Stabilization of Pickering Emulsions by Hairy Nanoparticles Bearing Polyanions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PS@PSS Hairy Nanoparticles
2.2.1. Synthesis of Crosslinked Polystyrene (PS) Core Emulsion
2.2.2. Synthesis of Hairy Nanoparticles
2.3. Preparation of Pickering Emulsion
2.4. Characterization
2.4.1. Dynamic Light Scattering (DLS)
2.4.2. Transmission Electron Microscopy (TEM)
2.4.3. Polarizing Microscope Observation
2.4.4. Contact Angle Analysis
3. Results and Discussion
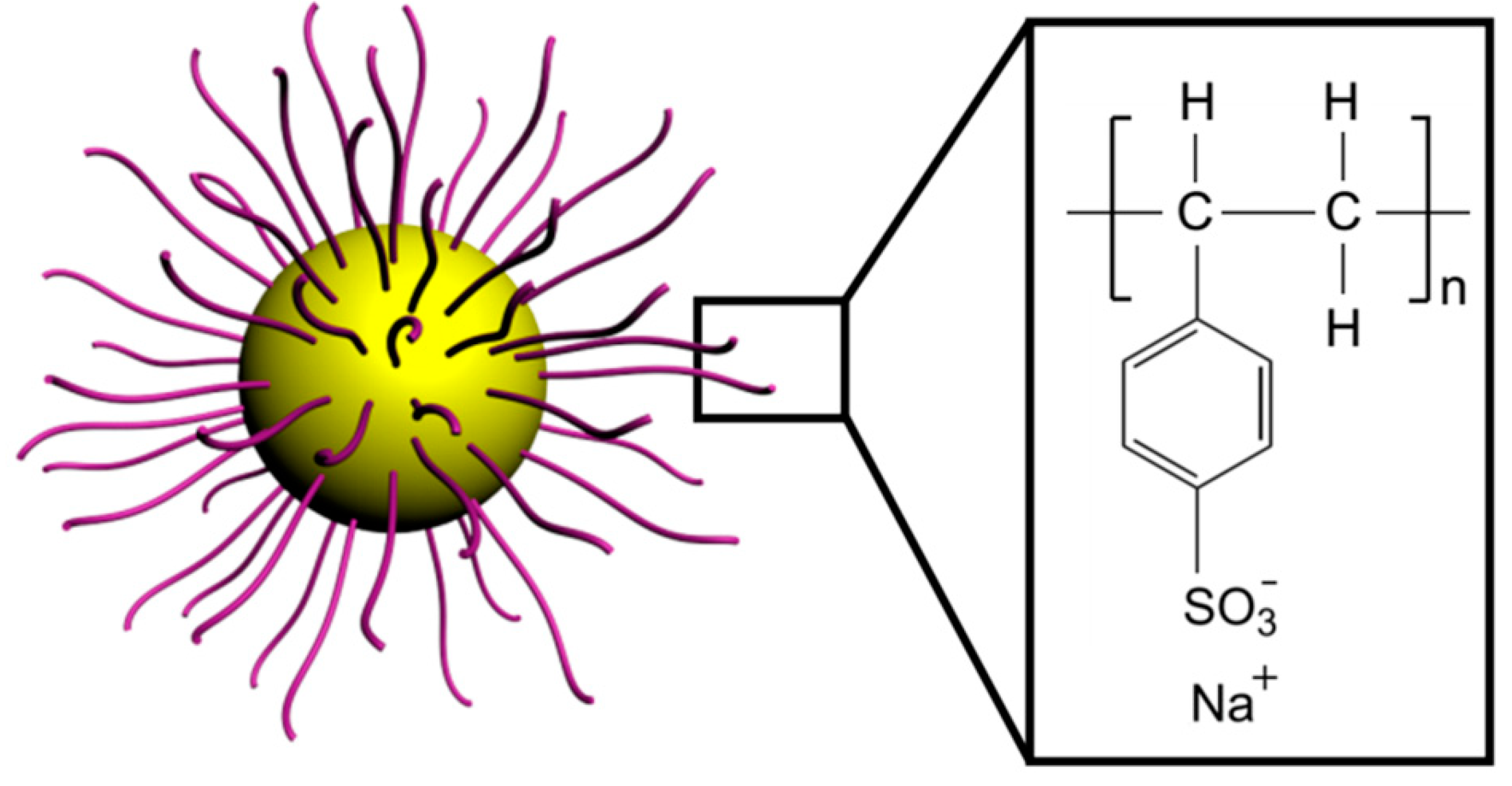
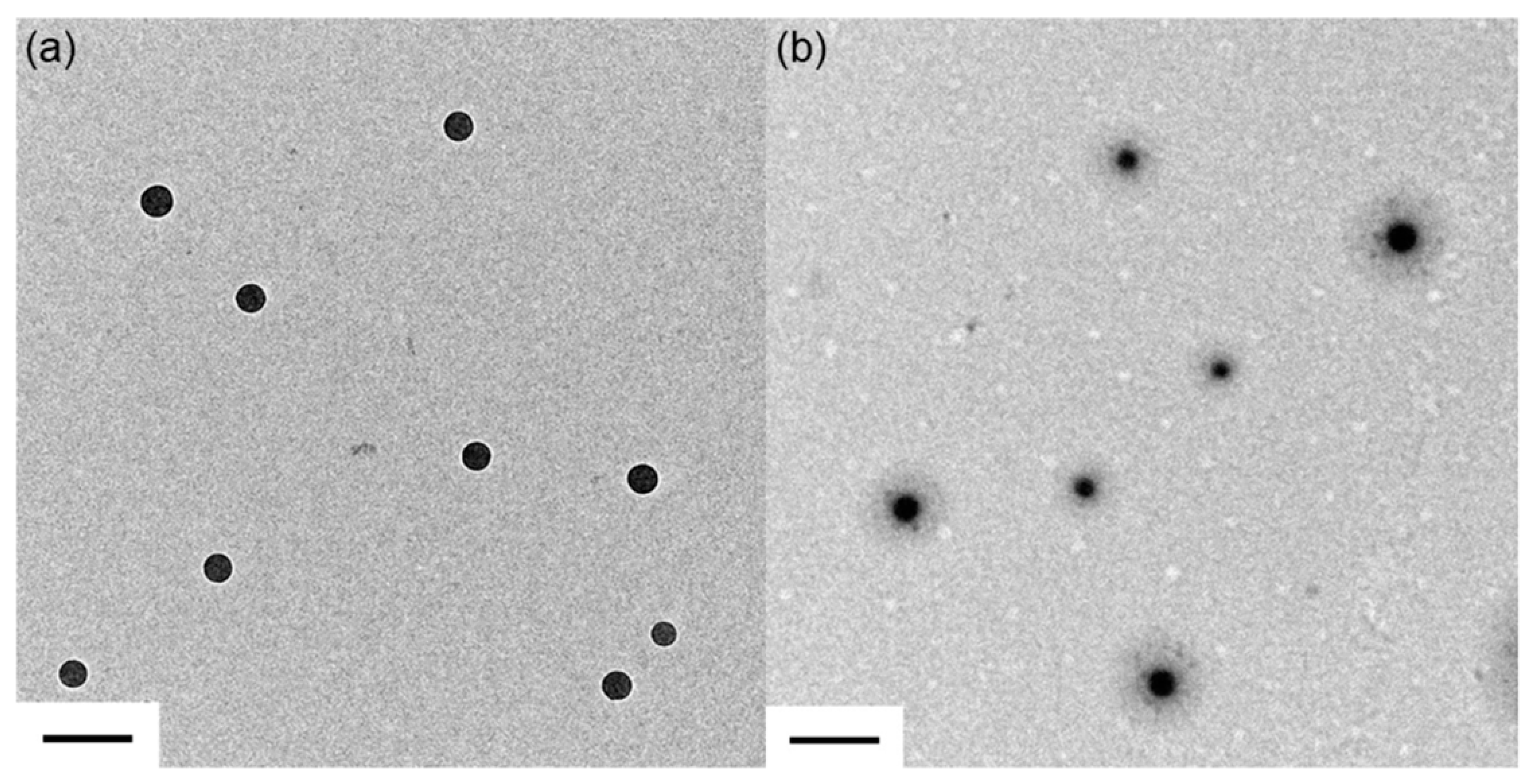
3.1. Synthesis of PS@PSS Hairy Nanoparticles
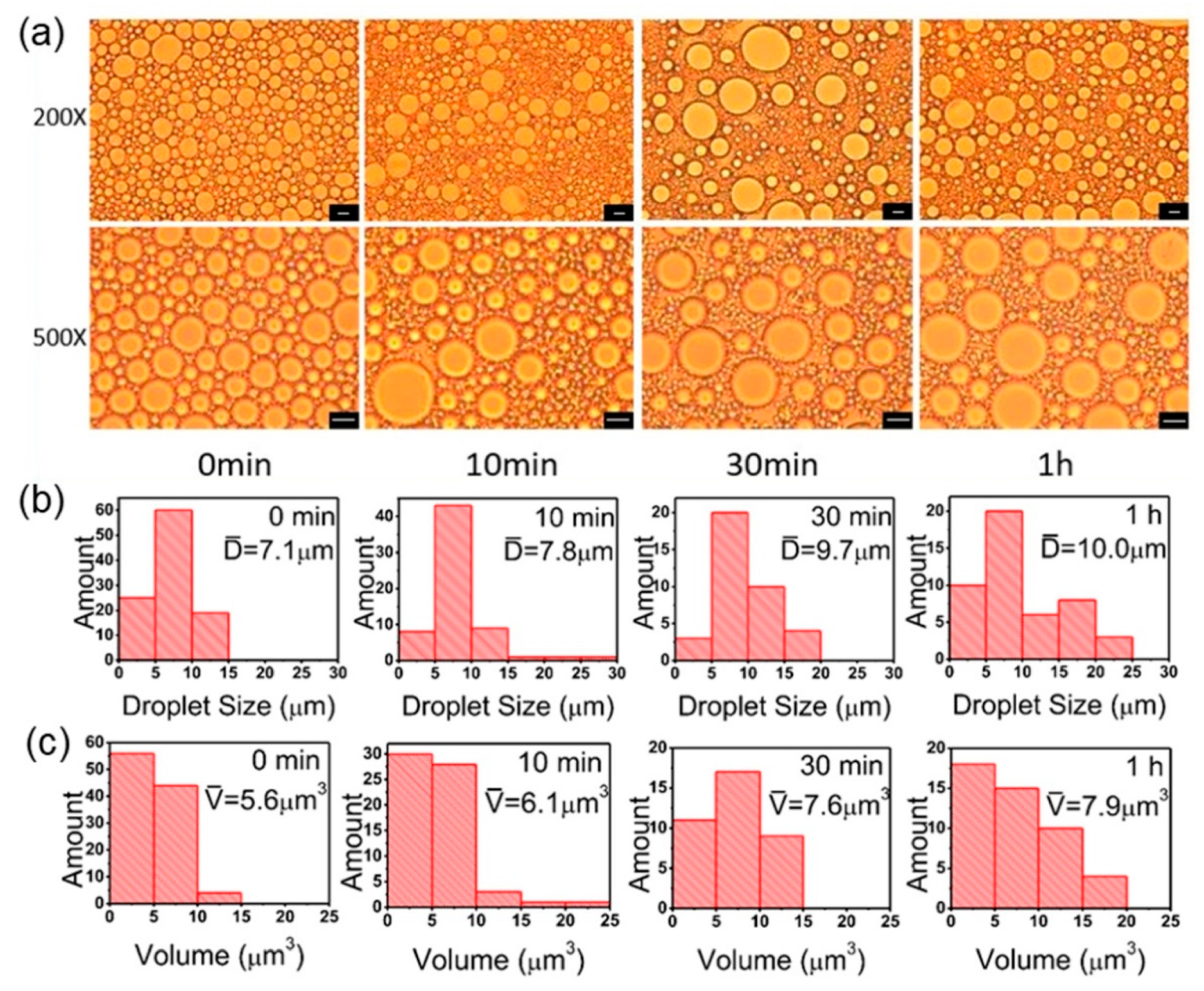
3.2. Ultrasonic Power and Time
3.3. Standing Time
3.4. Oil Phase
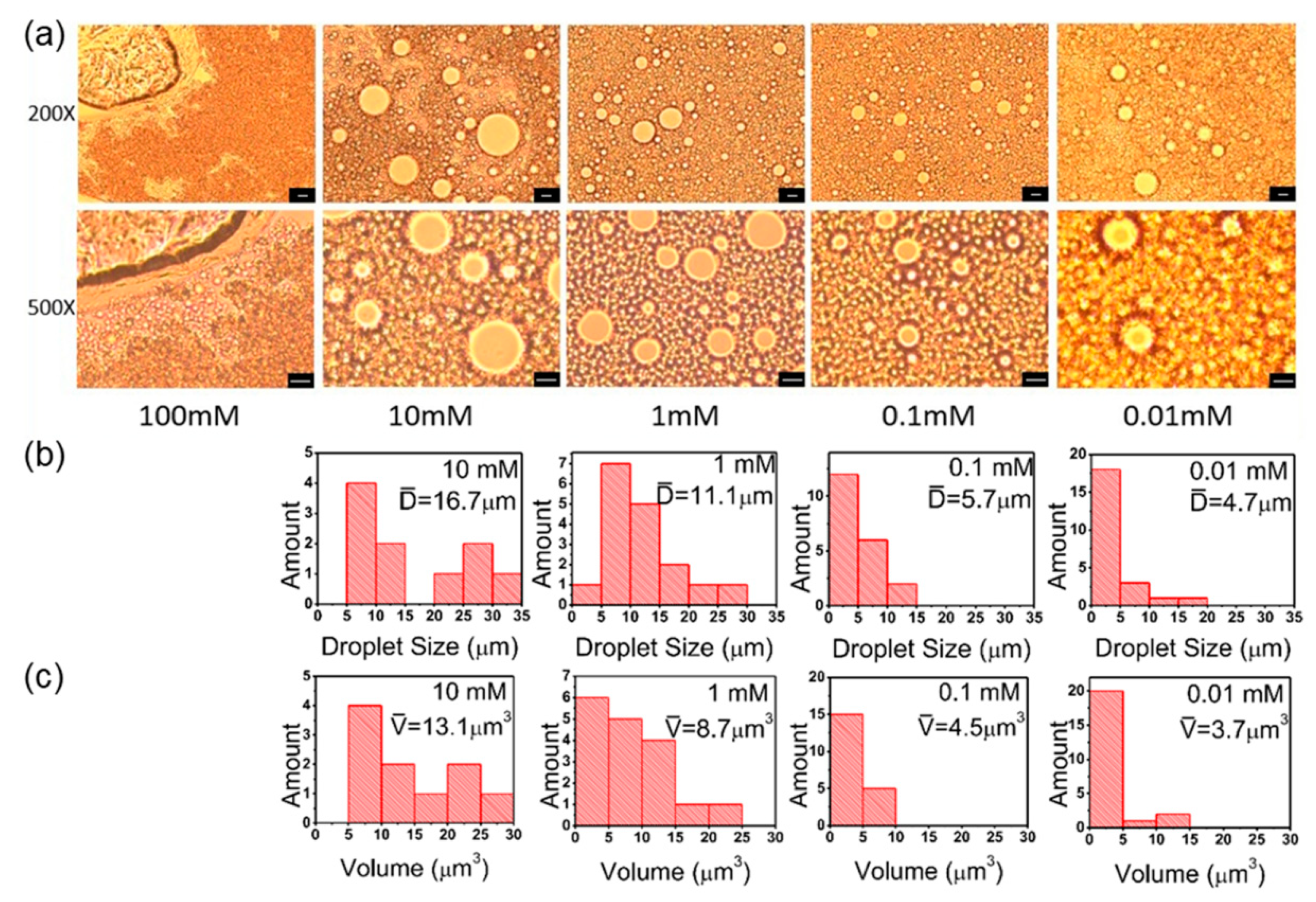
3.5. Salt Concentration
3.6. Water:Oil Ratio
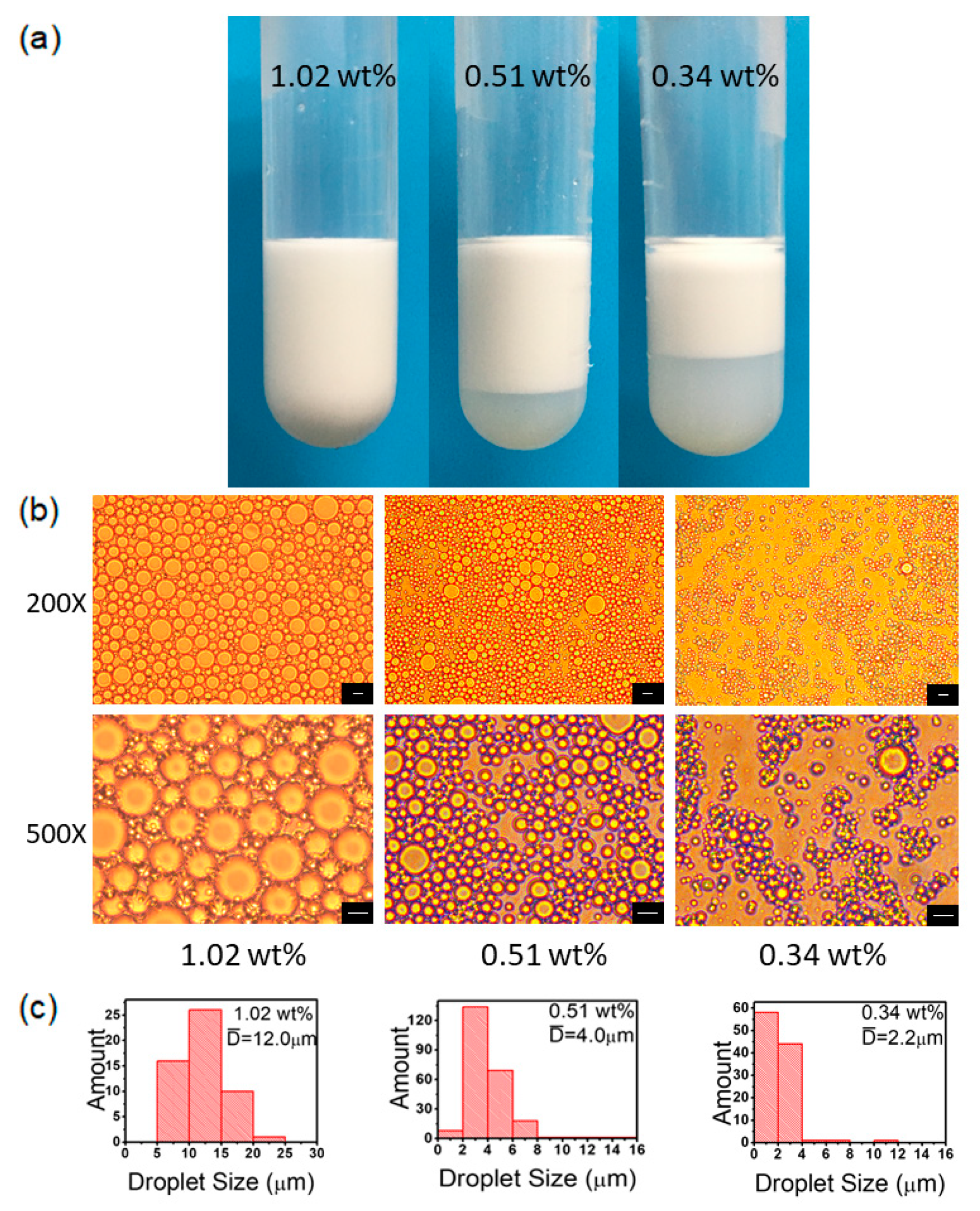
3.7. PS@PSS Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Bai, Y.; Chen, S.; Ju, J.; Li, Y.; Wang, T.; Wang, Q. Stimuli-Responsive Composite Particles as Solid-Stabilizers for Effective Oil Harvesting. ACS Appl. Mater. Interfaces 2014, 6, 13334–13338. [Google Scholar] [CrossRef]
- Pickering, S.U. Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Marto, J.; Ascenso, A.; Simoes, S.; Almeida, A.J.; Ribeiro, H.M. Pickering Emulsions: Challenges and Opportunities in Topical Delivery. Expert Opin. Drug Deliv. 2016, 13, 1093–1107. [Google Scholar] [CrossRef]
- Marquis, M.; Alix, V.; Capron, I.; Cuenot, S.; Zykwinska, A. Microfluidic Encapsulation of Pickering Oil Microdroplets into Alginate Microgels for Lipophilic Compound Delivery. ACS Biomater. Sci. Eng. 2016, 2, 535–543. [Google Scholar] [CrossRef]
- Nonomura, Y.; Fukuda, K.; Komura, S.; Tsujii, K. Self-Assembly of Surface-Active Powder at the Interfaces of Selective Liquids. 2: Behavior of an Organic-Crystalline Powder. Langmuir 2003, 19, 10152–10156. [Google Scholar] [CrossRef]
- Nonomura, Y.; Sugawara, T.; Kashimoto, A.; Fukuda, K.; Hotta, H.; Tsujii, K. Self-Assembly of Surface-Active Powder at the Interfaces of Selective Liquids. 1: Multiple Structural Polymorphism. Langmuir 2002, 18, 10163–10167. [Google Scholar] [CrossRef]
- Kargar, M.; Fayazmanesh, K.; Alavi, M.; Spyropoulos, F.; Norton, I.T. Investigation into the Potential Ability of Pickering Emulsions (Food-Grade Particles) to Enhance the Oxidative Stability of Oil-in-Water Emulsions. J. Colloid Interface Sci. 2012, 366, 209–215. [Google Scholar] [CrossRef]
- Dinsmore, A.D.; Hsu, M.F.; Nikolaides, M.G.; Marquez, M.; Bausch, A.R.; Weitz, D.A. Colloidosomes: Selectively Permeable Capsules Composed of Colloidal Particles. Science 2002, 298, 1006–1009. [Google Scholar] [CrossRef]
- Thompson, K.L.; Williams, M.; Armes, S.P. Colloidosomes: Synthesis, Properties and Applications. J. Colloid Interface Sci. 2015, 447, 217–228. [Google Scholar] [CrossRef]
- Li, Z.; Ming, T.; Wang, J.; Ngai, T. High Internal Phase Emulsions Stabilized Solely by Microgel Particles. Angew. Chem. Int. Ed. 2009, 48, 8490–8493. [Google Scholar] [CrossRef]
- Li, Z.; Wei, X.; Ming, T.; Wang, J.; Ngai, T. Dual Templating Synthesis of Hierarchical Porous Silica Materials with Three Orders of Length Scale. Chem. Commun. 2010, 46, 8767–8769. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, Z.; Wang, C.; Tong, Z. Lignin-Based Pickering HIPEs for Macroporous Foams and Their Enhanced Adsorption of Copper(II) Ions. Chem. Commun. 2013, 49, 7144–7146. [Google Scholar] [CrossRef]
- Silverstein, M.S. PolyHIPEs: Recent Advances in Emulsion-Templated Porous Polymers. Prog. Polym. Sci. 2014, 39, 199–234. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Stability of Oil-in-Water Emulsions Stabilised by Silica Particles. Phys. Chem. Chem. Phys. 1999, 1, 3007–3016. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-Water Emulsions Stabilized by Chitin Nanocrystal Particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Kim, D.-M.; Song, I.-H.; Choi, J.-Y.; Jin, S.-W.; Kim, B.-J.; Jeong, J.-W.; Jang, C.-E.; Chu, K.; Chung, C.-M. An Oligoimide Particle as a Pickering Emulsion Stabilizer. Polymers 2018, 10, 1071. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Z.; Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Yuan, W.; Yang, Y.; Xie, Y.; Chen, Y. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef]
- Saigal, T.; Dong, H.; Matyjaszewski, K.; Tilton, R.D. Pickering Emulsions Stabilized by Nanoparticles with Thermally Responsive Grafted Polymer Brushes. Langmuir 2010, 26, 15200–15209. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Wang, H.; Pei, Y.; Shi, Y.; Wang, J. Visible Light-Controlled Inversion of Pickering Emulsions Stabilized by Functional Silica Microspheres. Langmuir 2018, 34, 2784–2790. [Google Scholar] [CrossRef]
- Madivala, B.; Vandebril, S.; Fransaer, J.; Vermant, J. Exploiting Particle Shape in Solid Stabilized Emulsions. Soft Matter 2009, 5, 1717–1727. [Google Scholar] [CrossRef]
- Katepalli, H.; John, V.T.; Tripathi, A.; Bose, A. Microstructure and Rheology of Particle Stabilized Emulsions: Effects of Particle Shape and Inter-Particle Interactions. J. Colloid Interface Sci. 2017, 485, 11–17. [Google Scholar] [CrossRef]
- Tsuji, S.; Kawaguchi, H. Thermosensitive Pickering Emulsion Stabilized by Poly(N-isopropylacrylamide)-Carrying Particles. Langmuir 2008, 24, 3300–3305. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y. Tuning Amphiphilicity of Particles for Controllable Pickering Emulsion. Materials 2016, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Zhao, B.; Zhu, L. Hairy Particles: Theory, Synthesis, Behavior, and Applications. J. Polym. Sci. Pol. Phys. 2014, 52, 1581–1582. [Google Scholar] [CrossRef]
- Cao, L.; Chen, K.; Qin, X.; Zhang, Y.; Li, K.; Guo, X. Effect of Block Sequence on Responsive Behavior of Core-Shell Diblock Polymer Brushes. Mater. Lett. 2018, 223, 116–119. [Google Scholar] [CrossRef]
- Amalvy, J.I.; Armes, S.P.; Binks, B.P.; Rodrigues, J.A.; Unali, G.F. Use of Sterically-Stabilised Polystyrene Latex Particles as a pH-Responsive Particulate Emulsifier to Prepare Surfactant-Free Oil-in-Water Emulsions. Chem. Commun. 2003, 15, 1826–1827. [Google Scholar] [CrossRef]
- Motornov, M.; Zhou, J.; Pita, M.; Tokarev, I.; Gopishetty, V.; Katz, E.; Minko, S. An Integrated Multifunctional Nanosystem from Command Nanoparticles and Enzymes. Small 2009, 5, 817–820. [Google Scholar] [CrossRef]
- Thompson, K.L.; Giakoumatos, E.C.; Ata, S.; Webber, G.B.; Armes, S.P.; Wanless, E.J. Direct Observation of Giant Pickering Emulsion and Colloidosome Droplet Interaction and Stability. Langmuir 2012, 28, 16501–16511. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Chen, K.; Cao, L.; Zhang, Y.; Li, L.; Guo, X. Antifouling Performance of Nano-Sized Spherical Poly(N-hydroxyethyl Acrylamide) Brush. Colloids Surf. B 2017, 155, 408–414. [Google Scholar] [CrossRef]
- Yang, Q.; Li, L.; Zhao, F.; Han, H.; Wang, W.; Tian, Y.; Wang, Y.; Ye, Z.; Guo, X. Hollow Silica-Polyelectrolyte Composite Nanoparticles for Controlled Drug Delivery. J. Mater. Sci. 2019, 54, 2552–2565. [Google Scholar] [CrossRef]
- Guo, X.; Weiss, A.; Ballauff, M. Synthesis of Spherical Polyelectrolyte Brushes by Photoemulsion Polymerization. Macromolecules 1999, 32, 6043–6046. [Google Scholar] [CrossRef]
- Guo, X.; Ballauff, M. Spatial Dimensions of Colloidal Polyelectrolyte Brushes as Determined by Dynamic Light Scattering. Langmuir 2000, 16, 8719–8726. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Li, D.S.; Ilavsky, J.; Kuzmenko, I.; Jeng, G.-S.; O’Donnell, M.; Pozzo, L.D. Ultrasound-Based Formation of Nano-Pickering Emulsions Investigated via in-situ SAXS. J. Colloid Interface Sci. 2019, 536, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.L.R.; Gomes, A.; Cunha, R.L. One-Step Ultrasound Producing O/W Emulsions Stabilized by Chitosan Particles. Food Res. Int. 2018, 107, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Salari, J.W.O.; Mutsaers, G.; Meuldijk, J.; Klumperman, B. Deformation of the Water/Oil Interface during the Adsorption of Sterically Stabilized Particles. Langmuir 2014, 30, 7327–7333. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S.; Price, G.J. Applications of Ultrasound to Materials Chemistry. Annu. Rev. Mater. Sci. 1995, 20, 29–34. [Google Scholar] [CrossRef]
- Zhang, R.; Hou, X.; Cang, Y.; Yu, Z.; Shen, Z.; Zhou, Z.; Guo, X.; Wang, J.; Zhu, X. Efficiently Amplified Ultrasonic Degradation of Spherical Polyelectrolyte Brushes by a Magnetic Field. RSC Adv. 2016, 6, 24549–24556. [Google Scholar] [CrossRef]
- Ashby, N.P.; Binks, B.P. Pickering Emulsions Stabilised by Laponite Clay Particles. Phys. Chem. Chem. Phys. 2000, 2, 5640–5646. [Google Scholar] [CrossRef]
- Kumar, A.; Li, S.; Cheng, C.-M.; Lee, D. Recent Developments in Phase Inversion Emulsification. Ind. Eng. Chem. Res. 2015, 54, 8375–8396. [Google Scholar] [CrossRef]
- Whitby, C.P.; Wanless, E.J. Controlling Pickering Emulsion Destabilisation: A Route to Fabricating New Materials by Phase Inversion. Materials 2016, 9, 626. [Google Scholar] [CrossRef]
- Zhai, X.; Gao, J.; Wang, X.; Mei, S.; Zhao, R.; Wu, Y.; Hao, C.; Yang, J.; Liu, Y. Inverse Pickering Emulsions Stabilized by Carbon Quantum Dots: Influencing Factors and Their Application as Templates. Chem. Eng. J. 2018, 345, 209–220. [Google Scholar] [CrossRef]
- Li, Z.; Ngai, T. Macroporous Polymer from Core-Shell Particle-Stabilized Pickering Emulsions. Langmuir 2010, 26, 5088–5092. [Google Scholar] [CrossRef]
- Fielding, L.A.; Armes, S.P. Preparation of Pickering Emulsions and Colloidosomes Using either a Glycerol-Functionalised Silica Sol or Core-Shell Polymer/Silica Nanocomposite Particles. J. Mater. Chem. 2012, 22, 11235–11244. [Google Scholar] [CrossRef]
- Liu, M.; Chen, X.; Yang, Z.; Xu, Z.; Hong, L.; Ngai, T. Tunable Pickering Emulsions with Environmentally Responsive Hairy Silica Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 32250–32258. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, C.; Yang, Z.; Wang, P.; Yue, Y.; Kitaoka, T. Thermally Tunable Pickering Emulsions Stabilized by Carbon-Dot-Incorporated Core-Shell Nanospheres with Fluorescence “On-Off” Behavior. Langmuir 2018, 34, 273–283. [Google Scholar] [CrossRef]
- Jiang, W.; Fu, Q.; Yao, B.; Ding, L.; Liu, C.; Dong, Y. Smart pH-Responsive Polymer-Tethered and Pd NP-Loaded NMOF as the Pickering Interfacial Catalyst for One-Pot Cascade Biphasic Reaction. ACS Appl. Mater. Interfaces 2017, 9, 36438–36446. [Google Scholar] [CrossRef]
- Tang, J.; Quinlan, P.J.; Tam, K.C. Stimuli-Responsive Pickering Emulsions: Recent Advances and Potential Applications. Soft Matter 2015, 11, 3512–3529. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Schliebe, C.; Stoychev, G.; Jakob, A.; Lang, H.; Synytska, A. Hybrid Hairy Janus Particles Decorated with Metallic Nanoparticles for Catalytic Applications. ACS Appl. Mater. Interfaces 2015, 7, 21218–21225. [Google Scholar] [CrossRef]
- Anjali, T.G.; Basavaraj, M.G. Influence of pH and Salt Concentration on Pickering Emulsions Stabilized by Colloidal Peanuts. Langmuir 2018, 34, 13312–13321. [Google Scholar] [CrossRef] [PubMed]




| Size and Size Distribution | PS Core | PS@PSS | Thickness (nm) |
|---|---|---|---|
| Diameter (nm) 1 | 74.2 ± 0.3 | 252.8 ± 1.2 | 89.3 ± 0.7 |
| PDI 1 | 0.001 ± 0.001 | 0.018 ± 0.009 | - |
| Entry | Diameter (nm) 1 | Thickness (nm) 2 | PDI 1 |
|---|---|---|---|
| PS@PSS (untreated) | 252 ± 1.2 | 89 ± 0.8 | 0.018 ± 0.009 |
| PS@PSS (100 W) | 249 ± 0.9 | 88 ± 0.6 | 0.027 ± 0.013 |
| PS@PSS (150 W) | 247 ± 1.4 | 86 ± 0.9 | 0.022 ± 0.008 |
| PS@PSS (200 W) | 239 ± 2.7 | 82 ± 1.5 | 0.056 ± 0.021 |
| PS@PSS (300 W) | 236 ± 1.8 | 81 ± 1.1 | 0.037 ± 0.015 |
| Entry | Diameter (nm) 1 | Thickness (nm) 2 | PDI 1 |
|---|---|---|---|
| PS@PSS (1 min) | 248 ± 0.9 | 87 ± 0.6 | 0.011 ± 0.002 |
| PS@PSS (2 min) | 246 ± 0.4 | 86 ± 0.4 | 0.032 ± 0.013 |
| PS@PSS (3 min) | 243 ± 0.4 | 85 ± 0.4 | 0.027 ± 0.010 |
| PS@PSS (4 min) | 242 ± 0.3 | 84 ± 0.3 | 0.047 ± 0.018 |
| PS@PSS (5 min) | 241 ± 0.2 | 83 ± 0.3 | 0.068 ± 0.024 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, K.; Cao, L.; Li, K.; Wang, Q.; Fu, E.; Guo, X. Stabilization of Pickering Emulsions by Hairy Nanoparticles Bearing Polyanions. Polymers 2019, 11, 816. https://doi.org/10.3390/polym11050816
Zhang Y, Chen K, Cao L, Li K, Wang Q, Fu E, Guo X. Stabilization of Pickering Emulsions by Hairy Nanoparticles Bearing Polyanions. Polymers. 2019; 11(5):816. https://doi.org/10.3390/polym11050816
Chicago/Turabian StyleZhang, Ying, Kaimin Chen, Lan Cao, Kai Li, Qiaoling Wang, Enyu Fu, and Xuhong Guo. 2019. "Stabilization of Pickering Emulsions by Hairy Nanoparticles Bearing Polyanions" Polymers 11, no. 5: 816. https://doi.org/10.3390/polym11050816
APA StyleZhang, Y., Chen, K., Cao, L., Li, K., Wang, Q., Fu, E., & Guo, X. (2019). Stabilization of Pickering Emulsions by Hairy Nanoparticles Bearing Polyanions. Polymers, 11(5), 816. https://doi.org/10.3390/polym11050816







