.org/10.3390/polym11050819





