Ionic Liquid Composite Polybenzimidazol Membranes for High Temperature PEMFC Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials.
2.2. Characterization
2.3. Experimental Procedures
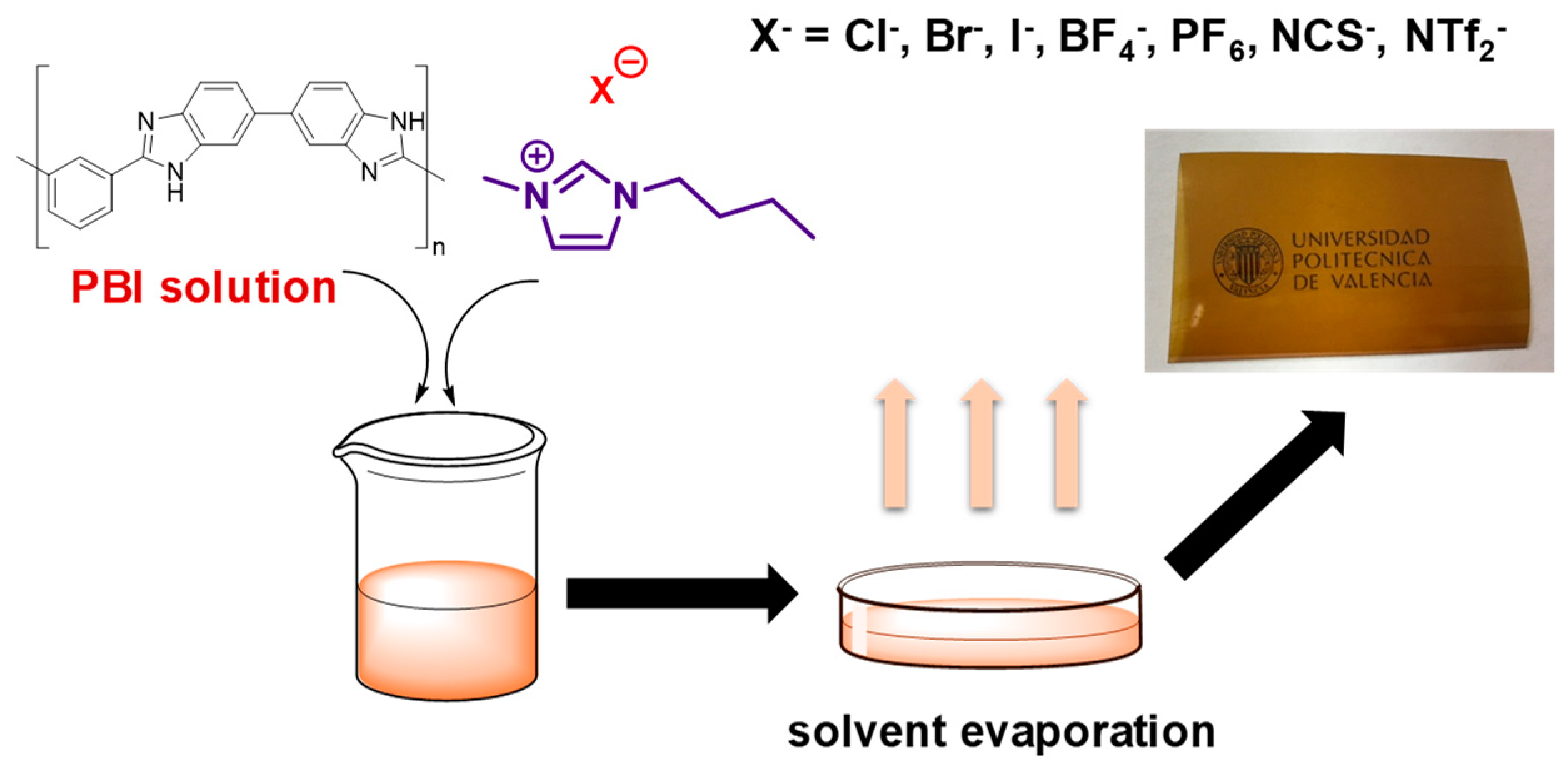
2.3.1. Preparation of the PBI Solution
2.3.2. Membrane Preparation
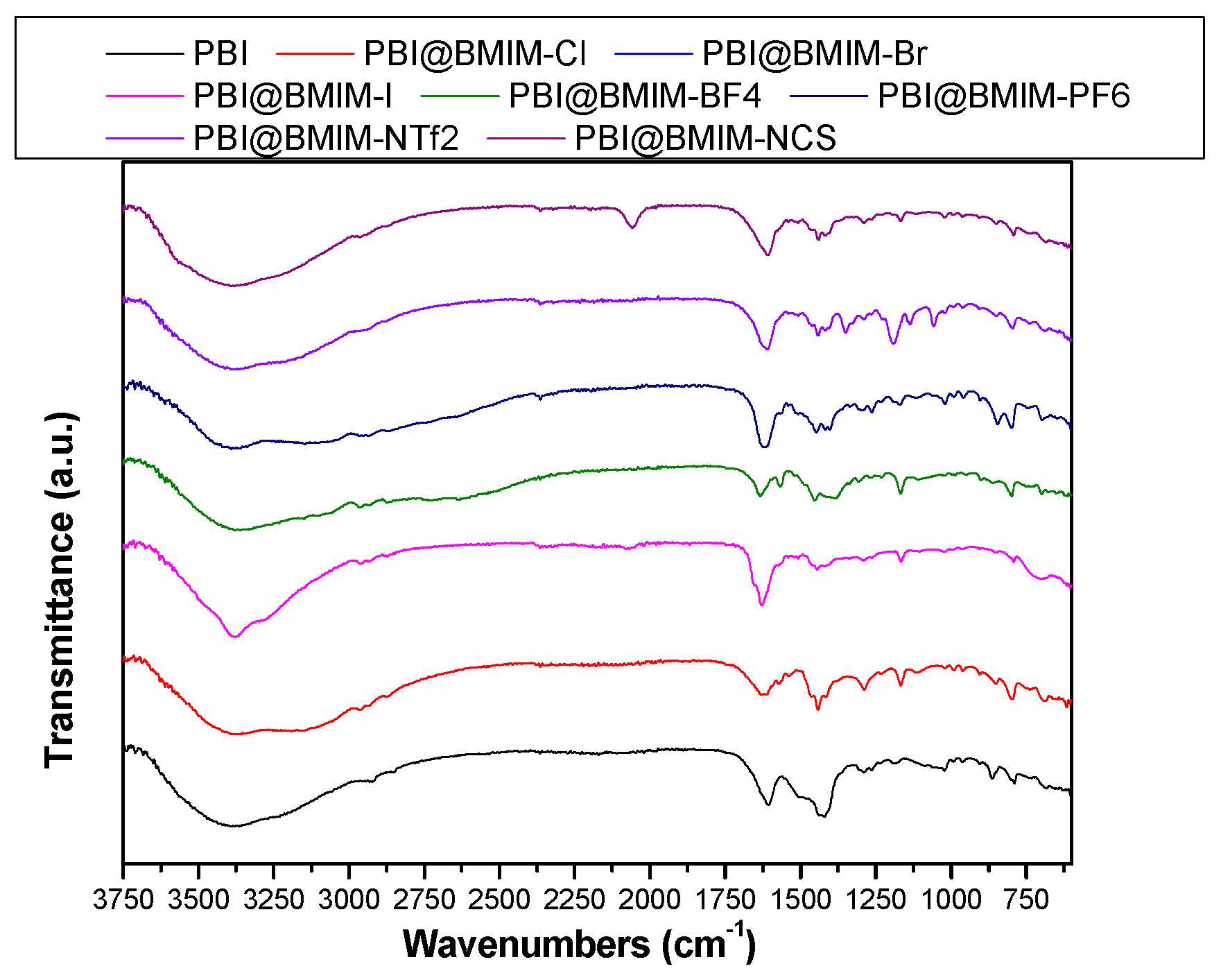
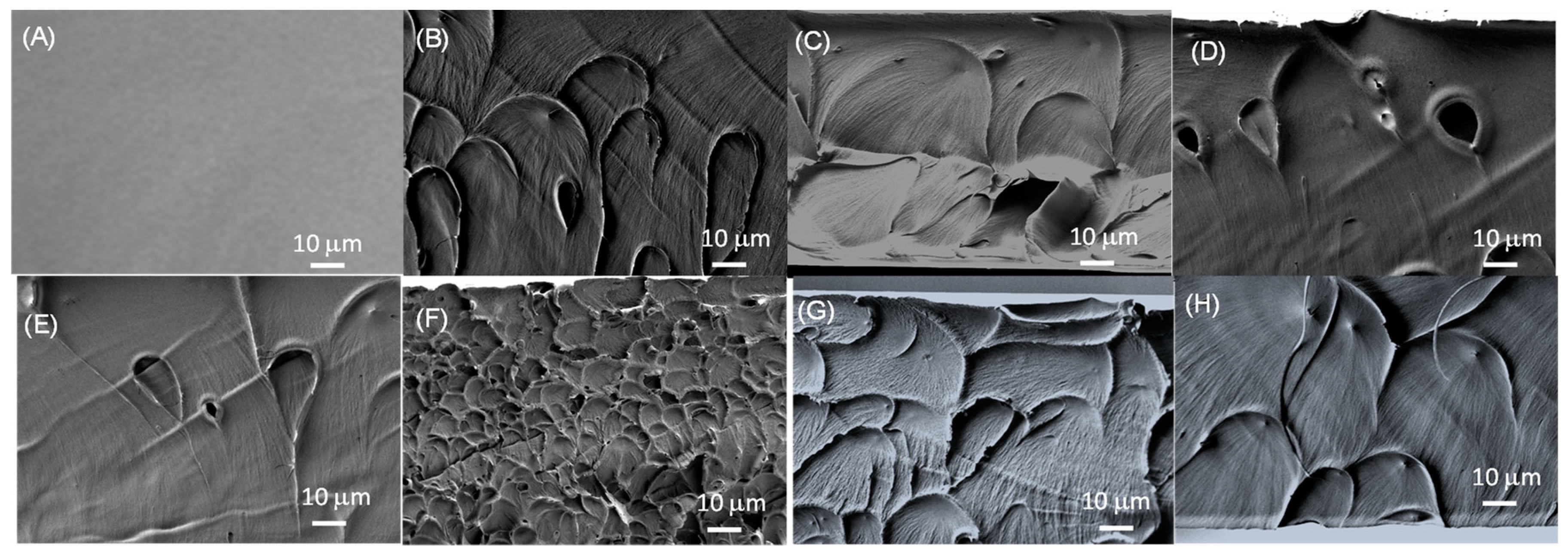
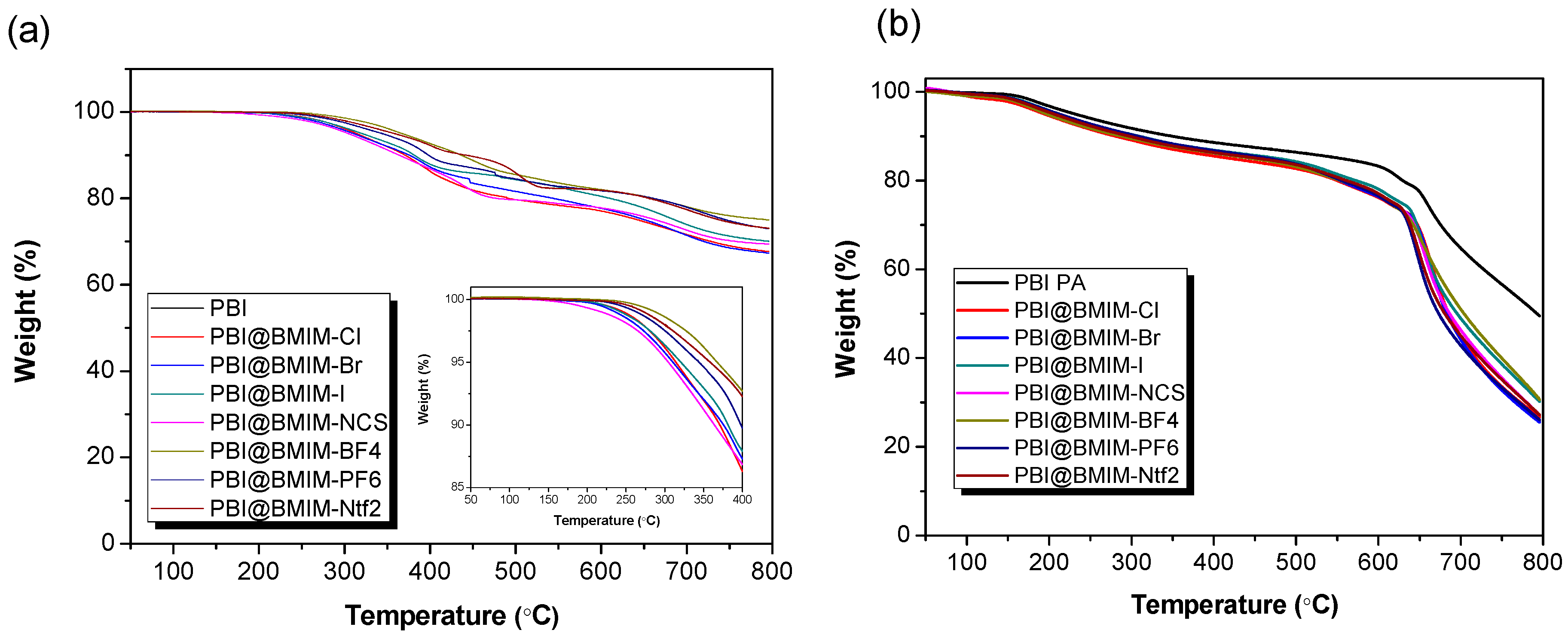
3. Results and discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ye, Y.-S.; Rick, J.; Hwang, B.-J. Water soluble polymers as proton exchange membranes for fuel cells. Polymers 2012, 4, 913–963. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Kreuer, K.-D. Proton conductivity: materials and applications. Chem. Mater. 1996, 8, 610–641. [Google Scholar] [CrossRef]
- Bakangura, E.; Wu, L.; Ge, L.; Yang, Z.; Xu, T. Mixed matrix proton exchange membranes for fuel cells: State of the art and perspectives. Prog. Polym. Sci. 2016, 57, 103–152. [Google Scholar] [CrossRef]
- Devanathan, R. Recent developments in proton exchange membranes for fuel cells. Energy Environ. Sci. 2008, 1, 101–119. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Savage, J.; Tse, Y.-L.S.; Voth, G.A. Proton transport mechanism of perfluorosulfonic acid membranes. J. Phys. Chem. C 2014, 118, 17436–17445. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, W.; Li, Y. An overview of the proton conductivity of Nafion membranes through a statistical analysis. J. Membr. Sci. 2016, 504, 1–9. [Google Scholar] [CrossRef]
- Xia, R.; Zhou, H.; Wu, R.; Wu, W.-P. Nanoindentation Investigation of Temperature Effects on the Mechanical Properties of Nafion® 117. Polymers 2016, 8, 344. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Review of advanced materials for proton exchange membrane fuel cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; et al. High temperature PEM fuel cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Choi, S.-W.; Park, J.O.; Pak, C.; Choi, K.H.; Lee, J.-C.; Chang, H. Design and synthesis of cross-linked copolymer membranes based on poly(benzoxazine) and polybenzimidazole and their application to an electrolyte membrane for a high-temperature PEM fuel cell. Polymers 2013, 5, 77–111. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Gnana Kumar, G.; Yoon, J.M.; Yoo, D.J. Toward improved mechanical strength, oxidative stability and proton conductivity of an aligned quadratic hybrid (SPEEK/FPAPB/Fe3O4-FGO) membrane for application in high temperature and low humidity fuel cells. RSC Adv. 2017, 7, 39034–39048. [Google Scholar] [CrossRef]
- Kim, A.R.; Yoo, D.J. A comparative study on physiochemical, thermomechanical, and electrochemical properties of sulfonated poly(ether ether ketone) block copolymer membranes with and without Fe3O4 nanoparticles. Polymers 2019, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Uregen, N.; Pehlivanoglu, K.; Ozdemir, Y.; Devrim, Y. Development of polybenzimidazole/graphene oxide composite membranes for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 2636–2647. [Google Scholar] [CrossRef]
- Wieser, C. Novel polymer electrolyte membranes for automotive applications—requirements and benefits. Fuel Cells 2004, 4, 245–250. [Google Scholar] [CrossRef]
- Rasheed, R.K.A.; Liao, Q.; Zhang, C.; Chan, S.H. A review on modelling of high temperature proton exchange membrane fuel cells (HT-PEIVIFCs). Int. J. Hydrogen Energy 2017, 42, 3142–3165. [Google Scholar]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kaer, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Zhao, Y.; Lu, W.; Shao, Z.; Yi, B. High-temperature proton-exchange-membrane fuel cells using an ether-containing polybenzimidazole membrane as electrolyte. ChemSusChem 2012, 5, 896–900. [Google Scholar] [CrossRef]
- Ghosh, S.; Maity, S.; Jana, T. Polybenzimidazole/silica nanocomposites: Organic-inorganic hybrid membranes for PEM fuel cell. J. Mater. Chem. 2011, 21, 14897–14906. [Google Scholar] [CrossRef]
- Singha, S.; Jana, T. Influence of interfacial interactions on the properties of polybenzimidazole/clay nanocomposite electrolyte membrane. Polymer 2016, 98, 20–31. [Google Scholar] [CrossRef]
- Fuentes, I.; Andrio, A.; Garcia-Bernabé, A.; Escorihuela, J.; Viñas, C.; Teixidor, F.; Compañ, V. Structural and dielectric properties of cobaltacarborane composite polybenzimidazole membranes as solid polymer electrolytes at high temperatura. Phys. Chem. Chem. Phys. 2018, 20, 10173–10184. [Google Scholar] [CrossRef]
- Ozdemir, Y.; Uregen, N.; Devrim, Y. Polybenzimidazole based nanocomposite membranes with enhanced proton conductivity for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 2648–2657. [Google Scholar] [CrossRef]
- Xu, C.X.; Wu, X.; Wang, X.; Mamlouk, M.; Scott, K. Composite membranes of polybenzimidazole and caesium-salts-of-heteropolyacids for intermediate temperature fuel cells. J. Mater. Chem. 2011, 21, 6014–6019. [Google Scholar] [CrossRef]
- Escorihuela, J.; Narducci, R.; Compañ, V.; Costantino, F. Proton conductivity of composite polyelectrolyte membranes with metal-organic frameworks for fuel cell applications. Adv. Mater. Interfaces 2019, 6, 1801146. [Google Scholar] [CrossRef]
- Barjola, A.; Escorihuela, J.; Andrio, A.; Giménez, E.; Compañ, V. Enhanced conductivity of composite membranes based on sulfonated poly(ether ether ketone) (SPEEK) with zeolitic imidazolate frameworks (ZIFs). Nanomaterials 2018, 8, 1042. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Gnana Kumar, G.; Yoo, D.J. Sulfonated graphene oxide/Nafion composite membranes for high temperature and low humidity proton exchange membrane fuel cells. RSC Adv. 2018, 8, 7494–7508. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, X.; Ma, Y.; Huang, Y.; Xiaobo Liu, X. Constructing continuous proton-conducting highways within sulfonated poly(arylene ether nitrile) composite membrane by incorporating amino-sulfo-bifunctionalized GO. Polymers 2018, 10, 1005. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.X.; Wang, S.; Li, J.S.; Tian, X.; Wang, X.; Chen, H.; Wang, Z. Polybenzimidazole/ionic-liquid-functional silica composite membranes with improved proton conductivity for high temperature proton exchange membrane fuel cells. J. Membr. Sci. 2017, 541, 492–499. [Google Scholar] [CrossRef]
- Altava, B.; Compañ, V.; Andrio, A.; Del Castillo, L.F.; Mollá, S.; Burguete, M.I.; García-Verdugo, E.; Luis, S.V. Conductive films based on composite polymers containing ionic liquids absorbed on crosslinked polymeric ionic-like liquids (SILLPs). Polymer 2015, 72, 69–81. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Ricka, J.; Hwang, B.-J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Chen, D.; Ying, W.; Guo, Y.; Ying, Y.; Peng, X. Enhanced Gas Separation through Nanoconfined Ionic Liquid in Laminated MoS2 Membrane. ACS Appl. Mater. Interfaces 2017, 9, 44251–44257. [Google Scholar] [CrossRef] [PubMed]
- Marrucho, I.M.; Branco, L.C.; Rebelo LP, N. Ionic Liquids in Pharmaceutical Applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- González-Mendoza, L.; Altava, B.; Burguete, M.I.; Escorihuela, J.; Hernando, E.; Luis, S.V.; Quesada, R.; Vicent, C. Bis(imidazolium) salts derived from amino acids as receptors and transport agents for chloride anions. RSC Advances 2015, 5, 34415–34423. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, J.; Huang, C.; Lei, Z. Ionic liquids in selective oxidation: catalysts and solvents. Chem. Rev. 2017, 117, 6929–6983. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Li, J.; Li, Z.; He, P.; Liu, H.; Li, J. Highly active horseradish peroxidase immobilized in 1-butyl-3-methylimidazolium tetrafluoroborate room-temperature ionic liquid based sol–gel host materials. Chem. Commun. 2005, 1778–1780. [Google Scholar] [CrossRef]
- González, L.; Escorihuela, J.; Altava, B.; Burguete, M.I.; Luis, S.V. Chiral room temperature ionic liquids as enantioselective promoters for the asymmetric aldol reaction. Eur. J. Org. Chem. 2014, 5356–5363. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of ionic liquids to energy storage and conversion materials and devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef]
- Díaz, M.; Ortiz, A.; Ortiz, I. Progress in the use of ionic liquids as electrolyte membranes in fuel cells. J. Membr. Sci. 2017, 469, 379–396. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H., Jr.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Sun, P.; Li, Z.; Jin, L.; Yang, Y.; Wang, S.; Yin, X.; Wang, Y. Pre-oxidized acrylic fiber reinforced ferric sulfophenyl phosphate-doped polybenzimidazole-based high-temperature proton exchange membrane. Macromol. Mater. Eng. 2017, 302, 1600468. [Google Scholar] [CrossRef]
- Hanke, K.; Kaufmann, M.; Schwaab, G.; Havenith, M.; Wolke, C.T.; Gorlova, O.; Johnson, M.A.; Prasad Kar, B.; Sander, W.; Sanchez-Garcia, E. Understanding the ionic liquid [NC4111][NTf2] from individual building blocks: an IR-spectroscopic study. Phys. Chem. Chem. Phys. 2015, 17, 8518–8529. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Liu, C.; Li, J.; Liu, F.; Tian, X.; Chen, H.; Mao, T.; Xu, J.; Wang, Z. Cage-like cross-linked membranes with excellent ionic liquid retention and elevated proton conductivity for HT-PEMFCs. Electrochimica Acta 2018, 283, 691–698. [Google Scholar] [CrossRef]
- Mack, F.; Aniol, K.; Ellwein, C.; Kerres, J.; Zeis, R. Novel phosphoric acid-doped PBI-blends as membranes for high-temperature PEM fuel cells. J. Mater. Chem. A 2015, 3, 10864–10874. [Google Scholar] [CrossRef]
- Cao, Y.; Tiancheng Mu, T. Comprehensive investigation on the thermal stability of 66 ionic liquids by thermogravimetric analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.J.; Ma, W.J.; Zhang, G.; Liu, Z.G.; Ni, J.; Li, M.Y.; Zhang, N.; Na, H. Preparation and properties of epoxy-cross-linked porous polybenzimidazole for high temperature proton exchange membrane fuel cells. J. Membr. Sci. 2012, 411–412, 54–63. [Google Scholar] [CrossRef]
- Chuang, S.W.; Hsu, S.L.C. Synthesis and properties of a new fluorine-containing polybenzimidazole for high-temperature fuel-cell applications. J. Polym. Sci. A 2006, 44, 4508–4513. [Google Scholar] [CrossRef]
- Wang, J.T.-W.; Hsu, S.L.-C. Enhanced high-temperature polymer electrolyte membrane for fuel cells based on polybenzimidazole and ionic liquids. Electrochim. Acta 2011, 56, 2842–2846. [Google Scholar] [CrossRef]
- Han, M.; Zhang, G.; Liu, Z.; Wang, S.; Li, M.; Zhu, J.; Li, H.; Zhang, Y.; Lew, C.M.; Na, H. Cross-linked polybenzimidazole with enhanced stability for high temperature proton exchange membrane fuel cells. J. Mater. Chem. 2011, 21, 2187–2193. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Ma, W.; Zhang, N.; Liu, Z.; Zhang, G.; Na, H. Macromolecular cross-linked polybenzimidazole based on bromomethylated poly (aryl ether ketone) with enhanced stability for high temperature fuel cell applications. J. Power Sources 2013, 243, 102–109. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Chen, H.; Li, J.; Tian, X.; Wang, X.; Mao, T.; Xu, J.; Wang, Z. Cross-linkable polymeric ionic liquid improve phosphoric acid retention and long-term conductivity stability in polybenzimidazole based PEMs. ACS Sustainable Chem. Eng. 2018, 6, 16352–16362. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Ma, W.; Zhang, N.; Zhang, Y.; Zhang, G.; Liu, Z.; Na, H. Silane-cross-linked polybenzimidazole with improved conductivity for high temperature proton exchange membrane fuel cells. J. Mater. Chem. A 2013, 1, 621–629. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Chen, D.; Ye, Z. Sulfonated binaphthyl-containing poly(arylene ether ketone)s with rigid backbone and excellent film-forming capability for proton exchange membranes. Polymers 2018, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Rewar, A.S.; Chaudhari, H.D.; Illathvalappil, R.; Sreekumar, K.; Kharul, U.K. New approach of blending polymeric ionic liquid with polybenzimidazole (PBI) for enhancing physical and electrochemical properties. J. Mater. Chem. A 2014, 2, 14449–14458. [Google Scholar] [CrossRef]
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerruma, N.J. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: an overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Sacco, A. Electrochemical impedance spectroscopy: Fundamentals and application in dye-sensitized solar cells. Renewable Sustainable Energy Rev. 2017, 79, 814–829. [Google Scholar] [CrossRef]
- Bode, H.W. Network Analysis and Feedback Amplifier Design; Van Nostrand: Princeton, NJ, USA, 1945. [Google Scholar]
- Reyes-Rodriguez, J.L.; Escorihuela, J.; García-Bernabé, A.; Giménez, E.; Solorza-Feria, O.; Compañ, V. Proton conducting electrospun sulfonated polyether ether ketone graphene oxide composite membranes. RSC Adv. 2017, 7, 53481–53491. [Google Scholar] [CrossRef]
- Ghatee, M.H.; Zolghadr, A.R. Local depolarization in hydrophobic and hydrophilic ionic liquids/water mixtures: Car−Parrinello and classical molecular dynamics simulation. J. Phys. Chem. C 2013, 117, 2066–2077. [Google Scholar] [CrossRef]
- Chen, B.-K.; Wu, T.-Y.; Wong, J.-M.; Chang, Y.-M.; Lee, H.-F.; Huang, W.-Y.; Chen, A.F. Highly sulfonated diamine synthesized polyimides and protic ionic liquid composite membranes improve PEM conductivity. Polymers 2015, 7, 1046–1065. [Google Scholar] [CrossRef]
- Pu, H.T.; Wang, L.; Pan, H.Y.; Wan, D.C. Synthesis and characterization of fluorine-containing polybenzimidazole for proton conducting membranes in fuel cell. J. Polym. Sci. Part A 2010, 48, 2115–2122. [Google Scholar] [CrossRef]
- Sun, P.; Li, Z.; Wang, S.; Yin, X. Performance enhancement of polybenzimidazole based high temperature proton exchange membranes with multifunctional crosslinker and highly sulfonated polyaniline. J. Membr. Sci. 2018, 549, 660–669. [Google Scholar] [CrossRef]
- Escorihuela, J.; Sahuquillo, O.; García-Bernabé, A.; Giménez, E.; Compañ, V. Phosphoric acid doped polybenzimidazole (PBI)/zeolitic imidazolate framework composite membranes with significantly enhanced proton conductivity under low humidity conditions. Nanomaterials 2018, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.E., Jr. Mobility and conductivity of ions in and into polymeric solids. Pure Appl. Chem. 1976, 46, 157–170. [Google Scholar] [CrossRef][Green Version]
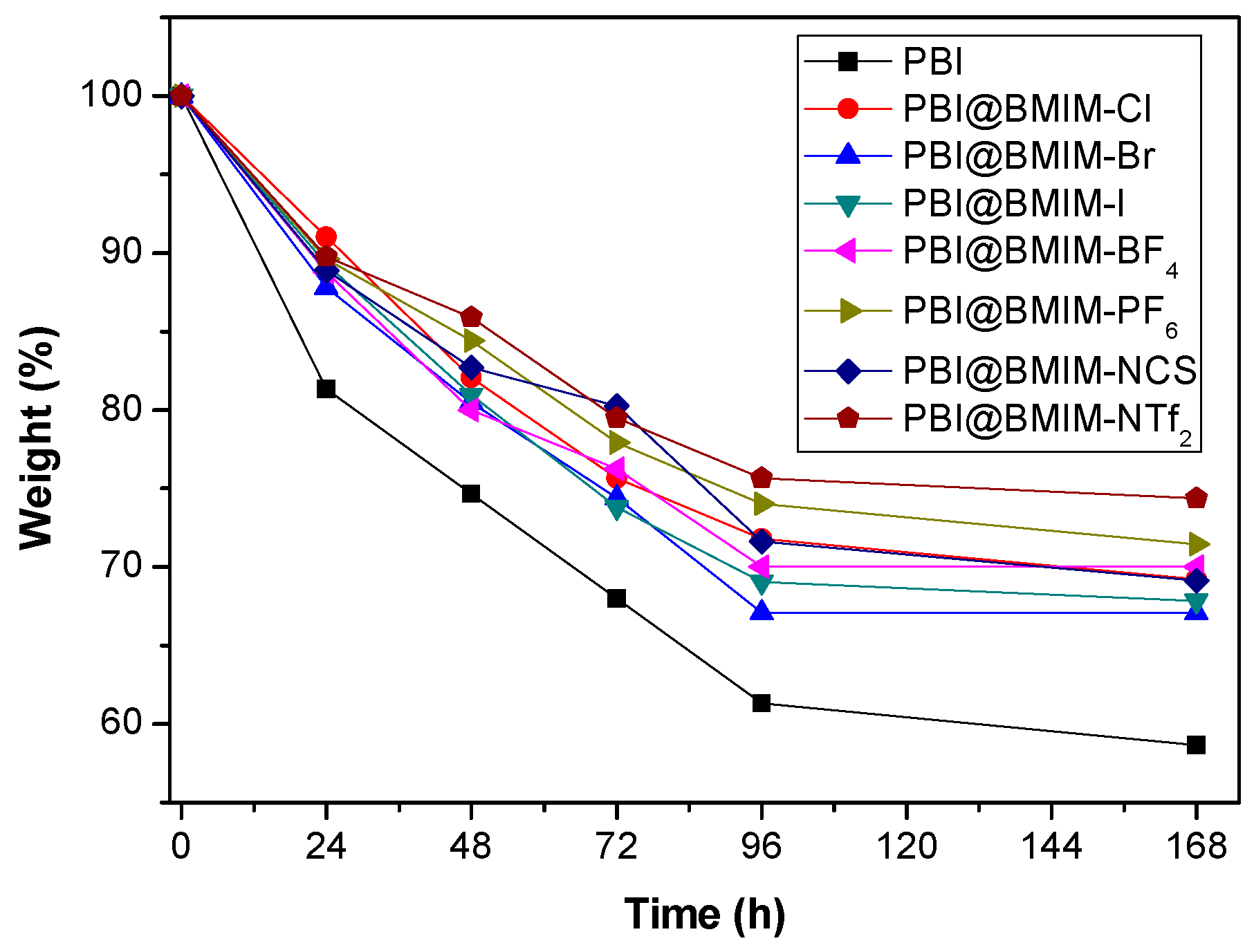
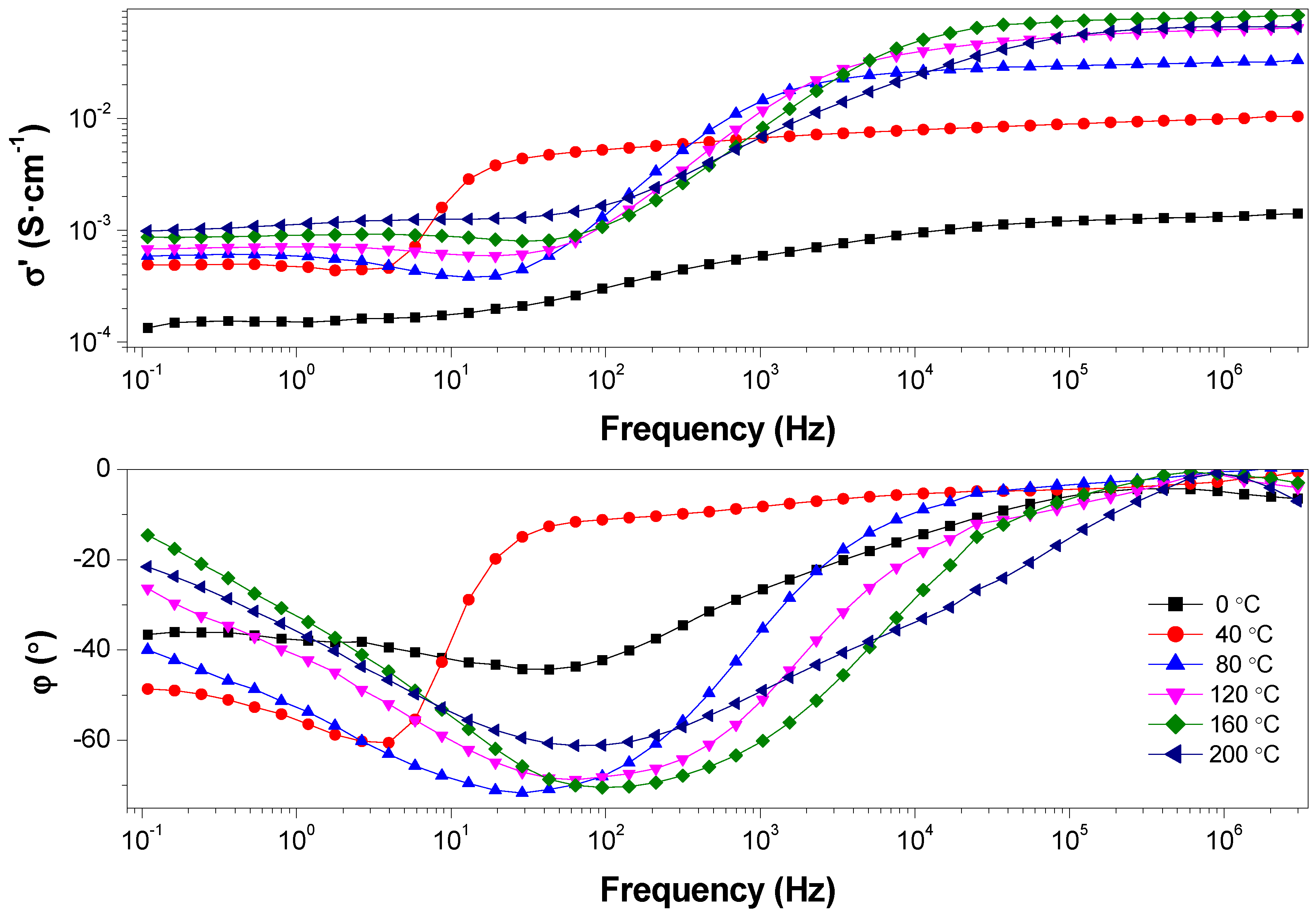
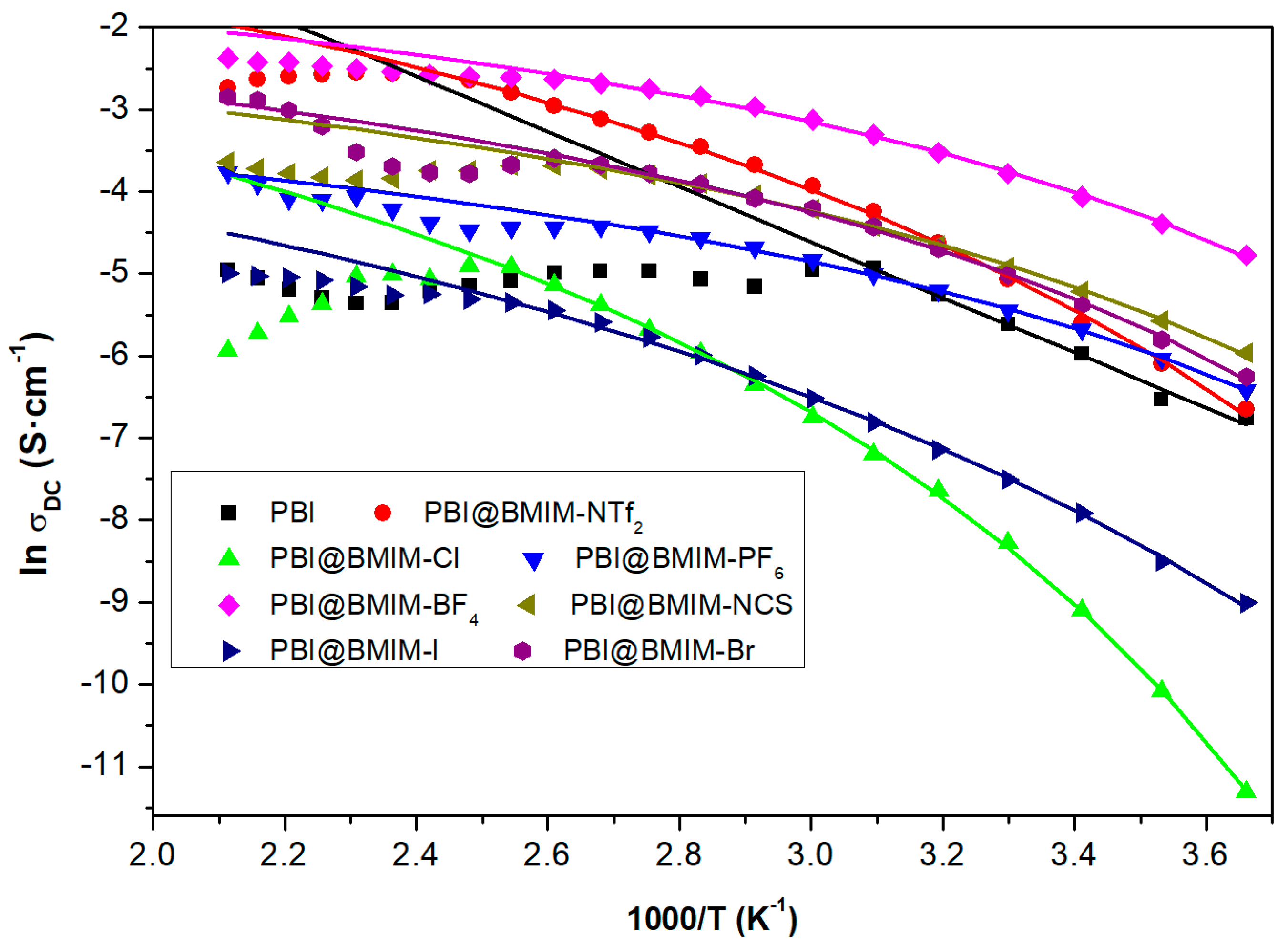
| Membrane | Young’s Modulus (GPa) | Tensile Stress (MPa) | Strain at break (%) |
|---|---|---|---|
| PBI | 2.6 ± 0.5 | 97 ± 4 | 27 ± 4 |
| PBI@BMIM-Cl | 3.7 ± 0.1 | 141 ± 3 | 9 ± 1 |
| PBI@BMIM-Br | 3.0 ± 0.3 | 128 ± 4 | 15 ± 3 |
| PBI@BMIM-I | 3.6 ± 0.1 | 131 ± 3 | 7 ± 1 |
| PBI@BMIM-BF4 | 2.8 ± 0.1 | 125 ± 4 | 17 ± 4 |
| PBI@BMIM-PF6 | 3.4 ± 0.3 | 124 ± 2 | 8 ± 1 |
| PBI@BMIM-NCS | 3.6 ± 0.4 | 131 ± 3 | 10 ± 1 |
| PBI@BMIM-NTf2 | 3.1 ± 0.3 | 127 ± 2 | 19 ± 1 |
| T (°C) | PBI | [Cl]− | [Br]− | [I]− | [BF4]− | [PF6]− | [NCS]− | [NTf2]− |
|---|---|---|---|---|---|---|---|---|
| 0 | 1.2 × 10−3 | 1.2 × 10−5 | 1.9 × 10−3 | 1.2 × 10−4 | 8.5 × 10−3 | 1.6 × 10−3 | 2.6 × 10−3 | 1.3 × 10−3 |
| 40 | 5.2 × 10−3 | 4.8 × 10−4 | 9.1 × 10−3 | 7.9 × 10−4 | 2.9 × 10−2 | 5.4 × 10−3 | 9.5 × 10−3 | 9.8 × 10−3 |
| 80 | 6.3 × 10−3 | 2.6 × 10−3 | 2.0 × 10−2 | 2.5 × 10−3 | 5.8 × 10−2 | 1.0 × 10−2 | 2.0 × 10−2 | 3.1 × 10−2 |
| 120 | 6.1 × 10−3 | 7.4 × 10−3 | 2.5 × 10−2 | 4.7 × 10−4 | 7.4 × 10−2 | 1.2 × 10−2 | 2.5 × 10−2 | 6.1 × 10−2 |
| 160 | 4.7 × 10−3 | 6.5 × 10−3 | 3.0 × 10−2 | 5.8 × 10−3 | 8.2 × 10−2 | 1.7 × 10−2 | 2.1 × 10−2 | 7.8 × 10−2 |
| 200 | 7.1 × 10−3 | 2.6 × 10−2 | 5.8 × 10−2 | 6.8 × 10−3 | 9.4 × 10−2 | 2.3 × 10−2 | 2.6 × 10−2 | 6.5 × 10−2 |
| Membrane | Ln σ∞ (S·cm−1) | T0 (K) | Eact (kJ·mol−1) |
|---|---|---|---|
| PBI@BMIM-Cl | −1.02 | 199 | 6.33 |
| PBI@BMIM-Br | −1.61 | 195 | 3.04 |
| PBI@BMIM-I | −2.19 | 172 | 5.80 |
| PBI@BMIM-BF4 | −0.97 | 194 | 2.53 |
| PBI@BMIM-PF6 | −2.72 | 192 | 2.51 |
| PBI@BMIM-NCS | −1.81 | 190 | 2.91 |
| PBI@BMIM-NTf2 | 0.24 | 181 | 5.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escorihuela, J.; García-Bernabé, A.; Montero, Á.; Sahuquillo, Ó.; Giménez, E.; Compañ, V. Ionic Liquid Composite Polybenzimidazol Membranes for High Temperature PEMFC Applications. Polymers 2019, 11, 732. https://doi.org/10.3390/polym11040732
Escorihuela J, García-Bernabé A, Montero Á, Sahuquillo Ó, Giménez E, Compañ V. Ionic Liquid Composite Polybenzimidazol Membranes for High Temperature PEMFC Applications. Polymers. 2019; 11(4):732. https://doi.org/10.3390/polym11040732
Chicago/Turabian StyleEscorihuela, Jorge, Abel García-Bernabé, Álvaro Montero, Óscar Sahuquillo, Enrique Giménez, and Vicente Compañ. 2019. "Ionic Liquid Composite Polybenzimidazol Membranes for High Temperature PEMFC Applications" Polymers 11, no. 4: 732. https://doi.org/10.3390/polym11040732
APA StyleEscorihuela, J., García-Bernabé, A., Montero, Á., Sahuquillo, Ó., Giménez, E., & Compañ, V. (2019). Ionic Liquid Composite Polybenzimidazol Membranes for High Temperature PEMFC Applications. Polymers, 11(4), 732. https://doi.org/10.3390/polym11040732








